Introduction
Seed dormancy can enhance seedling survival by preventing germination under unfavourable conditions or those that are only ephemerally favourable (Bewley, Reference Bewley1997). It is a critical regulator of the seasonal timing of germination by providing a temporal context for the perception of environmental cues, such that a cue perceived soon after dispersal, when seeds are dormant, will not have the same effect on germination probability as cues perceived sometime after dispersal, when seeds have lost dormancy. It is well known that germination-inducing factors, such as water and permissible germination temperatures, interact with dormancy to regulate the timing of germination under natural conditions (reviewed in Baskin and Baskin, Reference Baskin and Baskin1998). How environmentally induced secondary dormancy alters these dynamics, however, is still poorly understood.
Seeds with physiological dormancy are shed in a dormant state (Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006; Holdsworth et al., Reference Holdsworth, Bentsink and Soppe2008), and dormancy is lost through a process of ‘afterripening’ (Finch-Savage et al., Reference Finch-Savage, Cadman, Toorop, Lynn and Hilhorst2007; Carrera et al., Reference Carrera, Holman, Medhurst, Dietrich, Footitt, Theodoulou and Holdsworth2008; Holdsworth et al., Reference Holdsworth, Bentsink and Soppe2008; Iglesias-Fernandez et al., Reference Iglesias-Fernandez, Rodrıguez-Gacio and Matilla2011). As afterripening proceeds, germination can occur under a wider range of environmental conditions. As a result, many winter annuals, such as Arabidopsis thaliana, are not able to germinate under ephemerally cold conditions of spring when seeds are first shed, nor under warmer temperatures of summer. By the time autumn arrives, afterripening allows germination under cooler temperatures, and plants exhibit a classic winter-annual life history (Reference Baskin and Baskin1972 Reference Baskin and Baskin1983). Therefore, primary dormancy prevents germination until after the risk of summer drought. Such regulation of germination time is a major determinant of fitness, and germination time has been shown to be under extremely strong natural selection (reviewed in Donohue et al., Reference Donohue, Rubio de Casas, Burghardt, Kovach and Willis2010).
The conditions of seed maturation, determined by the season of flowering and fruit set, influence the level of primary dormancy that is induced in seeds (reviewed in Gutterman, Reference Gutterman and Fenner1992; Donohue and Schmitt, Reference Donohue, Schmitt, Mousseau and Fox1998; Donohue, Reference Donohue2009). For example in A. thaliana, cold temperatures during seed maturation induce strong primary dormancy (Donohue et al., Reference Donohue, Heschel, Chiang, Butler and Barua2007; Chiang et al., Reference Chiang, Bartsch, Barua, Nakabayashi, Debieu, Kronholm, Koornneef, Soppe, Donohue and de Meaux2011; Kendall and Penfield, Reference Kendall and Penfield2012; Penfield and Springthorpe, Reference Penfield and Springthorpe2012). Cold seed-maturation temperatures occur during early spring, or during autumn for genotypes that do not require vernalization to flower (Thompson, Reference Thompson1994; Donohue, Reference Donohue2009). As such, seed-maturation temperature provides information on seasonal timing; seeds matured under cold conditions may experience cold temperatures before summer, whereas seeds matured under warmer temperatures would experience cold temperatures only after summer has passed. It is therefore important to know how seed-maturation temperature influences responses to other seasonal cues, such as water availability and temperature fluctuations during imbibition, and whether it does so exclusively by altering levels of primary dormancy.
Even after primary dormancy is lost, seeds can acquire secondary dormancy if conditions are still unfavourable for germination, and dormancy cycling can occur under natural conditions (Reference Baskin and Baskin1983 Reference Baskin and Baskin1998; Bewley and Black, Reference Bewley and Black1994; Footitt et al., Reference Footitt, Douterelo-Soler, Clay and Finch-Savage2011, Reference Footitt, Huang, Clay, Mead and Finch-Savage2013; Penfield and Springthorpe, Reference Penfield and Springthorpe2012). Secondary dormancy can be induced by specific environmental factors, such as moisture and temperature, that vary seasonally. Water limitation, which frequently occurs during the summer in temperate climates, can induce secondary dormancy. In naturalized populations of Acacia saligna seeds, for example, secondary dormancy induction is regulated by humidity, with low moisture content inducing secondary dormancy (Tozer and Ooi, Reference Tozer and Ooi2014). Low moisture exposure due to low soil water content can accelerate germination once moisture becomes available again. For instance, seeds of Polygonum aviculare were induced into deep secondary dormancy by exposure to low-moisture conditions, but after seeds were hydrated, dormancy breakage was more pronounced in those seeds than in the ones that were in a constantly moist soil (Batlla and Benech-Arnold, Reference Batlla and Benech-Arnold2006; Batlla et al., Reference Batlla, Nicoletta and Benech-Arnold2007).
Exposure to wet incubation at different temperatures under darkness can also induce secondary dormancy. In A. thaliana, secondary dormancy can be induced by imbibition at high temperature (Donohue et al., Reference Donohue, Heschel, Chiang, Butler and Barua2007) or prolonged imbibition at cold temperatures (Penfield and Springthorpe, Reference Penfield and Springthorpe2012; Rubio de Casas et al., Reference Rubio de Casas, Kovach, Dittmar, Barua, Barco and Donohue2012; Debieu et al., Reference Debieu, Tang, Stich, Sikosek, Effgen, Josephs, Schmitt, Nordborg, Koornneef and de Meaux2013). The seasonal context of these responses is poorly understood, but such responses are likely to be important determinants of the seasonal patterns of dormancy cycling under natural conditions (Footitt et al., Reference Footitt, Douterelo-Soler, Clay and Finch-Savage2011, Reference Footitt, Huang, Clay, Mead and Finch-Savage2013) that ultimately determine the seasonal timing of seed germination.
To understand how environmental factors that regulate secondary dormancy actually influence the timing of seed germination under natural seasonal conditions, it is necessary to know how they interact with other factors that vary seasonally, such as seed-maturation conditions and afterripening. After ripening is an indicator of the time since seed dispersal, and seed-maturation temperature is a cue of when during a season seeds were dispersed. Determining how seasonally variable, secondary-dormancy regulating factors interact with afterripening and seed-maturation temperature will therefore provide important information on the mechanisms regulating the seasonal timing of seed germination.
