Introduction
Tachinid flies (Diptera: Tachinidae) are natural enemies of many lepidopteran insects (Stireman & Singer, Reference Stireman and Singer2003; Dindo, Reference Dindo2011). These koinobiont parasitoids allow their hosts to continue to feed and develop after being parasitized and develop to the late-larval stage by feeding on the host tissues (Stireman et al., Reference Stireman, O'Hara and Wood2006). The tachinid fly Drino inconspicuoides (Baranov) is a gregarious and ovolarviparous endoparasitoid that parasitizes a wide range of lepidopteran larvae (Baranov, Reference Baranov1934; Shima, Reference Shima1999). D. inconspicuoides larvae hatch within 1 min after oviposition on the body surface of the host, penetrate the host body cavity, attach themselves to the cuticle and create a cap-like respiration organ called a funnel (part of the sheath; Dindo, Reference Dindo2011). The early larvae remain attached with the funnel until they reach the 3rd larval instar, when they begin to wander freely inside the host haemocoel, eventually killing the host and leaving the remains to undergo pupation.
Insects that are attacked by tachinid flies possess an immune system that can eliminate invading foreign organisms (Lackie, Reference Lackie1988). One cellular immune reaction against invading parasitoid eggs and larvae is encapsulation with haemocytes (Lavine & Strand, Reference Lavine and Strand2002), which involves granular cells (an adherent haemocyte cell type) attaching to and forming a single layer around the target, following which plasmatocytes (another adherent haemocyte cell type) aggregate to form a multi-layered capsule; the granular cells attaching to the outermost layer of plasmatocytes undergo apoptosis (Pech & Strand, Reference Pech and Strand2000). Therefore, parasitoids that penetrate into the host haemocoel will come face to face with this immune reaction.
Some tachinid flies escape the host immune response by migrating out of the haemocoel. For example, larvae of Compsilura concinnata move into the space between the midgut cells and the periplasmic membrane immediately after hatching in the host haemocoel (Ichiki & Shima, Reference Ichiki and Shima2003), while Blepharipa sericariae larvae enter the host by ingestion and migrate from the gut to the ganglion, passing through the haemocoel (Hirose, Reference Hirose2005). Moreover, hatched Zenillia libatrix larvae move into the abdominal muscles or silk glands of host lepidopteran insects (Dowden, Reference Dowden1934). However, D. inconspicuoides larvae spend the entire larval period (approximately 8 days at 25°C) developing inside the host haemocoel (Kalyebi & Nakamura, Reference Kalyebi and Nakamura2006), forcing them to deal with this immune response in a different way.
Salt (Reference Salt1968) postulated that such tachinid flies survive the host immune response by moulding the host haemocytes that are engaging in encapsulation into a funnel to avoid suffocation. However, asphyxia is not the only cause of death for encapsulated organisms. For example, Nappi et al. (Reference Nappi, Vass, Frey and Carton1995) suggested that toxic reactive oxygen species (ROS) produced by the host also kill parasitoids, and the production of various types of ROS has been associated with melanization (Dubovskii et al., Reference Dubovskii, Grizanova, Chertkova, Slepneva, Komarov, Vorontsova and Glupov2010; Satyavathi et al., Reference Satyavathi, Minz and Nagaraju2014). According to Dudzic et al. (Reference Dudzic, Kondo, Ueda, Bergman and Lemaitre2015), two prophenoloxidases (PPOs) contribute to the melanization of the capsules that are formed around parasitoids: PPO2, which is produced by crystal cells (exhibiting similar characteristics to lepidopteran oenositoids; Ribeiro & Brehélin, Reference Ribeiro and Brehélin2006); and PPO3, which is produced by lamellocytes (exhibiting similar characteristics to lepidopteran plasmatocytes; Ribeiro & Brehélin, Reference Ribeiro and Brehélin2006). Therefore, since the sheath that was reported by Salt (Reference Salt1968) only covers the posterior end of the larva, leaving the other end open, it seems unlikely that it plays a vital role in protecting tachinid larvae from the host immune reactions although it may contribute to protect the larvae partially. In this study, we investigated whether other defence mechanisms exist in D. inconspicuoides larvae using Mythimna separata (Lepidoptera: Noctuidae) as a host.
Materials and methods
Insects
The colony of D.inconspicuoides originated from parasitized larvae of the fall webworm Hyphantria cunea (Drury) (Lepidoptera: Erebidae), which had been collected in Tsukuba, Japan (36°03′N, 140°05′), in September and October 2010. D. inconspicuoides was reared by using a method of Kalyebi & Nakamura (Reference Kalyebi and Nakamura2006). The adult flies were supplied with sugar cubes and water and were maintained at 20°C. Mated D. inconspicuoides females were provided with 4-day-old final (6th) instar larvae of M. separata as a host. Following oviposition, the host larvae were reared on an artificial diet, Silkmate (Nihon Nosanko), at 25°C under a 16 h light:8 h dark photoperiod.
