Introduction
Depression is one of the most prevalent psychopathologies and a cause of morbidity and mortality in the world. Furthermore, it is a serious and recurrent disorder often manifested with psychological, behavioural and physiological symptoms. It affects 17–20% of the world's population resulting in premature death, major social deficits and economic consequences Reference Kessler, McGonagle and Zhao(1). Among people with major depression, 75–85% have recurrent episodes Reference Mueller, Leon and Keller(2) and 10–30% recover incompletely, presenting persistent and residual depressive symptoms Reference Mann(3).
Recently, several studies have been carried out in order to develop a better treatment for major depression, considering that the adequate management of this psychopathology remains a challenge. The primary reasons for the unsuitable treatment include the incidence of undesired side-effects produced by currently available antidepressants, the apparent delay in achieving therapeutic benefits and a high percentage of treatment-resistant patients Reference Skolnick, Popik, Janowsky, Beer and Lippa(4). Thus, there is a compelling need for the development of novel antidepressant therapies with improved pharmacological actions Reference Skolnick(5).
The challenges for the design of new agents to treat depression are the rapid onset of antidepressant response, broader efficacy and fewer adverse effects. While progress has been made to reduce side-effects, currently available antidepressants do not show convincing evidence of neither rapid onsets of therapeutic actions nor improved efficacy in the treatment of major depression Reference Nutt(6).
Modulation and dysfunction of the glutamatergic system seem to be involved in depression. Glutamate is the primary excitatory neurotransmitter in the mammalian brain. Glutamatergic neurotransmission may be modulated in the brain by different receptor types, including ionotropic and metabotropic receptors. In this context, studies have pointed to an important role for the ionotropic glutamate N-methyl-d-aspartate (NMDA) receptor in the aetiology of psychopathologies such as anxiety and major depression Reference Javitt(7,Reference Krystal, D’Souza and Petrakis8).
Ketamine is a non-competitive antagonist of NMDA glutamate receptor, which works in a voltage-dependent manner. Ketamine binds to the ionic channel of NMDA receptor, blocking the entrance of Ca2+ into the neuron Reference Danysz and Parsons(9). However, distinct from other NMDA receptor antagonists, ketamine also interacts with voltage-sensitive Ca2+ channels and opioid, monoaminergic and muscarinic receptors Reference Hirota and Lambert(10). Recently, a renewed interest in the glutamatergic system as a treatment option for major depression emerged from the fact that the NMDA antagonist ketamine leads to a rapid improvement of depressive symptoms Reference Murck, Schubert, Schmid, Schüssler, Steiger and Auer(11).
Several recent works support the hypothesis that metabolism impairment is also involved in the pathophysiology of depression Reference Kanarik, Matrov, Kõiv, Eller, Tõnissaar and Harro(12,Reference Stanyer, Jorgensen, Hori, Clark and Heales13). Mitochondrial oxidative phosphorylation is the major adenosine triphosphate (ATP) producing pathway, which supplies more than 95% of the total energy requirement of the cells Reference Boekema and Braun(14). Damage in the mitochondrial electron transport chain has been suggested as an important factor in the pathogenesis of a range of neuropsychiatric disorders such as bipolar disorder, depression and schizophrenia Reference Rezin, Amboni, Zugno, Quevedo and Streck(15). In this context, we have recently reported that mitochondrial respiratory chain complexes I, III and IV were inhibited in the cerebral cortex and cerebellum of rats after 40 days of chronic mild stress (CMS) Reference Rezin, Cardoso and Gonçalves(16). CMS is an animal model of depression which consists of exposing animals to a variety of mild and unpredictable stressors (e.g. isolation, water and food deprivation, restraint, forced swimming and flashing light exposure) for several days Reference Gamaro, Manoli, Torres, Silveira and Dalmaz(17). We have also shown that complex II and creatine kinase were not affected in this animal model Reference Rezin, Cardoso and Gonçalves(16). Besides, we have also shown that inhibition of complexes I, III and IV caused by CMS was reversed by acute administration of ketamine Reference Rezin, Gonçalves and Daufenbach(18).
Considering that we have recently shown that mitochondrial respiratory chain complexes I, III and IV were inhibited in the cerebral cortex and cerebellum of rats after 40 days of CMS and that acute administration of ketamine reversed this inhibition, we investigated whether the inhibition of these enzymes may be reversed by chronic ketamine administration.
