INTRODUCTION
Concentrations of the greenhouse gas carbon dioxide (CO2) continue to increase in the Earth’s atmosphere and most predictions are that this will lead to a rise in global temperature of at least 1.5°C before the end of the current century (IPCC 2013). Although it is accepted that anthropogenic activities are driving the increase in CO2 levels (IPCC 2013), sources of CO2 to the atmosphere are complex and include both direct (e.g. fossil fuel emissions) and indirect (e.g. land-use change) anthropogenic emissions, as well as natural emissions of the Earth’s carbon cycle. Moreover, the response of the carbon cycle to climatic warming, and whether this will result in the loss of carbon stores that have been “locked away” for centuries/millennia, adds further complexity, but is important because it could lead to a positive feedback to further increase the rate of warming (Walker et al. Reference Walker, Garnett, Ward, Oakley, Bardgett and Ostle2016).
Radiocarbon (14C) analysis of CO2 provides unique information on the age (since photosynthetic fixation from the atmosphere) of emissions and rate of carbon turnover and can help to identify the contribution of different sources (Levin and Hesshaimer Reference Levin and Hesshaimer2000). For example, Campeau et al. (Reference Campeau, Bishop, Billett, Garnett, Laudon, Leach, Nilsson, Öquist and Wallin2017) found that aquatic emissions of carbon dioxide from a boreal fen were predominantly modern (i.e. with a 14C concentration >100 pMC) implying that they were substantially composed of carbon that had been fixed within the last ca. 60 years. In contrast, Newsham et al. (Reference Newsham, Garnett, Robinson and Cox2018) reported that discrete taxa of fungi respired carbon from Antarctic soil that was over a thousand years old, providing direct evidence for the release of old organic carbon to the atmosphere.
Cartridges containing zeolite molecular sieve (Hardie et al. Reference Hardie, Garnett, Fallick, Rowland and Ostle2005; Garnett and Murray Reference Garnett and Murray2013) are convenient for collecting CO2 samples for 14C analysis and are particularly useful when the CO2 occurs at less than several thousand ppm. The porous molecular sieve has a crystalline structure, which, when a gas flows through it, precludes large molecules from entering (Breck Reference Breck1974). In addition, the adsorption properties of the zeolite are such that unlike other gases (e.g. nitrogen and oxygen) the polar molecule CO2 is rapidly trapped and concentrated into a relatively small and therefore highly portable volume (Breck Reference Breck1974). The sampled CO2 can be released from the zeolite by heating and purified using routine cryogenic methods (Hardie et al. Reference Hardie, Garnett, Fallick, Rowland and Ostle2005; Garnett and Murray Reference Garnett and Murray2013).
Molecular sieves have been used for collecting carbon dioxide for 14C analysis since at least the 1960s (Godwin and Willis Reference Godwin and Willis1964). At the NERC Radiocarbon Facility (East Kilbride, UK), we have used cartridges containing zeolite molecular sieves for sample collection for over 20 years, with development of the technique being documented at various stages (Bol and Harkness Reference Bol and Harkness1995; Hardie et al. Reference Hardie, Garnett, Fallick, Rowland and Ostle2005; Garnett and Murray Reference Garnett and Murray2013). The cartridges have been utilized in novel field sampling methods to enable the 14C analysis of soil-respired CO2 (Hardie et al. Reference Hardie, Garnett, Fallick, Rowland and Ostle2005), aquatic CO2 (Garnett et al. Reference Garnett, Dinsmore and Billett2012) and atmospheric CO2 (Garnett and Hartley Reference Garnett and Hartley2010). Development of our molecular sieve methods continues, most recently with a focus on further improving the reliability of the method and quality of results, and to increase capacity and throughput to meet demand from users. Here, we describe the latest refinements to our methods, present the results of tests used to verify our latest procedures, and report the results of all known 14C content quality assurance standards processed alongside samples over the last two years.