Dissection of parasitized M. separata larvae
The parasitized M. separata larvae were dissected at 2, 3, 4, 24, 48, 52 and 72 h after the invasion and the parasitizing larvae were observed under a stereoscopic microscope, MZ12 (Leica). The surrounding materials and the sheath were peeled from the excised larvae using forceps and were used for genetic analyses. The surrounding materials at 3, 4, 24 and 48 h after the invasion were also surgically separated into the inner and outer layers.
4′,6-diamidino-2-phenylindole (DAPI) staining
The material surrounding the tachinid larvae at 24 h after the invasion was stained for 5 min with DAPI (Life Technologies), which binds to nuclear DNA, and was observed under a phase-contrast microscope, DM IRB (Leica).
Cloning of the ribosomal internal transcribed spacer (ITS) region
Genomic DNAs were extracted from D. inconspicuoides and M. separata larvae with the Wizard® Genomic DNA Purification Kit (Promega). A primer set for amplifying the ITS region was designed based on the conserved ITS sequences described by Ji et al. (Reference Ji, Zhang and He2003): 5′-TACACACCGCCCGTCGCTACTA-3′ (CAS18sF1 primer) and 5′-ATGTGCGTTCRAAATGTCGATGTTCA-3′ (CAS5p8sB1d primer). The polymerase chain reaction (PCR) was performed using GoTaq® DNA polymerase (Promega) under the following conditions: 94°C for 5 min followed by 35 cycles at 94°C for 15 s, 60°C for 15 s and 72°C for 15 s. The PCR products that were amplified from the genomic DNAs of D. inconspicuoides and M. separata were subcloned into the pGEM®-T Easy Vector (Promega) and sequenced with an ABI 3130 Genetic Analyzer (Applied Biosystems). Based on this ITS sequence information, the following specific primer sets were used to amplify the ITS regions for D. inconspicuoides and M. separata: 5′-AGAACTAAGACATTCGCAACA-3′ (forward primer for D. inconspicuoides), 5′-TGTCGCAGTAGTCGATCTAG-3′ (forward primer for M. separata), and 5′-TGTGTCCTGCAGTTCACAC-3′ (reverse primer for both species).
Amplification of genomic DNA by PCR
Genomic DNAs from the ‘cloak’ and the sheath were amplified with the specific primer sets for the ITS regions of D. inconspicuoides and M. separata. The PCR was performed using Paq5000 DNA polymerase (Agilent) with approximately 2.5 ng of each genomic DNA sample. The PCR programme consisted of initial denaturation at 94°C for 5 min followed by 35 cycles at 94°C for 30 s, 65°C for 30 s and 72°C for 30 s.
Transplantation experiments
Polystyrene beads (Φ600 µm; Polysciences) or intact D. inconspicuoides larvae that had been excised from the host 24 h after the invasion and killed by freezing were transplanted into the abdominal leg of ice-anaesthetized 6th instar larvae of M. separata, following which the leg was immediately tied with thread to prevent haemolymph leakage. The host larvae were then dissected at 2 h and 24 h after transplantation, the transplanted objects were excised, and the capsules around them were separated for genetic analysis.
Cloning of a gene encoding aldehyde dehydrogenase (aldh) in M. separata
To find a candidate marker gene for distinguishing fat body cells from haemocytes, suppression subtractive hybridization (SSH) was performed using the GeneFishing DEG Kit (Seegene) following the manufacturer's instructions. Briefly, RNAs were extracted from the fat body and haemocytes of 6th instar larvae of M. separata with TRIzol reagent (Invitrogen) and adaptor-linked cDNAs were synthesized. PCR was then conducted using Taq™ HS DNA polymerase (Takara) with a primer set that contained the adaptor sequence. The PCR products that were amplified from only the cDNA of the fat body were subcloned into the pGEM-T Easy Vector and sequenced.