Experimental procedure
Animals
Male Wistar rats (300 g) were obtained from Central Animal House of Universidade do Extremo Sul Catarinense. The animals had free access to food (rat chow) and water, except for the stressed group during the period when the stressor applied required no food or water. The rats were maintained on a 12-h light–dark cycle (lights on at 7:00 am), at a temperature of 23 °C ± 1 °C. The experiments were carried out in accordance with the National Institutes of Health Guide for the Use and Care of Laboratory Animals and the Brazilian Society for Neuroscience and Behavior (SBNeC) recommendations for animal care, with the approval of Universidade do Extremo Sul Catarinense (UNESC) Ethics Committee. Moreover, all efforts were made to minimise animal suffering as well as to reduce the number of animals.
Chronic mild stress (CMS) model
CMS protocol was adapted from the procedure described by Gamaro et al. Reference Gamaro, Manoli, Torres, Silveira and Dalmaz(17). The animals were divided into control and stressed groups. Controls were kept undisturbed in their home cages during the 40 days of the experiment; a 40-day variate-stressors paradigm was used for the animals in the stressed group. Stress application started at different times everyday, in order to minimise its predictability.
Drugs and treatment
After 40 days of CMS, the animals were divided into four groups as follows: (a) control + saline, (b) control + ketamine, (c) CMS + saline and (d) CMS + ketamine. Ketamine (Fort Dodge Animal Health, Fort Dodge, IA) was administered at a dose of 15 mg/kg to evoke antidepressant-like effects. The chronic treatment with ketamine was performed during the anhedonia test, once a day, during 7 days, 60 min prior to behavioural assessment.
Consumption of sweet food
After 40 days of CMS, consumption of sweet food was measured to verify anhedonia. The animals were placed in a lightened rectangular box (40 cm × 15 cm × 20 cm) with a glass ceiling and floor and side walls made of wood. This test was performed between 8:00 a.m. and 12:00 a.m. Ten Froot Loops (Kellogg's®, pellets of wheat and corn starch and sucrose) were placed in each extremity of the box. Animals were submitted to five trial sessions of 3 min, once a day, in order to become familiarised with this food Reference Ely, Dapper and Marasca(19). After the habituation, the animals were exposed to two test sessions of 3 min each. After the fifth training session the number of ingested pellets was counted. Consumption of even part of the Froot Loops (e.g. 1/3 or 1/4), was also considered. These two evaluations were made with the animals submitted to fasting (during a period of 22 h prior to the behavioural task) or with animals fed ad libitum. These evaluations were made since food deprivation, which is used in many behaviour tasks as a motivating stimulus, may also be an acute stressor Reference Katz(20).
Adrenal gland and body weight
Body weight was measured at the beginning and at the end of the experiment. After killing the animals, the adrenal gland weight was evaluated as an indirect parameter of hypothalamic-pituitary-adrenal axis activation. Adrenal gland weight is usually found increased in chronic stress as a result of glucocorticoid hormones release by the adrenals in response to physical and psychological stressors.
Tissue and homogenate preparation
The animals were killed by decapitation, the brain was removed and the cerebellum and cerebral cortex were homogenised (1:10, w/v) in Sucrose-EDTA- Trizma base-Heparin (SETH) buffer, pH 7.4 (250 mM sucrose, 2 mM ethylenediaminetetraacetic acid (EDTA), 10 mM Trizma base, 50 IU/ml heparin). The homogenates were centrifuged at 800 ×g for 10 min, and the supernatants were stored at −70 °C until used for the determination of enzymes activities. The maximal period between homogenate preparation and enzyme analysis was always less than 5 days. Protein content was determined by the method described by Lowry et al. Reference Lowry, Rosebough, Farr and Randall(21) using bovine serum albumin as the standard.
Mitochondrial respiratory chain enzymes activities
Nicotinamide adenine dinucleotide (NADH) dehydrogenase (complex I) was evaluated by the method described by Cassina and Radi Reference Cassina and Radi(22) by the rate of NADH-dependent ferricyanide reduction at 420 nm. The activity of succinate cytochrome c oxidoreductase (complex III) was determined according to the method of Fischer et al. Reference Fischer, Ruitenbeek and Berden(23), measured by cytochrome c reduction using succinate as the substrate. The activity of cytochrome c oxidase (complex IV) was assayed according to the method described by Rustin et al. Reference Rustin, Chretien and Bourgeron(24), measured by following the decrease in absorbance at 550 nm because of the oxidation of previously reduced cytochrome c. The activities of the mitochondrial respiratory chain complexes were expressed as nanomole per minute per milligram of protein.
