INTRODUCTION
Horses (i.e., family Equidae) are key taxa in palaeontological and evolutionary studies due to their abundance and widespread presence in the fossil record (MacFadden, Reference MacFadden1992, Reference MacFadden2005; Orlando, Reference Orlando2015). Among the different equid lineages, Hipparionini and Equini tribes have traditionally received much interest because of their importance as stratigraphical, biochronological, and palaeoecological markers (Strömberg, Reference Strömberg2006; van Asperen, Reference van Asperen2012; Bernor et al., Reference Bernor, Göhlich, Harzhauser and Semprebon2017; Boulbes and van Asperen, Reference Boulbes and van Asperen2019; Rook et al., Reference Rook, Bernor, Avilla, Cirilli, Flynn, Jukar, Sanders, Scott and Wang2019). Turkey has an excellent palaeontological record of both Hipparionini and Equini equids (Tekkaya et al., Reference Tekkaya, Atalay, Gürbüz, Ünay and Ermumcu1975; Koufos and Kostopoulos, Reference Koufos and Kostopoulos1994; Forsten and Kaya, Reference Forsten and Kaya1995; Eisenmann and Sondaar, Reference Eisenmann and Sondaar1998; Kaya and Forsten, Reference Kaya and Forsten1999; Geraads et al., Reference Geraads, Gulec and Kaya2002; Saraç et al., Reference Saraç, Kaya and Geraads2002; Bernor et al., Reference Bernor, Scott, Fortelius, Kappelman, Sen, Fortelius, Kappelman, Sen and Bernor2003; Kaya et al., Reference Kaya, Geraads and Tuna2005a, Reference Kaya, Mayda and Saraç2005b, Reference Kaya, Mayda, Kostopoulos, Alcicek, Merceron, Tan, Karakutuk, Giesler and Scott2012; Koufos and Vlachou, Reference Koufos and Vlachou2005; Mayda et al., Reference Mayda, Sotnikova, Tesakov, Tan and Kaya2015). However, most of the studies performed so far on Miocene and Pleistocene Turkish horses have focused on the taxonomy and systematics of the different species (e.g., Kaya et al., Reference Kaya, Mayda, Kostopoulos, Alcicek, Merceron, Tan, Karakutuk, Giesler and Scott2012), whereas palaeobiological and diagenetic aspects still remain poorly explored.
Bone histology has proved to be a valuable tool for inferring palaeobiological and taphonomic information of prehistoric animals (e.g., Chinsamy-Turan, Reference Chinsamy-Turan2005; Turner-Walker, Reference Turner-Walker, Pinhasi and Mays2008; Kendall et al., Reference Kendall, Eriksen, Kontopoulos, Collins and Turner-Walker2018). Despite the host of information that bone histology is able to provide for archaeological and palaeontological vertebrates, it is still not widely addressed in these fields. On the one hand, histo-taphonomical works have traditionally focused on archaeological material (e.g., Jans, Reference Jans2005), and only a few studies thoroughly reported microscopic alterations in the bone tissue of palaeontological samples (Davis, Reference Davis1997; Trueman and Martill, Reference Trueman and Martill2002; Chinsamy-Turan, Reference Chinsamy-Turan2005; Chinsamy-Turan and Ray, Reference Chinsamy-Turan, Ray and Chinsamy-Turan2012; Turner-Walker, Reference Turner-Walker2012; van der Sluis et al., Reference van der Sluis, Hollund, Buckley, De Louw, Rijsdijk and Kars2014; Tomassini et al., Reference Tomassini, Miño-Boilini, Zurita, Montalvo and Cesaretti2015; Lyras et al., Reference Lyras, Giannakopoulou, Lillis and van der Geer2019; Mayer et al., Reference Mayer, Hubbe, Botha-Brink, Ribeiro, Haddad-Martim and Neves2020). Conversely, histological studies on palaeontological bones have generally aimed at inferring life history information of extinct species (Chinsamy-Turan, Reference Chinsamy-Turan2005; Kolb et al., Reference Kolb, Scheyer, Veitschegger, Forasiepi, Amson, Van der Geer, Van den Hoek Ostende, Hayashi and Sánchez-Villagra2015b), an approach less investigated within archaeological material.