We used A. thaliana as a study system for investigating these dynamics because of the abundant knowledge of seed dormancy and germination at the molecular level (Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006; Bentsink and Koornneef, Reference Bentsink and Koornneef2008; Holdsworth et al., Reference Holdsworth, Bentsink and Soppe2008), knowledge of the ecological context of seed dormancy (Donohue et al., Reference Donohue, Dorn, Griffith, Schmitt, Kim and Aguilera2005b; Huang et al., Reference Huang, Schmitt, Dorn, Griffith, Effgen, Takao, Koorneef and Donohue2010; Footitt et al., Reference Footitt, Douterelo-Soler, Clay and Finch-Savage2011, Reference Footitt, Huang, Clay, Mead and Finch-Savage2013), and how seed dormancy dynamics translate to seasonal life histories in this species (Reference Baskin and Baskin1972 Reference Baskin and Baskin1983; Thompson, Reference Thompson1994; Griffith et al., Reference Griffith, Kim and Donohue2004, Donohue et al., Reference Donohue, Dorn, Griffith, Kim, Aguilera and Schmitt2005a). A. thaliana is native to Europe and Eurasia (Sharbel et al. Reference Sharbel, Haubold and Mitchell-Olds2000; Hoffman Reference Hoffman2002), and exhibits life-history variation across its native and introduced range, including winter annuals, spring annuals, autumn flowering and rapid cycling (Ratcliffe, Reference Ratcliffe1965; Thompson, Reference Thompson1994; Donohue, Reference Donohue2009). It most typically exhibits a winter-annual life history, germinating in autumn and flowering in spring, but spring germinants are observed, even in populations that appear to be dominated by winter annuals (Griffith et al., Reference Griffith, Kim and Donohue2004; Donohue, Reference Donohue2009). Flowering has also been observed to occur in the autumn (Thompson, Reference Thompson1994; Donohue, Reference Donohue2009), and can contribute to a rapid-cycling life history. Therefore, flowering can occur during the cool conditions of late autumn or early spring, or during the warm conditions of mid to late spring or early autumn. In many locations throughout its natural and introduced range, A. thaliana experiences summer drought and hot temperatures, which have strongly deleterious effects on survival (e.g. Donohue et al., Reference Donohue, Dorn, Griffith, Schmitt, Kim and Aguilera2005b). Dormancy is thought to play a key role in escaping such adverse conditions, with dormant genotypes of Iberian populations found in locations with hotter summers (Montesinos-Navarro et al., Reference Montesinos-Navarro, Xavier Picó and Tonsor2012), and with more dormant alleles of the major dormancy locus DELAY OF GERMINATION-1 found in more arid locations (Kronholm et al., Reference Kronholm, Xavier Pico, Alonso-Blanco, Goudet and de Meaux2012). Thus dormancy dynamics appear to play an important role in regulating the timing of germination to occur under conditions of adequate moisture and permissive temperatures in this species.
To explore how different environments that induce secondary dormancy can influence the seasonal timing of seed germination, we examined how environmental cues that vary seasonally affect secondary dormancy induction in A. thaliana, and how their effects change with seed-maturation temperature and afterripening. Specifically, we address: (1) how low water potential interacts with seed-maturation temperature and afterripening duration to influence secondary dormancy induction; (2) how the duration of dark imbibition at high temperature interacts with afterripening duration to influence secondary dormancy induction; (3) how the duration of dark imbibition at cold temperatures interacts with seed-maturation temperature to influence secondary dormancy induction; and (4) how dark imbibition at diverse temperatures interacts with both seed-maturation temperature and afterripening duration to influence secondary dormancy induction and the probability of germination.
Materials and methods
To assess the effect of different environmental factors on the induction of secondary dormancy, we used seeds with different primary dormancy levels (either matured at different temperatures, or afterripened for different durations) and manipulated seed-incubation conditions such as water potential (Experiment 1); hot stratification, by incubation in the dark at 35°C (Experiment 2); and cold stratification by incubation at a range of cold temperatures (Experiment 3). To assess interactions among environmental factors, we also imposed different combinations of seed-maturation temperature, afterripening duration, and different stratification temperatures and durations of stratification (Experiment 4). In some of these manipulations (as in Experiments 1 and 3), seed-maturation temperature was de-coupled from the expressed level of primary dormancy by imposing other dormancy-breaking treatments that led to full germination before secondary-dormancy treatments were imposed; this permitted us to investigate the effect of seed-maturation temperature that could be independent of its effects on primary dormancy.
Seed production
We used seeds of the standard ecotype, Columbia (Col-0), for all experiments. Seeds were obtained from The Arabidopsis Information Resource seed-stock center at Ohio State University. To obtain seeds for Experiments 1, 3 and 4, seeds were matured at two temperatures to induce different levels of primary dormancy: 14°C, which induces strong primary dormancy, or 25°C, which does not induce strong primary dormancy. These temperatures are within the range experienced during seed maturation in A. thaliana under field conditions. To synchronize the harvest of seeds across seed-maturation treatments, seed-sowing was staggered across treatments. After 7 d of dark stratification at 4°C, seeds were sown into pots filled with Metromix 360 (Scotts Sierra, Marysville, Ohio, USA) and then moved to full-spectrum light at 20°C in a 12 h light cycle, to allow germination. After 10 d, seedlings were vernalized (4°C, 10 h light cycle) for 28 d before being placed into either 14°C or 25°C. Plants were grown under the two constant temperature regimes in a 12 h light cycle in EGC Model GC8-2 Plant Growth Chambers (Environmental Growth Chambers, Chagrin Falls, Ohio, USA). Twelve maternal plants were grown at each temperature. Replicate plants were randomly distributed over three replicate chambers containing four maternal replicate plants, and pot positions were rotated on a weekly basis within each chamber. Plants were fertilized twice before bolting with a 300 ppm solution of Peter's Professional 20–20–20 fertilizer (The Scotts Company, Marysville, Ohio, USA). Watering was withheld for 2 weeks when siliques approached maturity and seed harvest occurred on the same day for both temperature treatments. Seeds were stored at room temperature in a dessicator (Secador® 4.0 Auto-Dessicator Cabinets, Bel-Art Products, Pequannock, New Jersey, USA) until used for germination assays. Experiments 1 and 4 used the same seed batch and Experiment 3 used a different batch produced under the same conditions.
To obtain seeds for Experiment 2, seeds were sown in 0.6% w/v agar plates, stratified at 4°C for 7 d in darkness and then allowed to germinate at 22°C in a 12 h light cycle. Seedlings were then transferred to pots with potting soil (Metromix 360 soil, Scotts Sierra) and put in EGC GCW-30 Plant Growth Chambers under a 12 h light cycle at 22°C until they bolted. The plants were then moved to short days (8 h light) at 14°C until the seeds were matured. Plants were fertilized every 14 d with a 300 ppm nitrogen solution of Blossom Booster Fertilizer (JR Peters, Allentown, Pennsylvania, USA). For logistical reasons, seeds were produced in three different batches from plants grown in the same conditions at different times (batch 1: fresh seeds; batch 2: 3- and 5-month-old seeds; and batch 3: 18-month-old seeds). Harvest of each batch occurred on a single day, and seeds were kept in dry storage either at room temperature (batches 1 and 3) or 4°C (batch 2) until they were used for germination assays. Because seeds with different afterripening durations were harvested from different plants and stored under different conditions, the comparison across afterripening durations is confounded with batch. However, the motivation for different afterripening treatments was to manipulate the degree of primary dormancy; the batches did differ significantly in dormancy, as intended, even though we cannot attribute this difference solely to afterripening. A summary of the conditions used for seed production as well as the stratification treatments and incubation conditions is presented in Supplementary Table S1.