Reverse transcription PCR (RT-PCR)
Total RNAs were extracted from the ‘cloak’, the capsules around the transplanted objects, and the host fat body and haemocytes with TRIzol reagent (Invitrogen), and 100 ng of each RNA were converted to cDNA with PrimeScript™ RTase (Takara) in a total volume of 20 µl. cDNA synthesis was confirmed using the housekeeping gene rp49 (submitted as RpL32, accession number: AB669190; Yamaguchi et al., Reference Yamaguchi, Matsumoto, Ochiai, Tsuzuki and Hayakawa2012). aldh was used as the target gene that was specifically expressed in the host fat body and was amplified using the following primer set: 5′-GCTGGTGTCATCACCAACGA-3′ and 5′-CTCCAAGTATTGTGTGATACCTTCCT-3′. The M. separata C-type lectin gene epl (Ishihara et al., Reference Ishihara, Maruyama and Furukawa2017) was used as the target gene that was specifically expressed in the host haemocytes and was amplified using the following primer set: 5′-ACGATCAGGCTGGTGGAATGAT-3′ and 5′-GATACAGAATTCTGCGGTTGCAT-3′. PCR was performed using Paq5000 DNA polymerase (Agilent) with 0.8 µl of the cDNA synthesis mix as a template. The PCR programme consisted of initial denaturation at 94°C for 5 min followed by 31 (aldh) or 25 (epl) cycles at 94°C for 40 s, 55 °C for 40 s and 72°C for 40 s.
Sudan black B staining
The fat body and inner and outer layers of the ‘cloak’ at 3 h and 24 h after the invasion were fixed with 50% MeOH for 15 min and stained with Sudan black B (Muto Pure Chemical Co., Ltd) at 37°C for 15 min. The specimens were then washed with 50% MeOH and observed under a stereoscopic microscope (Leica).
Results
Characteristics of D. inconspicuoides larvae in the host haemocoel