METHODS
Design of the Molecular Sieve Cartridge
An earlier version of our molecular sieve cartridge was built from quartz glass (Hardie et al. Reference Hardie, Garnett, Fallick, Rowland and Ostle2005), but since cartridge temperature does not exceed 500°C we now use tubular borosilicate glass. The cartridge has a central compartment for containing the molecular sieve material (Figure 1) and is designed for processing using a tube furnace (MTF 10/15/130, Carbolite, UK). The dimensions of the three compartments are: centre (14 mm OD, 11 mm ID by 80 mm long), inlet (10 mm OD, 7 mm ID by 95 mm long), exhaust (6 mm OD, 4 mm ID by 95 mm long). At either end of the cartridge, 50 mm lengths of Iso-versinic® tubing (770260-05 and 770360-05, Saint-Gobain, France) are attached into which couplings (cPMCD22-03 and cPLCD170-06, Colder Products Company, USA) are inserted to facilitate connection to sample collection and recovery equipment. The couplings have auto-shutoffs that automatically seal when disconnected. Primary sealing of the cartridge is achieved by placing clips (PA50, Weloc, Scandinavia Direct, UK) across the Iso-versinic® tubing at both ends. The molecular sieve material (3–4 g, 1.6-mm pellets, Type 13X zeolite, 334340, Sigma-Aldrich, UK) is held within the central compartment using quartz wool. Prior to cartridge construction, the molecular sieve material is combusted in a furnace at 500ºC for 24 hr.

Figure 1 Molecular sieve cartridges used at the NERC Radiocarbon Facility (East Kilbride) for routine samples.
Processing of Molecular Sieve Cartridges
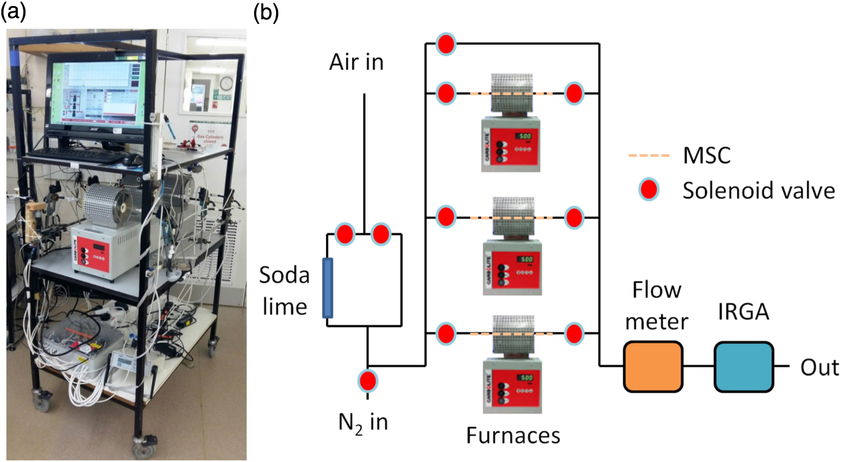
Cartridges are prepared for sample collection by purging with high purity nitrogen (Research Grade 5.0, BOC, UK) and CO2-free air while being heated (500°C) over a period of at least 30 min. First, the heated cartridge is purged using nitrogen (150 mL/min) for 8 min, which desorbs most of the CO2 trapped on the zeolite. The purge gas is then replaced with CO2-free air (ca. 400 mL/min; generated by pumping lab air through soda lime) for 2 min to oxidize any contaminants that may be present within the zeolite (Table 1). The purge gas is then switched back to nitrogen for at least another 20 min to further desorb CO2 from the zeolite. At this stage, an infrared gas analyzer (PP Systems EGM-4, Hitchin, UK) is used to ensure that the CO2 concentration in the exhaust gas is below 50 ppm, and if so, heating of the molecular sieve is stopped. However, because a small amount of CO2 continues to desorb from the zeolite until cooled to ca. 430ºC, purging with nitrogen continues for another 10 min by which time the molecular sieve temperature has fallen to less than 400°C and CO2 is no longer being desorbed. Valves are then closed to isolate the molecular sieve cartridge, which is left to cool for a further 20 min. Next, the cartridge is tested for leaks by flushing with nitrogen (150 mL/min) for 0.5 min and checking the CO2 concentration of the purge gas exiting the molecular sieve (i.e. to determine if ingress of atmospheric CO2 has occurred). Finally, the cartridge is filled with nitrogen to ca. 1 bar above atmospheric pressure and the cartridge removed from the furnace. These stages are all performed using a bespoke automated system (Figure 2) built using solenoid valves (PU220AR-01, Shako, Taiwan), microcontrollers (Arduino Uno and Mega, Arduino, Italy; www.arduino.cc) and PC software written in the Processing 2 programming language (www.processing.org). To maintain positive pressure within the cartridge, a further addition of nitrogen is performed manually once the cartridge has achieved room temperature. Using these procedures, we have found that the same molecular sieve cartridge can be recharged and reused for multiple samples, without any loss in performance.
Table 1 Summary of molecular sieve charging and sample recovery procedures.


Figure 2 (a) Photograph and (b) schematic of the automated rig for charging molecular sieve cartridges at the NERC Radiocarbon Facility (East Kilbride). MSC = molecular sieve cartridge. IRGA = infrared gas analyzer.