Experiment 1: Water potential
To examine the conditions under which water limitation affects secondary dormancy induction, we manipulated the seed-maturation temperature (14°C or 25°C), the afterripening duration (1 or 5 months), and the water potential of the solution in which the seeds were incubated ( − 1.0, − 1.2, − 1.5 and − 1.8 MPa) before being transferred to pure water (0 MPa). Germination assays were conducted at 16°C constant temperature and 12 h light/12 h dark cycle in Percival Model GR41LX incubation chambers (Percival Scientific Inc., Perry, Iowa, USA). Twelve seeds were sown in 60-mm Petri plates containing Whatman P5 filter paper saturated with one of four polyethylene glycol (PEG 8000) concentrations (EMD Chemicals Inc., Gibbstown, New Jersey, USA) to generate the range of water potentials. After 8 d of pre-incubation in PEG solution, seeds were rinsed and transferred to new 35-mm plates containing filter paper and fresh distilled water (0 MPa). Eleven biological replicates (different mother plants) were assayed per maternal treatment at each water potential. At the time of transfer to fresh water, control plates were prepared by placing seeds that experienced no pre-incubation into plates containing fresh, sterile, distilled water. Final germination (radicle protrusion) was recorded when germination had reached a clear plateau (20 d after the beginning of incubation in fresh distilled water). The final number of germinants and the total number of viable seeds were recorded to give the final germination proportion for each plate.
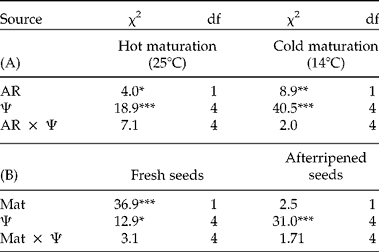
To test for significant effects of the pre-incubation treatment, and to test how those effects differed among seed-maturation and afterripening treatments, the final germination proportion was analysed using the GENMOD procedure in SAS 9.3 (SAS Institute, Cary, North Carolina, USA) to fit a generalized linear model with a binomial distribution and logit link function. Overdispersion, a common phenomenon encountered when modelling data with a binomial distribution, was corrected for, by using the deviance to estimate the scale parameter, and used for likelihood ratio tests. The LOGISTIC Procedure in SAS 9.3 was also conducted, with a Firth Penalized Likelihood approach to accommodate potential issues of data separation. As the results did not differ qualitatively, only the results from GENMOD are reported. The number of germinated seeds divided by the total number of viable seeds was used as the dependent variable, and seed-maturation temperature (Mat), duration of afterripening (AR) and water potential (Ψ) were treated as fixed factors. First, a full model including all interactions was analysed in order to test for significant three-way interactions. The AR × Mat interaction was significant, so each afterripening level was analysed separately, with Mat, Ψ, and Mat × Ψ as predictors. In addition, each Mat treatment was also analysed separately, with AR, Ψ and AR × Ψ as predictors.
Experiment 2: Hot stratification
To test the effect of high-temperature incubation on secondary dormancy induction, and whether it interacts with primary dormancy, we manipulated the duration of afterripening and the duration of exposure to high temperature (35°C) in the dark. Seeds, either fresh (3 d after harvest) or afterripened for 3, 5 or 18 months, were sown in 35-mm Petri plates with 0.6% w/v agar–agar and immediately incubated in darkness at 35°C for 1, 3, 5 or 7 d in Percival Model GR41LX incubation chambers. After the period of hot stratification, plates were incubated at 10, 22 or 31°C in a 12 h light/12 h dark cycle [photosynthetically active radiation (PAR): 120–150 μmol m− 2s− 1] in EGC Model GC8-2 Plant Growth Chambers (Environmental Growth Chambers). Seeding was staggered so that all plates in the different durations of hot stratification were transferred to light at the same time. At that time, control plates with no pre-incubation were prepared by sowing seeds in fresh agar plates and were immediately incubated in the conditions described above. For all the germination assays, 20 seeds per plate were used for every treatment and replicate. The number of biological replicates (different mother plants) was six for the experiments with fresh seeds, nine for the ones with seed batches afterripened for 3 months, and four for the seeds afterripened for 5 and 18 months. The final germination proportion (seeds showing radicle protrusion/total number of viable seeds) was recorded at 14 d after transfer to light, after germination had reached a clear plateau.
To test for effects of the duration of hot stratification (Hot Strat), and to test how those effects differed among incubation temperatures (Incub Temp) and afterripening treatments (AR), we fitted generalized linear models with logit link functions using ‘glm’ implemented in the ‘R’ statistical package (R Core Team, 2013), then performed type-III likelihood ratio tests using the ‘Anova’ function in the ‘car’ package (Fox and Weisberg, Reference Fox and Weisberg2011). A correction for multiple comparisons using the Holm method of ‘p.adjust’ in ‘stats’ package was used when appropriate. The three independent variables were treated as fixed factors. A full model that included all interactions was analysed first to test for a significant three-way interaction. To interpret interactions with afterripening, each afterripening group was analysed separately. Because of significant interactions between Hot Strat and Incub Temp within afterripening treatments, we then tested for an effect of Hot Strat within each combination of AR and Incub Temp.
Experiment 3: Cold stratification
To examine how the temperature and duration of low-temperature dark stratification influences whether dormancy is broken or induced, and to test whether that is affected by seed-maturation temperature, fresh seeds matured at 14°C or 25°C were stratified at one of four low temperatures (2°C, 4°C, 8°C and 12°C) for one of three durations (4, 8 or 12 d). Twelve seeds per biological replicate were sown in plates with filter paper (Whatman P5) soaked in 250 μl sterile distilled water. Plates were immediately put in darkness in the different stratification treatments. After stratification, plates were transferred to 22°C in a 12 h light/12 h dark cycle in Percival Model GR41LX incubation chambers. Control plates were seeded in the same way but were immediately transferred, without stratification, to the common incubation condition in the light. Final germination proportion (seeds showing radicle protrusion/total number of viable seeds) was recorded at 17 d after transfer to light, after germination had reached a clear plateau.