Invading D. inconspicuoides larvae remained near the penetration point throughout the observation period. At 2 h after penetration, the larvae were surrounded by a cloudy material (fig. 1a), following which a cottony mass gradually coagulated around them (fig. 1b). The size of this cottony mass increased over 24 h to completely conceal the moulted 2nd instar larvae (fig. 1c). We named the distinctive cloudy and cottony mass the ‘cloak’. Beneath the ‘cloak’, there was a brownish sheath with a blackened funnel surrounding the surface of the larvae (fig. 1d). The larvae inside the sheath were not melanized. The larvae moulted into 3rd instar larvae after approximately 52 h, by which time no further thickening of the ‘cloak’ occurred. Consequently, the ‘cloak’ no longer surrounded the entire larvae, with only a small portion of debris being observed on the sheath (fig. 1e), although the sheath still completely surrounded the larvae. Approximately 72 h after the invasion, the larvae increased in size so that their anterior half protruded from the sheath (fig. 1f).
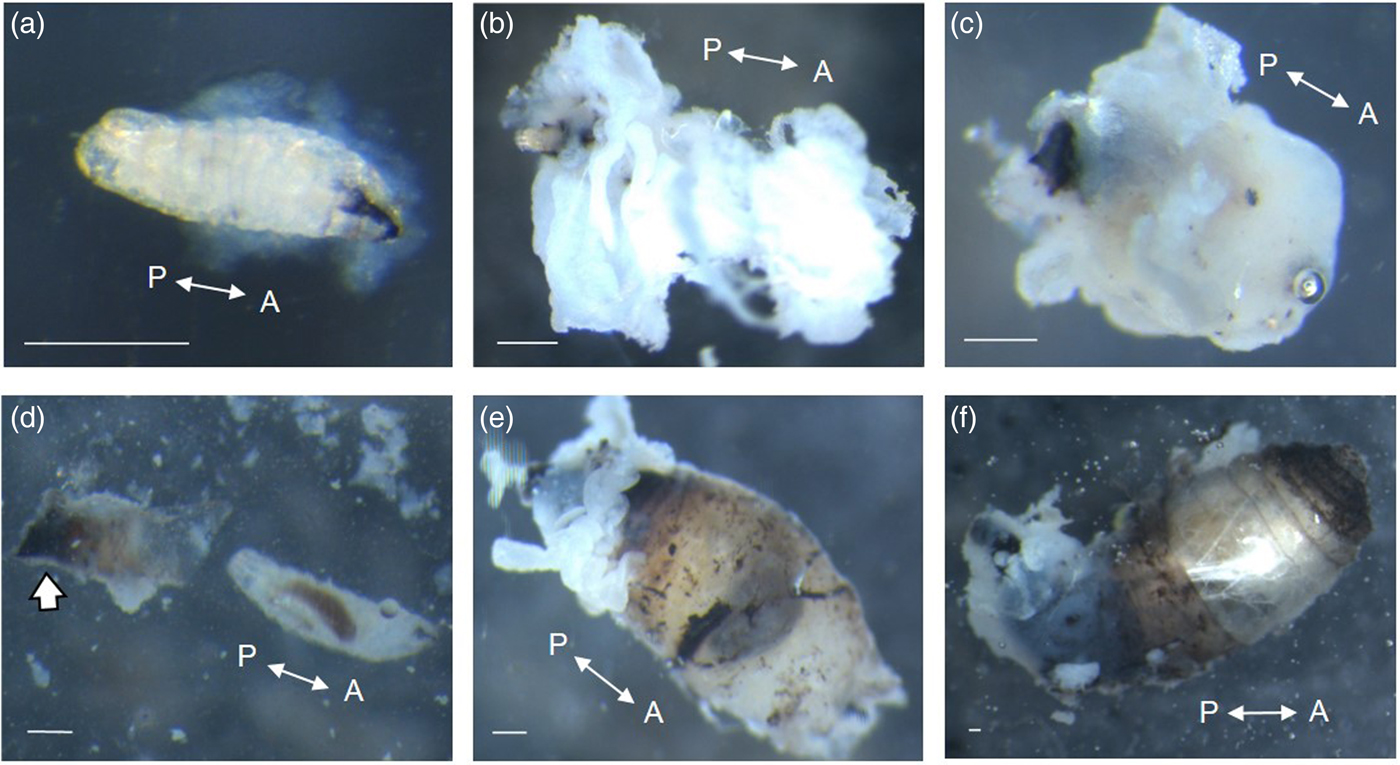
Fig. 1. Parasitizing Drino inconspicuoides larvae excised from Mythimna separata host larvae at (a) 2 h, (b) 3 h, (c) 24 h, (e) 52 h and (f) 72 h after the invasion. (d) Photograph of the sheath (left) and larva (right) inside the surrounding structure at 24 h after the invasion. Scale bars = 0.25 mm. Double-headed arrows indicate the anterior-posterior (A/P) axes of the larvae. The arrow in (d) indicates the funnel.
DNA analysis of the ‘cloak’
The DAPI-stained ‘cloak’ removed at 24 h after invasion emitted a multitude of blue fluorescent signals (fig. 2a), suggesting that it consisted of cell aggregates. To identify the origins of the ‘cloak’, we obtained the ITS sequences of both the parasitoid D. inconspicuoides (accession number: LC361445) and the host M. separata (accession number: LC361446). Only the host primer set amplified the specific DNA products from the ‘cloak’, with no DNA products being amplified by the D. inconspiduoides primer set (fig. 2b), suggesting that the ‘cloak’ was composed of M. separata cells. The sheath also contained only host cells.