Collection of samples and quality assurance standards on cartridges is usually undertaken by active sampling (at a flow rate of ca. 500 mL/min) using a molecular sieve CO2 sampling system originally described by Hardie et al. (Reference Hardie, Garnett, Fallick, Rowland and Ostle2005), but with the following improvements. First, all Tygon E-3603 tubing has been replaced with Iso-versinic® tubing (due to its greater rigidity and lower CO2 permeability). Second, instead of calcium sulphate, we now use cartridges containing magnesium perchlorate (0.7–1.2-mm grains, Elemental Microanalysis, UK), which we believe to be superior for drying the sample gas before collection on the molecular sieve. Third, for processing quality assurance standards (CO2 in sealed 6 mm OD glass tubes) a strong 750 mL glass bottle (an empty standard wine bottle cleaned using carbon-free detergent; Decon90®, Decon Laboratories Limited, UK) fitted with a rubber bung and two stainless steel sampling ports is used to provide an air-tight chamber into which the gas standards can be released. This is achieved by firstly removing atmospheric CO2 from the bottle by circulating the headspace through soda lime (Hardie et al. Reference Hardie, Garnett, Fallick, Rowland and Ostle2005), and then by shaking the bottle to break the scored glass tube against the bottle wall, thus releasing the standard gas.
Recovery of CO2 from molecular sieve cartridges is performed by purging (150 mL/min) the molecular sieve with high purity nitrogen while heating the cartridge inside a tube furnace, followed by cryogenic collection. The method is similar to that described by Garnett and Murray (Reference Garnett and Murray2013), except for the following changes. First, prior to heating, the cartridge is connected to the vacuum rig and evacuated (1x10-1 mbar) at both ends up to the clips, and then flooded with N2 (Table 1). Second, the CO2 concentration is no longer monitored using an infrared gas analyzer during desorption, and instead, all cartridges are simply desorbed for a set time of 15 min. Third, cartridges are now desorbed at 425ºC and not 500ºC as in the earlier method.
Tests of Current Cartridge Design and Procedures
The current molecular sieve cartridge and procedures were tested using ca. 3 mL (STP; standard temperature and pressure) CO2 standards of known carbon isotope concentration produced from reference materials (Iceland Spar Calcite (ISC), background; TIRI Barley Mash (TBM), 116.35 ± 0.0084 pMC; Gulliksen and Scott Reference Gulliksen and Scott1995; FIRI Belfast Cellulose (FBC), 57.22 ± 0.04 pMC; Boaretto et al. Reference Boaretto, Bryant, Carmi, Cook, Gulliksen, Harkness, Heinemeier, McClure, McGee, Naysmith, Possnert, Scott, van der Plicht and van Strydonck2002). The first tests were performed on freshly prepared cartridges for which the charging, gas collection, and gas recovery were undertaken within one week (i.e. storage time = 0 months). To assess the cartridges after a period of storage, tests were also performed on cartridges that had been charged at least three months before being used to trap CO2 standards (storage time = 3 months; i.e. the cartridges were charged, left three months, used to trap CO2 standards that were then recovered within the following week). To test for sample carry-over, we ensured that some of the previous CO2 stored on the molecular sieve cartridge had a 14C or 13C concentration that contrasted with the chosen standard. We also performed tests using CO2 produced from the abovementioned standard materials with each batch of molecular sieve samples submitted for analysis. For these tests, the volume of the standard used was selected to be similar to the samples and where possible undertaken using molecular sieve cartridges that had been prepared at the same time as those used for the samples, and often had accompanied the submitter to the field site.
After recovery, the volume of CO2 was determined using a pressure transducer and a calibrated volume, and split into aliquots. One aliquot was used for measurement of δ13C using isotope ratio mass spectrometry (Thermo-Fisher Delta V, Germany) with results expressed in per mil relative to VPDB. A second aliquot was transformed to graphite using Fe-Zn reduction (Slota et al. Reference Slota, Jull, Linick and Toolin1987) and analyzed by accelerator mass spectrometry (AMS) at the Scottish Universities Environmental Research Centre. Following convention, 14C measurements were corrected for isotopic fractionation by normalising to a δ13C of –25 ‰ using the measured δ13C values and reported as both pMC and conventional 14C ages (in BP = years before AD 1950; Stuiver and Polach Reference Stuiver and Polach1977).