The final germination proportion was analysed using the GENMOD and LOGISTIC procedure in SAS 9.3, as described for Experiment 1. Results from both procedures were qualitatively similar, but GENMOD experienced some difficulties with convergence because of low variance in some treatments, so only the results from LOGISTIC are reported. First, to test the effects of the primary dormancy manipulation, the germination of unstratified seeds was compared between the two seed-maturation temperatures. Second, to examine whether a short, cold stratification broke primary dormancy, the proportion of germination of unstratified control seeds was compared to that of seeds that experienced 4 d of cold stratification (at all temperatures). Third, to examine effects of stratification on secondary dormancy induction, we analysed only (initially non-dormant) stratified seeds. A full model was run with all interactions, with seed-maturation temperature (Mat), stratification temperature (Strat Temp) and stratification duration (Duration) as fixed factors. The effects of Strat Temp and Duration were analysed separately within each Maturation treatment, because of significant interactions with Maturation. Finally, because of interactions between Strat Temp and Duration within the cold Maturation treatment, the effect of Strat Temp was analysed within each Duration in cold-matured seeds.
Experiment 4: Interaction between seed-maturation, afterripening and stratification conditions
To test how different primary dormancy inducing factors interact with seed stratification conditions to influence secondary dormancy, we manipulated seed-maturation temperature (14°C or 25°C), afterripening duration (3 or 19 weeks), seed-stratification temperature (4°C, 16°C and 31°C) and seed-stratification duration (4 or 11 d). Twelve seeds of a given treatment were sown in 35-mm Petri plates containing Whatman P5 filter paper saturated with sterile, double-distilled water. Twelve biological replicate plates, each containing seeds from a single maternal plant, were used for all treatment combinations. After seeding, seeds were immediately placed in darkness for their stratification treatments. After stratification, plates were transferred to 22°C in a 12 h light/12 h dark cycle in Percival Model GR41LX incubation chambers. At that time, an unstratified treatment was prepared from each seed-maturation × afterripening treatment and used as a control to quantify the starting dormancy of seeds in each treatment. For all assays, final germination proportion (seeds showing radicle protrusion/total number of viable seeds) was recorded after 14 d of incubation at 22°C in the light, after germination had reached a clear plateau.
To test for effects of seed-maturation temperature (Mat), duration of afterripening (AR), stratification temperature (Strat Temp) and stratification duration (Duration), we used the ‘glm’ procedure in the ‘R’ statistical package, as described for Experiment 2. The four independent variables were treated as fixed factors. In each model, unstratified control seeds were included for each Mat × AR combination. A full model that included all interactions and a reduced model lacking the four-way interaction were fit first. To interpret interactions with afterripening treatment, we analysed each afterripening group separately (with Mat, Strat Temp, Duration and all interactions). To examine interactions with Mat, we analysed each Mat treatment separately (with AR, Strat Temp, Duration and all interactions). Finally, to interpret the interactions within those sub-models, the effects of Strat Temp and Duration were analysed within each of the four AR × Mat combinations separately.
Results
Secondary dormancy induction by limited water availability (Experiment 1)
Control seeds, without pre-incubation at low water potentials, exhibited high germination proportions in all seed-maturation and afterripening treatments, indicating low primary dormancy. However, fresh seeds matured in cold temperatures had slightly lower germination than afterripened seeds (AR × Mat: χ2 =7.34, df=1, P <0.001). Pre-incubation at low water potentials reduced germination significantly compared to the control (Fig. 1; Ψ: χ2 =31.52, df=4, P <0.0001), and this effect did not depend on afterripening (non-significant AR × Ψ interaction; Table 1A) or seed-maturation temperature (non-significant Mat × Ψ interaction; Table 1B).

Figure 1 Effect of water limitation on secondary dormancy induction (Experiment 1). (A) Experimental design scheme. (B–E) Final germination proportion (mean ± SE) for seeds matured at 14°C (left panels, B and D) and 25°C (right panels, C and E) at two stages of afterripening: 1 month (upper panels, B and C) and 5 months (lower panels, D and E), incubated at different water potentials (Ψ). Open squares indicate the control treatment, without pre-incubation, and dotted lines indicate the mean of the control treatment for comparison. Asterisks denote significant differences observed when seeds are imbibed at the indicated water potential compared to the control at 0 MPa (with no pre-treatment). *P< 0.05, **P< 0.01.
Table 1 Effect of low water potential on secondary dormancy induction (Experiment 1). χ 2 based on Type III analysis of likelihood ratios from a logistic regression to test whether effects of water potential (Ψ) depend on afterripening (AR) or seed-maturation temperature (Mat). (A) Results of model fit to test for effects of afterripening and water potential (Ψ) on final germination proportion for each level of seed-maturation temperature separately. (B) Results of model fit to test for effects of maturation temperature and water potential on final germination proportion, for each level of afterripening separately. *P< 0.05, **P < 0.01, ***P< 0.001.

The induction of secondary dormancy by low water potential was less effective when water potential was − 1.5 MPa or lower (Fig. 1). Specifically, seeds pre-incubated at higher water potentials (less negative) showed lower germination proportions than the control, while seeds pre-incubated at lower water potentials (more negative) did not. The effect was especially apparent in cold-matured seeds (although the Mat × Ψ interaction was not significant, Table 1B), where germination significantly decreased after pre-incubation at − 1.0 and − 1.2 MPa and did so consistently across afterripening stages (Fig. 1B, D). In hot-matured seeds, germination decreased compared to the control in fresh seeds that were pre-incubated at − 1.2 MPa and in afterripened seeds that were pre-incubated at − 1.0 MPa (Fig. 1C, E). In summary, low water potential induced secondary dormancy, but only if the pre-incubation environment was not too dry.
Secondary dormancy induction by hot stratification (Experiment 2)
Seeds pre-incubated in darkness at 35°C (hot stratified) showed reduced germination compared to control seeds that never experienced hot stratification (Fig. 2), indicating that hot stratification can induce secondary dormancy. There was a non-monotonic relationship between the germination proportion and the duration of hot stratification, with 3 d inducing the greatest level of dormancy, and more prolonged stratification being less effective in most afterripening treatments (AR). The effect of hot stratification on final germination proportion depended on the afterripening duration and the temperature at which the seeds were incubated afterwards in the light (Fig. 2, significant Hot Strat × Incub Temp × AR interaction, χ2= 20, df = 4, P< 0.001), so each AR treatment was analysed separately to interpret the interaction. Incubation in the light at 31°C led to almost no germination of any seeds, including all afterripening treatments, even in control seeds that were not stratified. Therefore, this condition provides no information on secondary dormancy induction because it is above the temperature at which even non-dormant seeds can germinate, and it was not included in the subsequent analyses (Fig. 2 and Table 2).