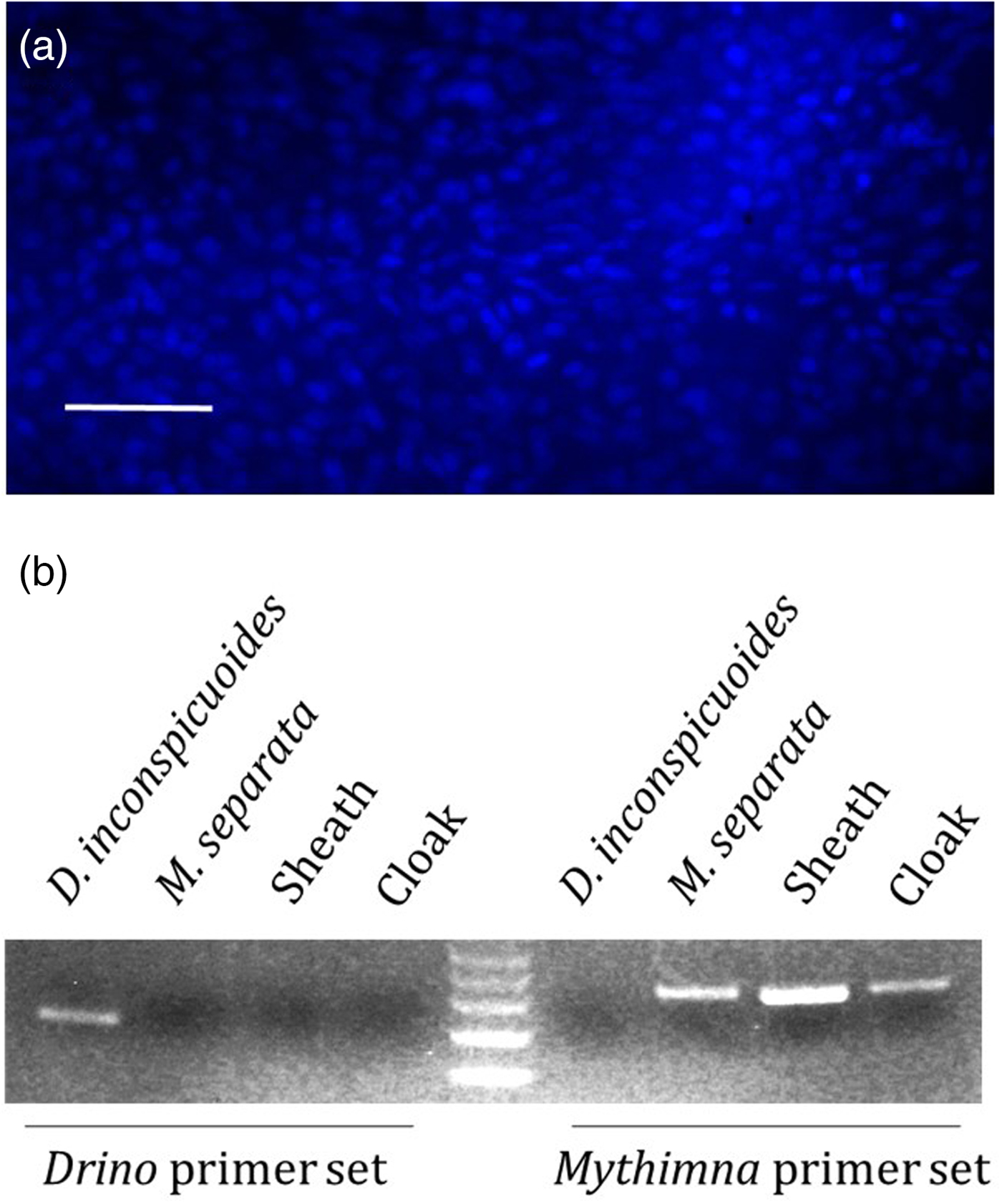
Fig. 2. Genomic analysis of the ‘cloak’. (a) ‘Cloak’ stained with 4′,6-diamidino-2-phenylindole (DAPI); scale bar = 0.05 mm. (b) Genomic polymerase chain reaction (PCR) with specific primer sets for the parasitoid Drino inconspicuoides and the host Mythimna separata.
Comparison with encapsulation capsules formed by the host
Since large foreign objects are usually encapsulated with host haemocytes, we considered it possible that the ‘cloak’ was a by-product of the cellular immune reactions. However, we observed lipid-like droplets within the cells of the cottony mass, leading us to hypothesize that production of the ‘cloak’ involved fat body cells. To verify this hypothesis, we identified the cell lineage of the ‘cloak’ and compared this with that of the encapsulation capsules.
The transplanted immune targets, micro-polystyrene beads and freeze-killed D. inconspicuoides larvae, became encapsulated with transparent capsules within 2 h (fig. 3a, b), at which time melanin deposition was visible on the surface of the dead larvae. The encapsulation capsules were completed by 24 h after insertion of the materials (fig. 3c, d).
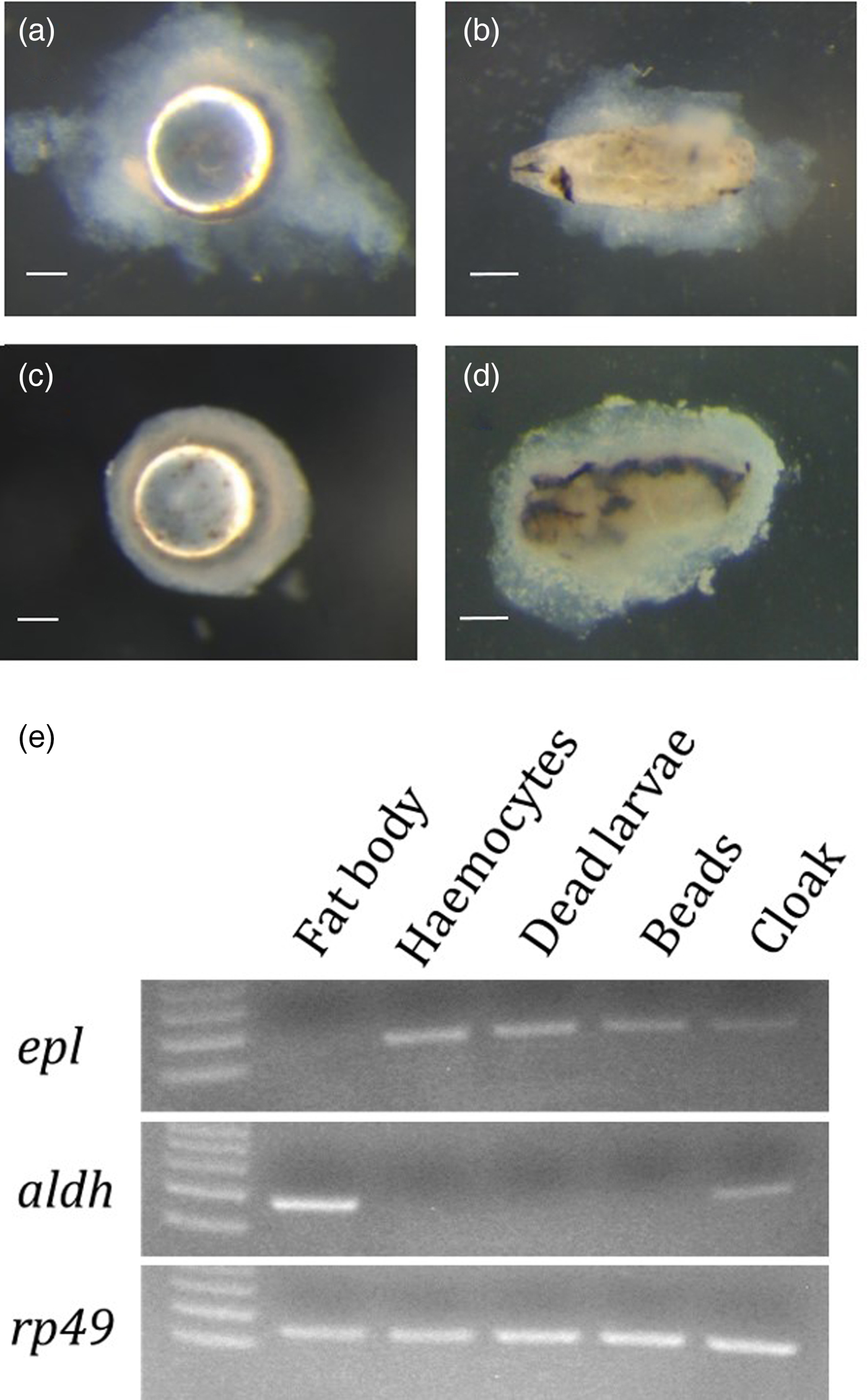
Fig. 3. Cell lineages of the encapsulation capsules that formed around transplanted objects and the ‘cloak’. (a–d) Encapsulation of the micro-polystyrene beads (a and c) and dead Drino inconspicuoides larvae (b and d) at 2 h and 24 h, respectively, after artificial transplantation into Mythimna separata larvae; scale bars = 0.25 mm. (e) Expression of haemocyte-specific (epl) and fat body-specific (aldh) genes in the capsules around the transplanted objects and the ‘cloak’ at 24 h after the invasion. rp49 gene is used as a control for cDNA synthesis.