RESULTS
Tests of the Current Cartridge Design and Procedures
The 14C concentration of the background standards (CO2 derived from Iceland Spar Calcite) used to test the latest cartridge design and procedures ranged from 0.41 to 0.77 pMC and are below the long-term background for molecular sieve cartridges at the NERC Radiocarbon Facility (1.0 ± 0.5 (SD) pMC based on n = 15 measurements between 2010 and 2015; Table 2). The 14C results for the non-background standards were all within 1 σ measurement uncertainty of consensus values, for both freshly-prepared and stored cartridges (Table 2). The δ13C values for all standards were also within measurement uncertainty (<1 σ) of the accepted values derived from measurements of bulk gas at NERC Radiocarbon Facility (TBM −26.9 ± 0.3 ‰; FBC −23.7 ± 0.3 ‰; ISC 2.4 ± 0.3 ‰).
Table 2 Results for aliquots of CO2 derived from 14C standard material trapped on molecular sieve cartridges and recovered using the latest procedures described in the text and Table 1. Consensus values for reference materials are: ISC = Iceland Spar Calcite, background; TBM = TIRI Barley Mash, 116.35 ± 0.0084 pMC (Gulliksen and Scott Reference Gulliksen and Scott1995); FBC = FIRI Belfast Cellulose, 57.22 ± 0.04 pMC (Boaretto et al. Reference Boaretto, Bryant, Carmi, Cook, Gulliksen, Harkness, Heinemeier, McClure, McGee, Naysmith, Possnert, Scott, van der Plicht and van Strydonck2002).

*Superscripts indicate previous gas stored on the molecular sieve cartridge: aatmospheric CO2, bTBM CO2, cISC CO2, dFBC CO2, eunknown.
The results for CO2 derived from Iceland Spar Calcite showed that during 2016–2017 the 14C background of the method was 1.08 ± 0.51 pMC (n = 10), which is similar to the long-term background for molecular sieve cartridges at the NERC Radiocarbon Facility (1.0 ± 0.5 pMC). During the same period a total of 14 TBM CO2 and 9 FBC CO2 standards were processed alongside samples. For TBM, all results except one differed from the consensus value (116.35 pMC) by <2 σ and the mean (116.13 ± 0.61 SD pMC) was not significantly different (p=0.20; 1-sample T-test, Minitab 18; Figure 3). For FBC, the mean (56.99 ± 0.24 SD pMC) was slightly lower than the consensus value (57.22 pMC; p=0.02), but with measurement uncertainty all results overlapped with the consensus value at <2 σ (Figure 3). δ13C values for all quality assurance standards (mean ± SD; TBM –26.8 ± 0.2‰; FBC –23.6 ± 0.1‰) agreed with accepted values.

Figure 3 Radiocarbon concentration of known 14C content quality assurance standards collected on, and recovered from, molecular sieve cartridges during 2016–2017. CO2 derived from TIRI Barley Mash (TBM; top) and FIRI Belfast Cellulose (FBC; bottom). Error bars are ±1 σ. Full lines represent the international consensus value for each standard and dashed line the mean for measured standards.
DISCUSSION
The benefit of cartridges containing zeolite molecular sieve for collecting carbon dioxide samples for 14C analysis is being increasingly recognized, with new systems having been recently reported from several other labs (Hämäläinen et al. Reference Hämäläinen, Fritze, Jungner, Karhu, Oinonen, Sonninen, Spetz, Tuomi, Vanhala and Liski2010; Palonen Reference Palonen2015; Walker et al. Reference Walker, Xu, Fahrni, Lupascu and Czimczik2015; Wotte et al. Reference Wotte, Wordell-Dietrich, Wacker, Don and Rethemeyer2017b). At the NERC Radiocarbon Facility, development of our molecular sieve method has continued with a focus on improving the reliability of the method and quality of results, and to increase capacity and throughput to meet demand from users. The analyses performed to test our latest cartridge design and procedures demonstrate that, within the operating parameters that we currently recommend (CO2 sample volume >3 mL, storage time < 3 months), our molecular sieve system is reliable. From a total of 29 measurements on international 14C standards over two years (6 new cartridge test standards plus 23 known 14C content standards accompanying samples), only one provided a 14C value that differed from the consensus value by >2 σ. Moreover, for the 6 test standards (Table 2) the agreement of δ13C values with consensus values show the absence of fractionation effects. Reliable 14C measurements from standards of disparate 14C concentrations demonstrate the absence of contamination and sample carry-over effects in both newly charged cartridges and cartridges that have been in storage for 3 months.