Statistical analysis
Data were analysed by one-way analysis of variance followed by the Tukey test when F was significant, and are expressed as mean ± standard deviation (SD). All analyses were performed using the Statistical Package for the Social Science (SPSS) software.
Results
Consumption of sweet food
CMS decreased the intake of sweet food in the stressed group when compared to the control group. Chronic administration of ketamine did not alter sweet food intake in control rats. Moreover, chronic administration of ketamine re-established sweet food intake in rats submitted to CMS (Figure 1).
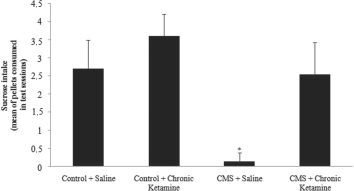
Fig. 1 Effects of CMS procedure on sweet food consumption (average of pellets consumed in 2 days of tests) in rats chronically treated with ketamine. Values are expressed as mean ± SD(n = 15). Different from control + saline: *p < 0.05 [one-way analysis of variance (ANOVA) followed by Tukey test].
Adrenal gland and body weights
Adrenal gland weight was significantly increased in stressed rats when compared to non-stressed rats. Moreover, chronic administration of ketamine re-established the normal range of adrenal gland weight in stressed rats. Body weights before and after 40 days of CMS were also measured. The results showed that the control group gained weight after 40 days but the stressed group did not gain weight after the same period (Figure 2). Moreover, stressed animals gained weight after chronic administration of ketamine, when compared to the body weight assessed at the beginning of the experiment (Figure 3).

Fig. 2 Effects of CMS procedure on body weight of rats chronically treated with ketamine. Values are expressed as mean ± SD(n = 15). Different from control + saline: *p < 0.05 [one-way analysis of variance (ANOVA) followed by Tukey test].

Fig. 3 Effects of CMS procedure on adrenal gland weight in rats chronically treated with ketamine. Values are expressed as mean ± SD(n = 15). Different from control + saline: *p < 0.05 [one-way analysis of variance (ANOVA) followed by Tukey test].
Activities of mitochondrial respiratory chain enzymes
As described previously Reference Rezin, Cardoso and Gonçalves(16), complexes I, III and IV were inhibited in stressed groups in the cerebral cortex and cerebellum. Moreover, chronic administration of ketamine reversed the inhibition of complexes I, III and IV activities in the cerebral cortex caused by CMS (Figures 4–6). However, chronic administration of ketamine reversed only the inhibition of complex IV activity in the cerebellum. Complexes I and III activities were not re-established by the chronic administration of ketamine in the cerebellum.
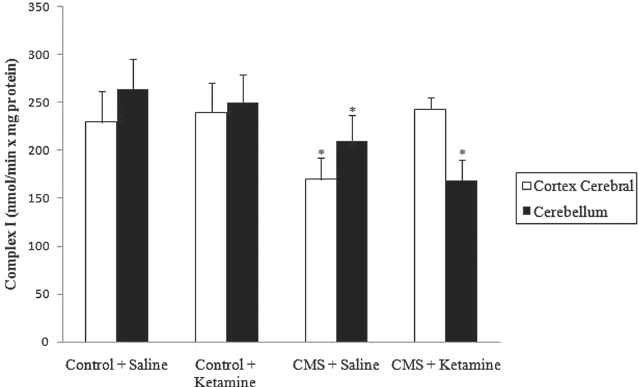
Fig. 4 Effect of chronic administration of ketamine on the activity of complex I in cerebral cortex and cerebellum of rats submitted to CMS. Values are expressed as mean ± SD(n = 6). Different from control + saline: *p < 0.05 [one-way analysis of variance (ANOVA) followed by Tukey test].