We confirmed that epl (Ishihara et al., Reference Ishihara, Maruyama and Furukawa2017) was specifically expressed in the haemocytes and not in the fat body cells. Therefore, we used this as the host haemocyte-specific marker gene. Use of the SSH technique identified a gene encoding an aldehyde dehydrogenase (aldh; accession number: LC361447) as a candidate fat body-specific marker gene. Since ALDH is known to detoxify acetaldehydes, which are generated through lipid metabolism (Chen et al., Reference Chen, Sun and Mochly-Rosen2010), we used this gene as the fat body-specific marker gene. RT-PCR analysis using the primer sets for the haemocyte- and fat body-specific marker genes showed that only the epl gene fragment was detected from the cDNAs of the encapsulation capsules surrounding both the micro-beads and freeze-killed D. inconspicuoides larvae (fig. 3e). By contrast, both the epl and aldh gene fragments were amplified from the cDNA of the ‘cloak’, indicating that it originated from both the haemocytes and fat body.
Sequential changes in the components of the ‘cloak’
RT-PCR analysis showed that the cloudy material and the inner layer of the ‘cloak’ expressed mainly the epl gene, whereas the outer layer expressed only the aldh gene at 3 h and 4 h after the invasion (fig. 4a). However, both genes were expressed in all layers after 24 h, suggesting that both the haemocyte and fat body cell lineages were mixed in the ‘cloak’. The staining of each layer with Sudan black B, which binds lipids, confirmed that those layers that expressed aldh (i.e. the outer layer at 3 h and both the inner and outer layers at 24 h after the invasion) included lipid-containing fat body cells (fig. 4b). Together, these findings indicate that haemocytes assembled around the larvae first and then the fat body cells covered the haemocyte layer, following which the two lineages progressively converged.