The performance of our molecular sieve sampling method compares well with similar systems developed in other laboratories. Radiocarbon concentrations of our background 14C standards are similar to those reported by Wotte et al. (Reference Wotte, Wischhöfer, Wacker and Rethemeyer2017a), and lower than those of Walker et al. (Reference Walker, Xu, Fahrni, Lupascu and Czimczik2015; calculated by Wotte et al. Reference Wotte, Wischhöfer, Wacker and Rethemeyer2017a). While our developments have enabled a reduction in the recommended sample size from ca. 10 mL (Hardie et al. Reference Hardie, Garnett, Fallick, Rowland and Ostle2005) to >3 mL for the current system, the results of Wotte et al. (Reference Wotte, Wischhöfer, Wacker and Rethemeyer2017a) suggest that a further reduction in sample size may be possible (although they acknowledge that smaller samples are vulnerable to contamination and carry-over effects).
We have previously demonstrated that our molecular sieve methods are reliable for stable-carbon and 14C measurement of CO2 (Garnett and Murray Reference Garnett and Murray2013). To construct more cartridges to meet higher demand from users, it has been necessary for us to source new supplies of Type 13X zeolite. Although molecular sieve traps can be reused, we had limited quantities of the BDH (British Drug Houses) 13X used in earlier cartridges (Hardie et al. Reference Hardie, Garnett, Fallick, Rowland and Ostle2005; Garnett and Murray Reference Garnett and Murray2013) and were unable to obtain new supplies. We found that the performance characteristics of new Type 13X zeolite from several suppliers differed slightly, and that they did not completely desorb all the CO2 that they had trapped when processed using the earlier reported methods (Garnett and Murray Reference Garnett and Murray2013), leading to significant sample carry-over (typically ca. 0.1 to 0.3 mg C). We found that we could overcome this problem when using Sigma-Aldrich Type 13X molecular sieve (used for all results presented here) by preparing the cartridges at 500ºC but recovering the sample CO2 at a lower temperature of 425ºC. Although this results in a slightly lower yield (by ca. 5%, presumably due to incomplete desorption at the lower temperature), it prevents significant sample carry-over and as shown in the results reported here, enables reliable 14C and 13C measurement (Table 2, Figure 3).
A considerable improvement to our molecular sieve method has been the construction of an automated charging rig for preparing the molecular sieve cartridges before use. This system ensures that all cartridges are prepared consistently, incorporates several different charging steps (purging with nitrogen and CO2-free air, slightly over-pressurizing cartridges with nitrogen) and performs a leak test to check the integrity of the cartridges. Moreover, after a minimal set up, the rig can be left to process three cartridges with no further operator input required until the charging processes is completed. Thus, up to 9 molecular sieve cartridges can be prepared in a standard working day, with a total operator time of less than one hour. An automated rig for recovering sample CO2 from molecular sieve cartridges is currently in the final phase of development and testing. This rig will be solely for CO2 recovery for analysis, with used cartridges being recharged on the existing automated charging rig as required.
Presently, we are undertaking tests to make cartridges more robust since even though they are provided to users in protective housing (polyethylene foam pipe insulation), they occasionally break in transit. Metal cartridges offer one solution (e.g. Wotte et al. Reference Wotte, Wordell-Dietrich, Wacker, Don and Rethemeyer2017b), though we have often found it useful to observe inside the cartridge to check for the presence of water (particularly when using the cartridges for passive sampling aquatic CO2; Garnett et al. Reference Garnett, Dinsmore and Billett2012) or contamination. For example, on at least one occasion a carbon-rich black deposit has formed on the 13X zeolite during sample recovery, which we suspect was due to the reduction of hydrocarbons (likely present on the molecular sieve due to the sample type). Since this was a potential contaminant for future samples processed using the cartridge, we incorporated the CO2-free air purge in the automated charging process (during the air-purge ca. 1 mL CO2 was observed in the exhaust gases and the black deposits were removed). Other modifications currently under trial include the replacement of quartz wool in the cartridges with stainless steel wool, which reduces back pressure during sampling, and should enable the cartridges to perform more consistently during sample collection.
Finally, the results presented here demonstrate the reliability of our methods under currently recommended operating parameters (>3 mL CO2, and <3 months storage time). Future investigations will seek to more accurately resolve the limitations of the current methods, and to find improvements that will enable reliable 14C measurement of smaller sample volumes (Walker et al. Reference Walker, Xu, Fahrni, Lupascu and Czimczik2015; Wotte et al. Reference Wotte, Wischhöfer, Wacker and Rethemeyer2017a).
ACKNOWLEDGMENTS
We thank staff at the NERC Radiocarbon Facility and SUERC AMS Facility. We are grateful to the UK Natural Environment Research Council for funding, and to the reviewers of the manuscript for their detailed comments.