Figure 2 Effect of hot stratification on secondary dormancy induction (Experiment 2). (A) Experimental design scheme. (B–E) Final germination proportion (mean ± SE) of seeds after a hot stratification treatment (pre-incubation at 35°C for 1, 3, 5 or 7 d) and subsequent incubation in the light at 10°C, 22°C or 31°C (see Key). (B) Fresh seeds; (C) seeds afterripened for 3 months; (D) seeds afterripened for 5 months; (E) seeds afterripened for18 months. Open symbols indicate the control treatment, without pre-incubation, and dotted lines indicate the mean of the control treatments for comparison. Asterisks within the graphs indicate a significant difference in germination proportion in 10°C versus 22°C within each duration of hot stratification. Asterisks in the rectangles to the right of each graph indicate a significant effect of the duration of hot stratification at each incubation temperature. ** P< 0.01, *** P< 0.001.
Table 2 Effect of duration of hot stratification (Hot Strat, incubation in the dark at 35°C) for each afterripening time (AR) and incubation temperature (Incub Temp) combination separately on the final germination proportion compared to the control with no hot stratification (Experiment 2). χ2 based on Type III analysis of likelihood ratios from a logistic regression to test for Hot Strat effects. ***P < 0.001

Considering each AR treatment separately, the effect of hot stratification duration differed significantly across incubation temperatures in fresh seeds and in those afterripened for 3 months (Hot Strat × Incub Temp interaction: χ2= 17.6, df = 4, P < 0.01, and χ2= 23.39, df = 4, P< 0.001, respectively), but not in seeds afterripened for longer durations (5 and 18 months: non-significant Hot strat × Incub Temp interaction). In general, although hot stratification reduced germination in almost all the treatments, it did so more in seeds incubated at 22°C than at 10°C (Fig. 2). Only seeds afterripened for 5 months and incubated at 10°C did not exhibit dormancy after hot stratification (Fig. 2D and Table 2). Hot stratification was most effective at inducing secondary dormancy in seeds afterripened for 18 months, impairing germination almost completely at both 10°C and 22°C after 3 d of stratification (Fig. 2E). This suggests that highly afterripened seeds may be induced into deeper secondary dormancy by hot stratification than fresher seeds.
Secondary dormancy induction by cold stratification (Experiment 3)
Unstratified seeds matured at 14°C were significantly more dormant than seeds matured at 25°C (Fig. 3, lines indicating controls; effect of seed maturation on unstratified seeds: χ2= 579.02, df = 1, P< 0.0001), but 4 d of stratification at any of the cold temperatures broke primary dormancy and led to full germination in seeds matured at both temperatures (effect of cold stratification: χ2= 1147.2, df = 4, P <0.0001, Fig. 3). Therefore, seeds from both maturation temperatures expressed no primary dormancy after short cold-stratification treatment. Nonetheless, seeds that were matured at 14°C and cold-stratified for longer durations had lower germination than those matured at 25°C, indicating that cold-matured seeds were more easily induced into secondary dormancy by longer cold stratification than warm-matured seeds (Fig. 3, effect of Mat in stratified seeds: χ2= 3.96, df = 1, P= 0.05). In fact, stratified seeds that were matured at 25°C had nearly 100% germination in all stratification treatments, with no effect of stratification temperature (effect of Strat Temp: χ2= 5.95, df = 3, P>0.05; Strat Temp × Duration, χ2= 3.60, P > 0.05).

Figure 3 Effect of cold stratification on secondary dormancy induction (Experiment 3). (A) Experimental design scheme. (B, C) Final germination proportion (mean ± SE) of seeds matured at 14°C (B) and 25°C (C) for each stratification duration (4, 8 and 12 d) and temperature (2°C, 4°C, 8°C and 12°C; see Key). Open squares indicate the control treatment, without stratification, and dotted lines indicate the mean of the control treatments for comparison. Asterisks denote significant dormancy induction (reduction in germination) compared to seeds stratified at 2°C for the comparable duration. *P < 0.05, **P < 0.01.
In seeds matured at 14°C, higher stratification temperatures (8°C and 12°C) induced secondary dormancy more effectively than the lower temperatures (2°C and 4°C) used in this experiment (Fig. 3B, effect of Strat Temp: χ2= 12.34, df = 3, P < 0.01), and this effect was more pronounced with longer stratification (Strat Temp × Duration, χ2= 12.47, df = 9, P< 0.05). After 4 d, no temperature induced secondary dormancy; after 8 d, only seeds stratified at 12°C showed secondary dormancy induction; after 12 d, seeds stratified at both 8°C and 12°C exhibited secondary dormancy. Thus for cold-matured seeds, longer durations of stratification at the warmer temperatures used in the range of this experiment (8°C and 12°C) were most effective at inducing secondary dormancy.
Interaction between primary dormancy factors and stratification temperature and duration (Experiment 4)
Seed maturation at 25°C (hot-matured) and longer afterripening each reduced primary dormancy; thus, baseline germination was lowest in cold-matured/fresher seeds, intermediate in hot-matured/fresher seeds, high in cold-matured/afterripened seeds, and highest in hot-matured/afterripened seeds (see controls in Fig. 4). A reduction in germination compared to these control data would indicate that stratification treatments induced secondary dormancy, while an increase in germination would indicate primary dormancy breakage by a stratification treatment.

Figure 4 Interaction between factors that influence primary dormancy (seed-maturation temperature and afterripening duration) and stratification (temperature and duration) (Experiment 4). (A) Experimental design scheme. (B–E) Final germination proportion (mean ± SE) is shown for seeds matured at 14°C (left panels) and 25°C (right panels) that were afterripened for 3 weeks (upper panels) or 19 weeks (lower panels) for each stratification duration (4 and 11 d) and temperature (4°C, 16°C and 31°C). Open squares indicate the control treatment, without stratification, and dotted lines indicate the mean of the control treatments for comparison.
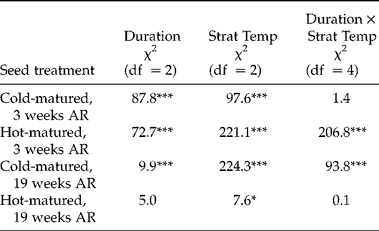
The effect of stratification temperature on seed germination depended on the maturation temperature and afterripening duration (significant Strat Temp × Mat × AR in the full model, χ2= 24.9, df = 2, P< 0.001). In seeds matured at 14°C (cold-matured), fresher seeds showed increased germination (i.e. primary dormancy breakage) after stratification at all three temperatures, with the coolest stratification temperature (4°C) being the most effective at reducing dormancy (Table 3A, Fig. 4B). In more afterripened seeds, secondary dormancy was induced only after longer incubation in the highest temperature (31°C, Fig. 4D). Thus, in cold-matured seeds, only the highest stratification temperature induced secondary dormancy, and it did so only in seeds afterripened for a longer period of time.