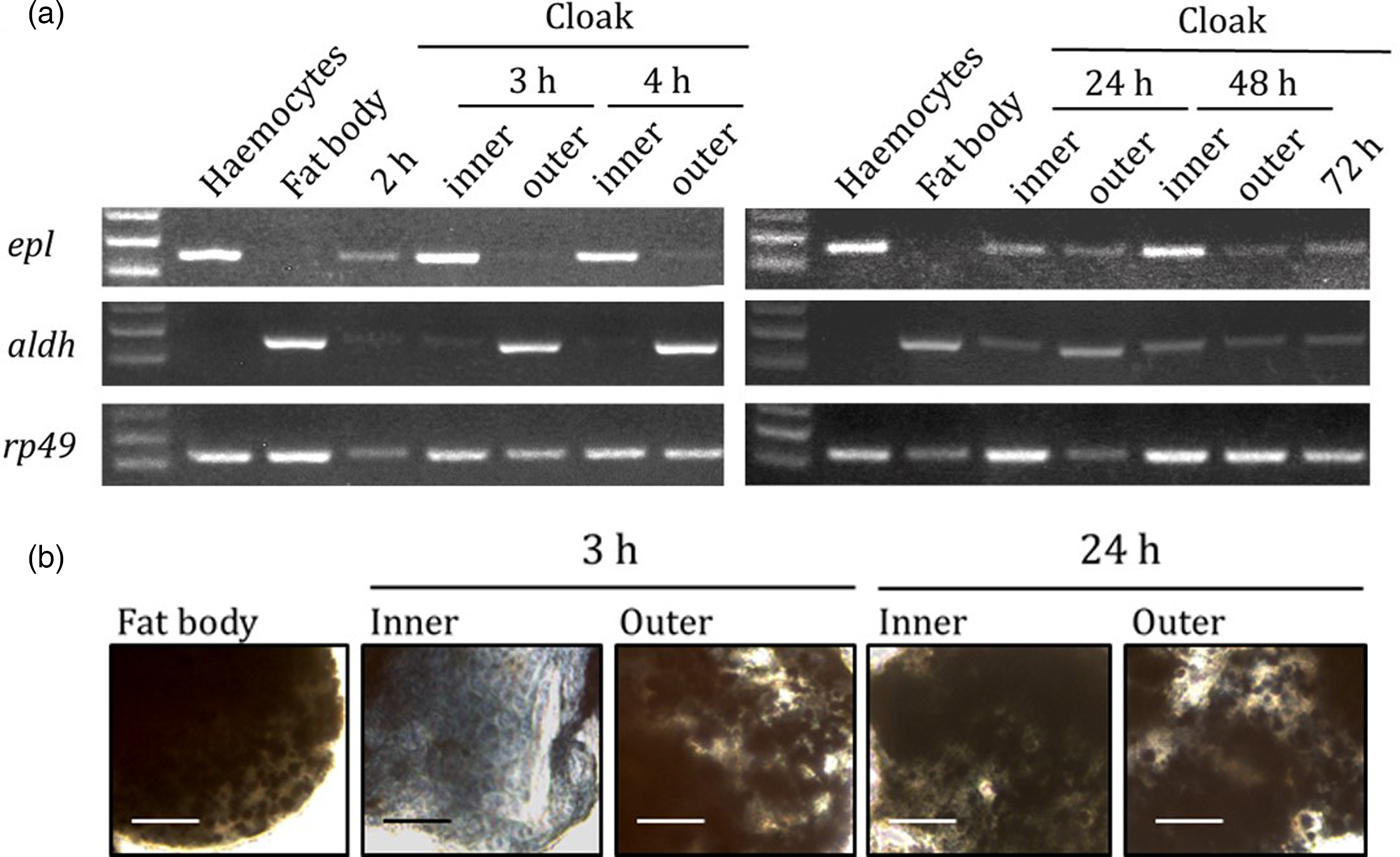
Fig. 4. Sequential changes in the cell lineage of the ‘cloak’. (a) Expression of haemocyte-specific (epl) and fat body-specific (aldh) genes in the ‘cloak’. rp49 gene is used as a control for cDNA synthesis. (b) Sudan black B staining of the fat body (control), and the inner and outer layers of the ‘cloak’ at 3 h and 24 h after the invasion. Scale bars = 0.25 mm.
Discussion
In this study, we discovered a unique structure that formed around D. inconspicuoides larvae several hours after the invasion, which we termed the ‘cloak’, and demonstrated that this structure was constructed from the host cells (fig. 2). A similar structure that is produced by the host tissue has been observed in the endoparasitoid Stichotrema dallatorreanum (Strepsiptera: Myrmecolacidae), the larvae of which camouflage themselves in a host-derived epidermal ‘bag’ by manipulating the host epidermal tissue (Kathirithamby et al., Reference Kathirithamby, Ross and Johnston2003), enabling them to avoid recognition and thus attack by the host immune system.