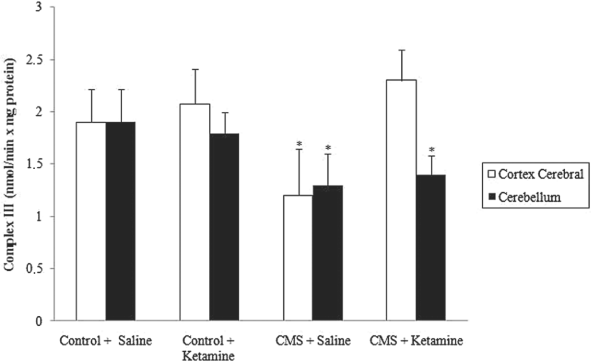
Fig. 5 Effect of chronic administration of ketamine on the activity of complex III in cerebral cortex and cerebellum of rats submitted to CMS. Values are expressed as mean ± SD(n = 6). Different from control + saline: *p < 0.05 [one-way analysis of variance (ANOVA) followed by Tukey test].
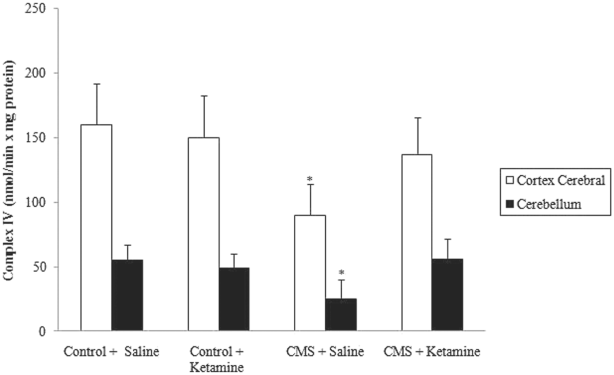
Fig. 6 Effect of chronic administration of ketamine on the activity of complex IV in cerebral cortex and cerebellum of rats submitted to CMS. Values are expressed as mean ± SD(n = 6). Different from control + saline: *p < 0.05 [one-way analysis of variance (ANOVA) followed by Tukey test].
Discussion
In the present study, we observed that rats submitted to CMS present with decreased sweet food intake and increased adrenal gland weight, without any change in the body weight, when compared to the control group. Several studies showed that chronic stress induced significant reduction in body weight gain, increased adrenal weight, as well as induced anhedonia (Reference Rezin, Cardoso and Gonçalves16,Reference Konarska, Stewart and McCarty25,Reference Harro, Tonissaar, Eller, Kask and Oreland26).
Moreover, chronic administration of ketamine re-established sweet food intake. In this context, previous studies showed that NMDA receptor antagonists such as AP-1 and MK801 increased the total amount of food consumed in rodents Reference Burns and Ritter(27,Reference Treece, Ritter and Burns28). This behavioural effect seems to be a mixture of central and visceral actions of NMDA receptor antagonists. Burns and Ritter Reference Burns and Ritter(27) have shown that the destruction of small unmyelinated visceral afferent neurons, which carry the satiety signals from the viscera to the satiety centre in the central nervous system (CNS), blocked the increase of food intake in MK801-treated rats. Additionally, Treece et al. Reference Treece, Ritter and Burns(28) have shown that the lesions of caudomedial subnucleus of the solitary tract and/or dorsal motor nucleus of the vagus in MK801-treated rats resulted in approximately the same amount of sucrose consumed as compared to animals that received saline.
Interestingly, repeated administrations of ketamine reversed the hypertrophy of adrenal gland in CMS rats. Distinct authors have already suggested an increase in rat adrenal weight after 14 Reference Harro, Tonissaar, Eller, Kask and Oreland(26) or 28 days Reference Konarska, Stewart and McCarty(25) of CMS procedure. The hypertrophy of adrenal gland could be mediated by the increase in adrenocorticotropic hormone (ACTH) circulating levels, which is released in high concentrations during stressful situations by the anterior pituitary gland Reference O’Connor, O’Halloran and Shanahan(29). It has been already shown that repeated administration of ketamine reversed these hormonal alterations in rats submitted to CMS, supporting the idea of a relevant role for the glutamatergic signalling, through NMDA receptors, in the mediation of hormonal aspects of stress.
Emotional changes such as exposure to stressful situations can influence feeding behaviour Reference Rezin, Cardoso and Gonçalves(16,Reference Bekris, Antoniou, Daskas and PapadopoulouDaifoti30). In this context, rats submitted to CMS and chronically treated with ketamine regain body weight when compared to CMS rats injected with saline. The rapid recovery of body weight in stressed rats may suggest that NMDA receptors modulate feeding behaviour during stressful situations.