Table 3 Interactions between factors that induce primary dormancy (seed-maturation temperature and afterripening duration) and seed stratification conditions (temperature and duration of stratification) (Experiment 4). χ2 based on Type III analysis of likelihood ratios from a logistic regression of germination proportions. (A) Effects of duration of stratification (Duration), stratification temperature (Strat Temp) and afterripening duration (AR) for each seed-maturation temperature (Mat). (B) Effect of Duration, Strat Temp and Mat for each afterripening duration separately. *P < 0.05, **P < 0.01, ***P < 0.001
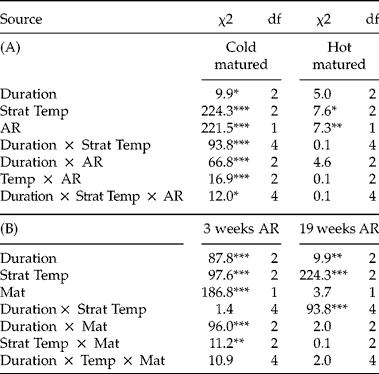
In seeds matured at 25°C (hot-matured), stratification at 31°C and prolonged stratification at 16°C both induced secondary dormancy in fresher seeds (Fig. 4C). Afterripening consistently increased germination and thereby decreased the effect of all three stratification temperatures on secondary dormancy induction (Table 3A), with a reduction in germination of more fully afterripened seeds only observed at the longest duration at 31°C (Fig. 4E). Combined, the results indicate that seed-maturation temperature drastically altered responses to stratification temperature in fresher seeds but not afterripened seeds (Table 3A). Specifically, in fresher seeds, stratification at all temperatures reduced dormancy of cold-matured seeds, but in hot-matured seeds, stratification at cooler temperatures reduced dormancy, while hot stratification increased dormancy. In contrast, in more afterripened seeds, stratification at 31°C induced secondary dormancy in both seed-maturation treatments.
The hottest stratification temperature (31°C) was generally the most effective at inducing secondary dormancy. Considering seeds with different levels of primary dormancy, in the most dormant group (cold-matured/fresher seeds; Table 4, Fig. 4B), all stratification temperatures increased germination (broke primary dormancy), with the largest effect observed for the coolest temperature (4°C). Longer stratification durations at all temperatures consistently reduced dormancy of cold-matured seeds. In seeds with intermediate dormancy (hot-matured/fresher seeds: Table 4, Fig. 4C), stratification temperatures differed in their effects on germination, and the effect of temperature depended on the length of stratification. The coolest stratification temperature (4°C) released dormancy consistently at both stratification durations. The intermediate temperature (16°C) first released dormancy, then induced dormancy at the longer stratification duration, returning seeds to a dormancy level similar to that of the control seeds. The hottest stratification temperature (31°C) marginally induced dormancy, the effect of which was consistent across stratification durations. In seeds with the second-least primary dormancy (cold-matured/afterripened seeds: Table 4, Fig. 4D), the cold (4°C) and intermediate (16°C) temperatures did not greatly induce dormancy at either duration. The hottest temperature (31°C) induced dormancy, and the effect increased with stratification duration, with complete dormancy induction (0% germination) by 11 d of stratification. In seeds with the least primary dormancy (hot-matured/afterripened seeds: Table 4, Fig. 4E), dormancy induction was only observed at the longest duration of stratification at 31°C. Therefore, dormancy induction by warmer stratification temperatures was most effective in seeds with intermediate dormancy.
Table 4 Test of effects of stratification duration (Duration) and temperature (Strat Temp) within each combination of seed-maturation temperature (Mat) and afterripening duration (AR) (Experiment 4). χ2 based on Type III analysis of likelihood ratios from a logistic regression of germination proportions. *P<0.05, ***P<0.001

Discussion
In regions with marked seasonal changes, seeds near the soil surface are subjected to cyclic fluctuations in soil water content and temperature. These environmental fluctuations interact with the dormancy status, causing seeds to cycle between different secondary dormant states in ways that can determine the timing of germination and hence the seasonal conditions for seedling establishment and plant growth (Baskin and Baskin, Reference Baskin and Baskin1998; Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006). In this paper, we show that secondary dormancy can be induced in seeds of A. thaliana by low water potential and by dark imbibition at a wide range of temperatures. The response to these treatments, however, depended on seed-maturation temperature and how long seeds had been afterripened – both cues of when, during a season, seeds are matured and shed. Therefore, the rate of loss of primary dormancy via afterripening, seed-maturation temperature and secondary dormancy inducing and reducing factors all interact in ways that can regulate precisely the seasonal timing of seed germination.
In nature, low soil water content can be a cue of a protracted dry season, unfavourable for germination and seedling survival. We found that secondary dormancy was induced by incubating seeds under conditions of reduced water availability, but not if the incubation environment was too dry (Fig. 1). Specifically, seeds that were pre-incubated at low water potential and then transferred to water had reduced germination compared to control seeds that were not pre-incubated, but when pre-incubation water potential was below − 1.2 MPa, germination was not affected. This suggests that there is a water potential threshold at which the seeds can sense that the environment is too dry and at which the resumption of metabolism required for germination is repressed via secondary dormancy induction, but that such repression cannot occur at increasingly lower water potentials. Cyclic fluctuations of soil water content that produce seed dehydration and rehydration have been shown to be more effective at inducing and reducing secondary dormancy than constant soil moisture (Batlla and Benech-Arnold, Reference Batlla and Benech-Arnold2006, Tozer and Ooi, Reference Tozer and Ooi2014). However, the mechanism by which secondary dormancy can be induced or reduced in response to rehydration is still uncertain. Our results also showed a trend for cold-matured seeds to be more easily induced into secondary dormancy than warm-matured seeds, although this difference was not statistically significant. This lack of significance may be partly because seed-maturation temperature did not cause differences in the germination proportions of these seeds. In fact, even differences in the duration of afterripening did not lead to differences in primary dormancy in this experiment, since even fresher control seeds had high germination proportions. We were therefore unable to assess how secondary dormancy induction by low water potential depends on primary dormancy status, so future study is necessary to examine that potential dynamic.