The ‘cloak’ observed in this study contained not only haemocytes but also fat body cells (figs 3 and 4). Namba et al. (Reference Namba, Nakamatsu, Tateishi, Miura and Tanaka2009) reported that the fat body is involved in the formation of the cuticular cyst that Autographa nigrisigna (Lepidoptera: Noctuidae) creates around parasitizing eggs of the endoparasitoid Campoletis chlorideae (Hymenoptera: Ichneumonidae), causing them to be discarded with the old cuticle at ecdysis. Therefore, we considered it possible that the fat body-containing ‘cloak’ observed in the present study was also specifically generated around D. inconspicuoides larvae as part of the host immune reactions. However, we found that the encapsulation capsules that formed around freeze-killed larvae and micro-polystyrene beads were composed of only haemocytes (fig. 3e), suggesting that formation of the fat body-containing ‘cloak’ does not specifically depend on the surface cuticle structure of D. inconspicuoides larvae but rather factors secreted by the living larva. It has previously been shown that the tachinid fly Exorista larvarum possesses large salivary glands (Gardenghi & Mellini, Reference Gardenghi and Mellini1995; Michalková et al., Reference Michalková, Valigurová, Dindo and Vanhara2009) that can secrete massive amounts of enzymes to extraintestinally digest the host tissue. Moreover, Hayakawa (Reference Hayakawa1986) reported that the tachinid fly Blepharipa sericariae secretes a factor that inhibits lipid transport in the host. Therefore, it is possible that living D. inconspicuoides larvae secrete factors that regulate the assembly of the host fat body to form the ‘cloak’. We have not yet determined whether the haemocytes in the ‘cloak’ were accumulated through some action of the parasitoid larvae or as a result of the host immune reactions. However, since the cloudy material is formed within 2 h of invasion, which is similar to the encapsulation capsule that surrounded dead tachinid larvae, this material may assemble as a result of the host immune reaction.
We found that dead larvae were melanized after transplantation (fig. 3b, d) whereas living larvae that were surrounded with the ‘cloak’ were not melanized (fig. 1d). In Drosophila species, PPO3 is specifically produced by haemocytes that engage in encapsulation and contributes to melanization around parasitoid eggs during encapsulation (Dudzic et al., Reference Dudzic, Kondo, Ueda, Bergman and Lemaitre2015). However, we currently have no evidence that PPOs activation is suppressed by the parasitoid larvae. Since normal encapsulation capsules contain only haemocytes and the composition of the ‘cloak’ was similar to that of fat body-contaminated encapsulation capsules, it is possible that the fat body physically interferes with the normal encapsulation process leading to suppression of the activities of PPOs. Alternatively, the secretion of chemicals from the larvae or the fat body cells within the ‘cloak’ may suppress the activation of PPOs.
Salt (Reference Salt1968) suggested that the sheath that forms around intruding larvae (fig. 1d, left) is made from the host haemocytes but he did not confirm this through genetic analyses. Here, we confirmed through genomic PCR that both the sheath and the ‘cloak’ are composed of only host cells. However, since the removal of the entire ‘cloak’ to prepare pure sheath material is difficult, we cannot exclude the possibility that there may have been some contamination of the sheath material by the ‘cloak’. In addition, since tachinid larvae moult twice within 72 h of invasion, it is likely that the cast-off cuticles are also incorporated into the sheath.
Once the D. inconspicuoides larvae became 3rd instar larvae, the ‘cloak’ no longer surrounded the entire larval body (fig. 1f). Therefore, it is likely that some other defence mechanism protects the larvae after this time. Alternatively, it is possible that they no longer require any particular means of defence because the host immune system has been sufficiently weakened by the consumption of immune tissues such as the fat body and haemocytes.
When females of endoparasitoid wasps deposit their eggs into the host, they also inject immune-suppressive factors such as polydnaviruses to allow their offspring to develop inside the host haemocoel (Moreau & Asgari, Reference Moreau and Asgari2015). However, parasitizing D. inconspicuoides larvae require the urgent activation of mechanisms that protect them from the host immune reactions in the absence of materials from their parents. Therefore, it may be that host-derived substances are used to form a barrier. Further studies that focus on the molecular mechanisms that are involved in the formation of the ‘cloak’ and the suppression of melanization will help further our understanding of the unique parasitization strategies of tachinid flies.
Acknowledgements
The authors thank Dr D. Taylor for critically reading the manuscript. This work was supported by JSPS KAKENHI Grant Number JP17K08157.