The CMS paradigm is a model of depression characterised by chronic unpredictable mild stressors Reference Wilner(31). In the CMS model, both consumption and preference for sucrose intake, as well as decreased intracranial self-stimulation behaviour, are used as markers of generalised decrease in sensitivity to reward and they are quite related to anhedonia Reference Gamaro, Manoli, Torres, Silveira and Dalmaz(17,Reference Bekris, Antoniou, Daskas and PapadopoulouDaifoti30). According to the literature, the present data confirm that rats submitted to CMS procedure consume less sweet food compared to non-stressed rats. These findings suggest that, under our experimental conditions, the CMS procedure induced anhedonic-like behaviour in our rats.
Additionally, several researchers hypothesised that metabolism impairment and dysfunction of the mitochondrial electron transport chain are important factors in the pathogenesis of a range of neuropsychiatric disorders, such as depression Reference Rezin, Amboni, Zugno, Quevedo and Streck(15). Gardner et al. Reference Gardner, Johansson and Wibom(32) showed a significant decrease in mitochondrial ATP production rates and mitochondrial enzyme activities in the muscle of major depressive disorder patients. Madrigal et al. Reference Madrigal, Olivenza and Moro(33) also reported that mitochondrial respiratory chain complexes I–III and II–III were inhibited in rat brains after chronic stress (immobilisation for 6 h during 21 days). In this scenario, we have also shown that the activity of complexes I, III and IV was inhibited in the cerebral cortex and cerebellum after 40 days of CMS Reference Rezin, Cardoso and Gonçalves(16). In addition, we showed that complexes I, III and IV inhibition caused by CMS was reversed by acute administration of ketamine Reference Rezin, Gonçalves and Daufenbach(18). However, in the cerebellum, only complex IV inhibition was reversed by chronic administration of ketamine. Unfortunately, at present, we cannot explain the reason as to why the inhibition of complexes I and III activities caused by CMS in the cerebellum was not reversed by ketamine chronic administration. On the basis of a previous study from our laboratory, we hypothesise that ketamine may modulate brain energy metabolism by increasing the activity of creatine kinase; this enzyme plays a crucial role in tissues like the brain tissue. In this study, we observed that ketamine increased creatine kinase activity in the brain of rats, especially in the cerebral cortex and cerebellum Reference Assis, Rezin and Comim(34).
Ketamine is an NMDA receptor antagonist which plays an important role in the aetiology of psycho-pathologies such as major depression Reference Javitt(7,Reference Krystal, D’Souza and Petrakis8). Berman et al. Reference Berman, Cappiello and Anand(35) showed that a single dose of ketamine produced antidepressant effects in patients suffering from major depression. Recently, Zarate et al. Reference Zarate, Singh and Carlson(36) extended this study to a higher number of patients, and they verified that the acute administration of ketamine rapidly improved depressive symptoms in patients with major depression. Therefore, these data strongly suggest that ketamine can induce robust and rapid antidepressant effects in depressed patients after a single intravenous injection.
In addition, the effects of ketamine on oxidative stress parameters are controversial. There are studies showing that ketamine present pro- Reference Zuo, Wu, Yao, Cao, Wu and Tanaka(37) and antioxidant effects Reference Saricaoglu, Dal, Salman, Doral, Kilinc and Aypar(38). Oxidative stress may occur by excessive formation of free radicals, which leads to oxidative damage to proteins, lipids and deoxyribonucleic acid Reference Arnaiz, Coronel and Boveris(39). It has also been shown that exposure to stress situations may stimulate numerous pathways leading to increased production of reactive oxygen species Reference Vasconcellos, Nieto and Crema(40). In this context, Madrigal et al. Reference Madrigal, Olivenza and Moro(33) reported glutathione depletion and lipid peroxidation in the brain of rats after chronic stress for 21 days. It is established that the impairment of the respiratory chain in mitochondria leads to an increase in the generation of free radicals, especially superoxide anion, and that these reactive oxygen species inhibit the activity of mitochondrial respiratory chain complexes, resulting in the generation of more reactive species, forming a cyclic phenomenon Reference Adam-Vizi(41).
In conclusion, we hypothesise that CMS inhibits the activity of complexes I, III and IV, and that chronic administration of ketamine administration partially reverses such an effect. It seems therefore reasonable to propose that ketamine administration might be a useful therapy for patients affected by major depression.