Besides water availability, the other major cue of seasonal conditions is temperature. We found that secondary dormancy can be induced by a wide range of temperatures, ranging from 8°C to 35°C depending on the persistence of the stimulus, with cooler temperatures requiring longer incubation periods to be effective at inducing dormancy. First, imbibition at cold temperatures is a well-known dormancy-breaking stimulus in many species, but when experienced for longer periods, it can induce secondary dormancy (Penfield and Springthorpe, Reference Penfield and Springthorpe2012; Rubio de Casas et al., Reference Rubio de Casas, Kovach, Dittmar, Barua, Barco and Donohue2012; Debieu et al., Reference Debieu, Tang, Stich, Sikosek, Effgen, Josephs, Schmitt, Nordborg, Koornneef and de Meaux2013). Our results show that after having an initial dormancy-breaking effect, extended incubation at 8°C and 12°C (for 12 d and 8 d, respectively) induces secondary dormancy in cold-matured seeds (Fig. 3). However, incubation at lower temperatures (2°C and 4°C) did not induce secondary dormancy in those seeds at all.
Next, we observed that a short period at high temperatures (hot stratification) induced secondary dormancy and reduced germination significantly (Fig. 2). Hot temperature is known to inhibit seed germination in many species during the process of thermoinhibition (Taylor et al., Reference Taylor, Hills, Gold, Stirk and van Staden2005; Argyris et al., Reference Argyris, Dahal, Hayashi, Still and Bradford2008; Watt et al., Reference Watt, Bloomberg and Finch-Savage2011, Toh et al., Reference Toh, Kamiya, Kawakami, Nambara, McCourt and Tsuchiya2012, Huo et al., Reference Huo, Dahal, Kunusoth, McCallum and Bradford2013). The physiological basis of secondary dormancy induction by hot temperature is not known, so it would be interesting to explore whether the physiological processes or genes involved in thermoinhibition of germination are also involved in secondary dormancy induction by high temperatures.
Secondary dormancy induction by hot stratification was more apparent when seeds experienced warmer temperatures (22°C) after the hot stratification treatment, suggesting that secondary dormancy onset by high temperature alters the probability of germination at specific temperatures, in a manner similar to primary dormancy. In A. thaliana (Reference Baskin and Baskin1972 Reference Baskin and Baskin1983) and other winter annuals, the duration of afterripening – and consequent loss of primary dormancy – alters the range of temperatures under which seeds can germinate; seeds first acquire the ability to germinate at low temperature and then can germinate at increasingly higher temperatures. Our results show that re-induction of dormancy (via secondary dormancy) likewise impedes germination at higher temperatures more than at lower temperatures.
We also found that the induction of secondary dormancy by high stratification temperature interacted with afterripening in a non-monotonic manner, suggesting that sensitivity to secondary dormancy induction fluctuates with dormancy cycling. Specifically, seeds afterripened for 3 and 18 months were more prone to be induced into secondary dormancy compared to fresh seeds and those afterripened for 5 months (note that the decrease in sensitivity from 3 to 5 months of afterripening, leading to the non-monotonic pattern, occurred in the same seed batch) (Fig. 2). Interestingly, this cyclic sensitivity occurred even though the germination propensity of the control seeds did not differ among afterripening times – that is, germination proportions of unstratified seeds were comparably high across all of our afterripening treatments. Our last experiment also showed a decrease in sensitivity of seeds to secondary dormancy induction by high temperature (31°C) in warm-matured seeds afterripened for almost 5 months compared to fresher seeds (Fig. 4). Why such cyclic sensitivity occurs is not known, as the genetic and metabolic processes occurring during afterripening are still largely unknown. Even less is known about how afterripening can influence secondary dormancy induction and whether they involve the same genetic pathways. Over the course of afterripening, small changes in the expression of genes involved in dormancy alleviation in dry seeds or seeds at low hydration were observed in wheat, sunflower and tobacco (Leubner-Metzger, Reference Leubner-Metzger2005; Meimoun et al., Reference Meimoun, Mordret, Langlade, Balzergue, Arribat, Bailly and El-Maarouf-Bouteau2014). In addition, imbibed seeds of A. thaliana that were afterripened for different durations had distinctly different gene expression profiles (Cadman et al., Reference Cadman, Toorop, Hilhorst and Finch-Savage2006). Moreover, secondary dormancy induction by dark imbibition at warm temperatures can re-set the gene expression profiles of afterripening-related genes and re-imposed an afterripening requirement for germination that was manifest as dormancy cycling (Cadman et al., Reference Cadman, Toorop, Hilhorst and Finch-Savage2006; Carrera et al. Reference Carrera, Holman, Medhurst, Dietrich, Footitt, Theodoulou and Holdsworth2008). Genes that were up-regulated in more dormant seeds were somewhat enriched in genes with abscisic acid (ABA)-responsive elements, implicating ABA in dormancy cycling. On the other hand, imbibed seeds of A. thaliana that were deficient in ABA synthesis or perception exhibited a similar pattern of regulation of genes that were associated with afterripening in wild-type seeds, even though the mutant seeds were capable of germinating without afterripening (Carrera et al., Reference Carrera, Holman, Medhurst, Dietrich, Footitt, Theodoulou and Holdsworth2008), suggesting that ABA may have a small role in gene regulation during afterripening. Even though there are several lines of evidence showing that afterripening could induce changes in gene expression, these could be confounded with decreases in mRNA caused by oxidation in response to non-enzymatic reactions happening during dry storage (Meimoun et al., Reference Meimoun, Mordret, Langlade, Balzergue, Arribat, Bailly and El-Maarouf-Bouteau2014). Regardless, it appears that afterripening and secondary dormancy induction interact non-additively: fresh seeds were not more easily induced into secondary dormancy, as would be expected if simple additions of dormancy-promoting factors (primary plus secondary dormancy) combined to establish dormancy levels. Nor is it simply the case that secondary dormancy can only be detected in seeds with low primary dormancy (high germination probability), since in our experiments, seeds at all stages of afterripening had high-enough germination percentages that could have been decreased by secondary dormancy induction. Our results suggest that sensitivity to secondary dormancy induction may vary with dormancy cycling over the course of afterripening, even when afterripening itself does not affect expressed germination proportions. Given the apparent decoupling of afterripening and germination propensity in our samples, combined with the cyclic sensitivity of secondary dormancy induction with afterripening, it would be interesting to examine whether sensitivity to secondary dormancy induction corresponds to changes in gene expression during afterripening (Cadman et al., Reference Cadman, Toorop, Hilhorst and Finch-Savage2006; Carrera et al., Reference Carrera, Holman, Medhurst, Dietrich, Footitt, Theodoulou and Holdsworth2008).
On the other hand, the interaction between primary and secondary dormancy inducing factors was also evident when afterripening was associated with a reduction in primary dormancy (Fig. 4). In this case, stratification temperatures that induced secondary dormancy in seeds with low primary dormancy (whether through afterripening or by seed maturation at higher temperatures) actually increased germination in seeds with high primary dormancy (Figs 3 and 4). Again, the physiological mechanism underlying this response cannot be explained solely by consistent changes to the hormone balance governing the dormancy level of the seeds imposed by these environmental stimuli. Instead, these temperature stimuli can have opposite effects, depending on the level of primary dormancy, implying a more complex regulatory mechanism.
Secondary dormancy induction was contingent not only on afterripening status, but also on the seed-maturation temperature. Cold seed-maturation induced strong primary dormancy, and this altered the response to secondary dormancy inducing factors, as just discussed. However, seed-maturation temperature altered responses to dark stratification even when differences in primary dormancy were not expressed because of afterripening (Experiment 4, Fig. 4) or dormancy-breaking cold stratification (Experiment 3, Fig. 3). In both cases, cold-matured seeds were more easily induced into secondary dormancy than the warm-matured ones.
Seed-maturation temperature is a cue of when during a season seeds are matured and shed. Cold seed maturation can occur in early spring in winter annuals, or in late autumn in autumn-flowering cohorts that occur in some populations (Thompson, Reference Thompson1994; Donohue, Reference Donohue2009). As such, seed-maturation temperature can interact with the loss of primary dormancy via afterripening and with secondary dormancy inducing stimuli, to influence the seasonal timing of seed germination. First, cold seed-maturation temperature causes deeper primary dormancy and slower rates of afterripening (Donohue et al., Reference Donohue, Heschel, Chiang, Butler and Barua2007; Kendall and Penfield, Reference Kendall and Penfield2012; Penfield and Springthorpe, Reference Penfield and Springthorpe2012; Murphey et al., Reference Murphey, Kovach, Elnaccash, He, Bentskink and Donohue2014). Second, cold-matured seeds are induced into secondary dormancy at lower temperatures than warm-matured seeds (Fig. 3). When interpreted in a seasonal context, this suggests for spring-flowering cohorts (Fig. 5A) that seeds matured and shed in early spring under cool conditions would be induced into secondary dormancy and be prevented from germinating before summer; seeds matured and dispersed later in the spring under warmer conditions would be induced into secondary dormancy by hot, dry conditions. Both cohorts could break dormancy in response to cold stratification in late autumn. Thus the effect of cold seed-maturation on secondary dormancy induction could synchronize germination of all spring-matured seeds (both early and late), leading to autumn germination and a winter-annual strategy.

Figure 5 Schematic of how seed-maturation temperature, primary dormancy and secondary dormancy are expected to influence the seasonal timing of germination. The diagrams show the progress towards germination (y-axis) as a function of primary dormancy loss and secondary dormancy induction. The x-axis indicates the months throughout the year. Seeds are matured and shed (cartoon plants) at different times of year, and therefore at different temperatures. Seeds matured under cold conditions are represented by dashed lines, and seeds matured under warmer temperatures are represented by solid lines. Seeds matured at lower temperature lose primary dormancy more slowly than seeds matured at higher temperatures. As seeds lose primary dormancy they increase in the probability of germination, indicated by their approach to the horizontal line that represents total loss of dormancy (‘germination threshold’); after they reach that line (lose enough dormancy), they can germinate. Grey lines indicate the loss of primary dormancy independently of secondary dormancy induction. Downward arrows indicate the induction of secondary dormancy by cold stratification (8°C or above, grey shaded box across the year; effective for cold-matured seeds only and hence outlined in dashed lines), hot stratification (31°C or above), and low soil water potential (time of low water potential is indicated by the shaded area during the summer months). Upward arrow indicates dormancy breakage by cold stratification. Black lines show dormancy status as determined by both primary dormancy loss and secondary dormancy induction. Note that we do not know the actual trajectory/depth of secondary dormancy induction because we did not test secondary dormancy breakage in this study. (A) Spring seed-maturation. Seeds are matured and shed in either early spring under cold temperatures (dashed line) or late spring under warm temperatures (solid line). Seeds matured at cold temperatures are prevented from germinating in late spring because of secondary dormancy induction by cold stratification. Seeds matured at warmer temperature experience hot conditions that induce secondary dormancy soon after dispersal. Cold stratification breaks dormancy of both seed types, leading to synchronized autumn germination. (B) Autumn seed-maturation. Note that the time line (x-axis) starts in June. Seeds matured at warmer temperature in early autumn are not induced into dormancy by cold stratification, so they may be able to germinate the same autumn, if temperatures remain permissive; if not, they can germinate as soon as temperatures become permissive in spring. Both strategies produce potential rapid cyclers (seedlings surrounded by solid circles). For seeds matured at cold temperature in late autumn, secondary dormancy induced by cold stratification prevents spring germination, and seeds germinate only after dormancy-breaking cold stratification the following autumn (seedling surrounded by dashed line). Therefore, the sensitivity of cold-matured seeds to secondary dormancy induction may prevent spring annuals and rapid cycling.
In autumn-flowering cohorts (Fig. 5B), seeds matured at higher temperatures early in autumn would be less dormant than seeds matured later at cooler temperatures. They therefore may be able to germinate that same autumn if permissive temperatures persist long enough, because these seeds would not experience the hot, dry conditions that induce secondary dormancy. If permissive temperatures do not persist, these seeds are expected to be capable of germinating in the following spring, since they are not induced into secondary dormancy by cold temperatures. These seeds could therefore exhibit a rapid-cycling life history. Seeds matured under cooler conditions later in autumn, however, are expected to be sensitive to secondary dormancy induction by cold temperatures in spring. Therefore, they may be prevented from germinating in spring, and would instead germinate after dormancy-breaking cold stratification in autumn. Thus for autumn-flowering cohorts, the effect of cold seed-maturation on secondary dormancy induction could prevent a spring annual or rapid-cycling life history. Exploring how genetic variation in dormancy levels interacts with the responses described in this paper, leading to modifications in the behaviour of the seeds, would lead to very interesting information to help us understand the changes in life-history expression in natural conditions.
In summary, the temperature during seed maturation, primary dormancy and secondary dormancy induction all interact to influence the probability of germination. The underlying mechanisms of these processes seem to be independent to some degree, increasing the precision of integration of environmental information, but they interact with each other in a manner that can finely regulate germination timing under natural seasonal conditions.
Acknowledgements
We thank the staff of the Duke University Phytrotron facilities for excellent care of the plants. We are grateful to Mercedes Zapata-Garcia, Anamika Saha and other undergraduate members of the Donohue Lab. for their assistance with these experiments.
Financial support
This work was supported by National Science Foundation grant DEB-1020963 to K.D. The work was also supported by fellowships to K.D. from the John Simon Guggenheim Foundation and the National Evolutionary Synthesis Center, National Science Foundation, EF-0905606.
Conflicts of interest
None.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0960258514000440