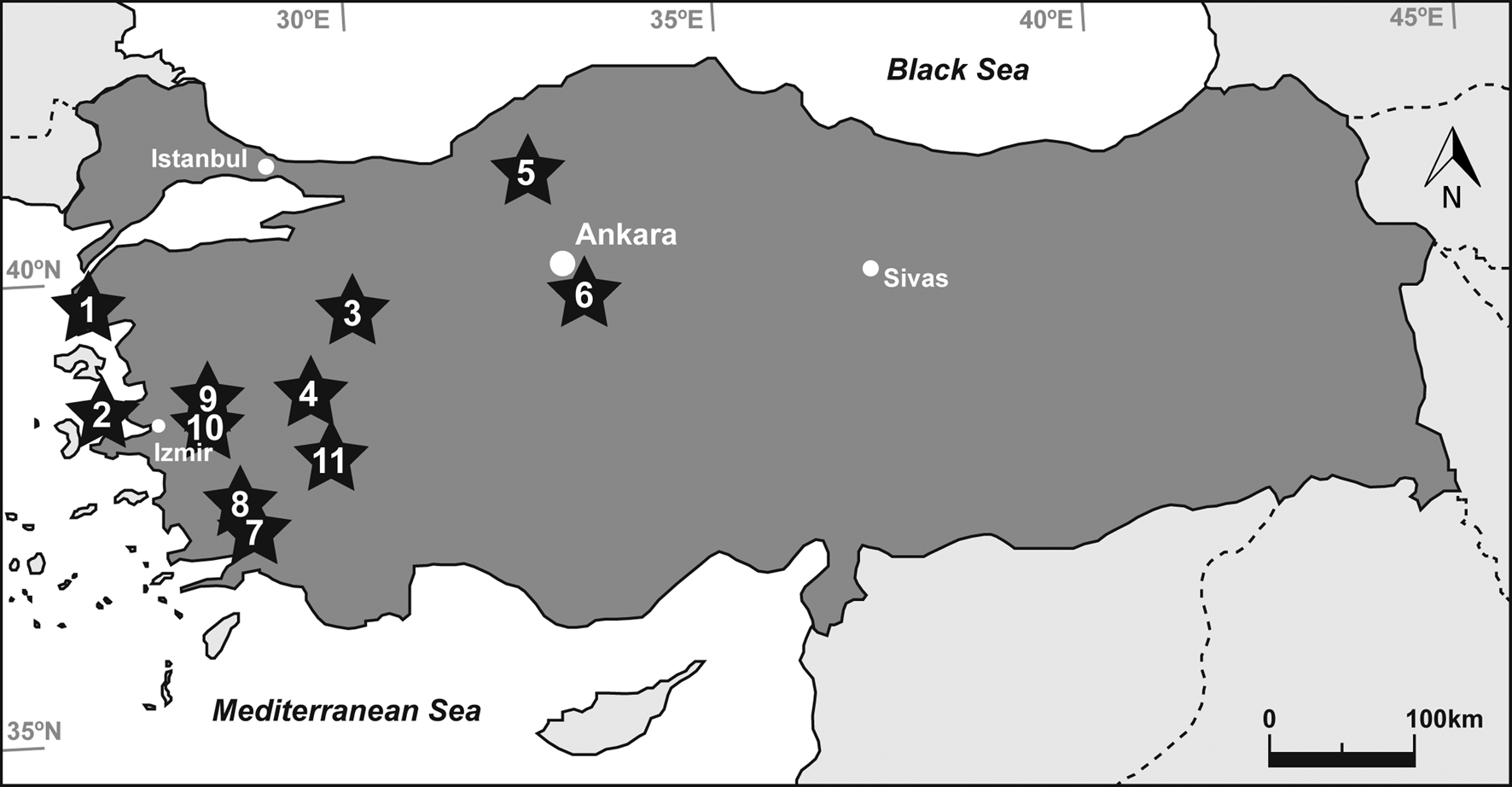
In this paper, we apply bone histology to several Equus and Hipparion specimens from the Miocene, Mio-Pliocene, and Pleistocene deposits of Turkey (Fig. 1, Table 1) with the objective of describing the bone histology and the microscopic diagenetic alterations of the samples. The main aims of our research are (1) to expand our knowledge of the palaeobiology of extinct equid species and (2) to shed light on the early taphonomic history of the fossils from 11 different Turkish localities.

Figure 1. Map of Turkey (dark grey) showing the locations of the localities from which the studied metapodia were recovered: (1) Çanakkale-Gülpınar, (2) Izmir-Karaburun, (3) Kütahya-Bayat, (4) Uşak-Kemiklitepe, (5) Çankırı-Yeniköy, (6) Kırşehir-Kaman, (7) Muğla-Yatağan-Salihpaşalar, (8) Muğla-Yatağan-Şerefköy, (9) Manisa-Düzpınar, (10) Manisa-Aşağıçobanisa, (11) Denizli-Pamukkale. Stars indicate fossil localities and circles depict main cities.
Table 1. Hipparion and Equus samples studied. ELMA = European Land Mammal Ages, based on Hilgen and colleagues (Reference Hilgen, Lourens, Van Dam, Beu, Boyes, Cooper, Krijgsman, Ogg, Piller, Wilson, Gradstein, Ogg, Schmitz and Ogg2012); MN = European Mammal Neogene Units; Ma = million years ago; Mt-III = third metatarsal; Mc-III = third metacarpal.

BACKGROUND
Hipparion and Equus palaeontology
Hipparionine horses are characterized by having three-toed limbs and isolated protocones in their upper cheek teeth (Christol, Reference Christol1832; Alberdi, Reference Alberdi, Prothero and Schoch1989; Bernor et al., Reference Bernor, Tobien, Woodburne, Lindsay, Fahlbusch and Mein1990). Originating in the Miocene of North America (MacFadden, Reference MacFadden1984, Reference MacFadden1985), they reached the Old World around 11 Ma (million years ago) during the migratory event usually referred to as “Hipparion datum” (Cormohipparion datum after Bernor et al., Reference Bernor, Göhlich, Harzhauser and Semprebon2017) (Bernor et al., Reference Bernor, Kovar-Eder, Lipscomb, Rögl, Sen and Tobien1988; Sen, Reference Sen, Lindsay, Fahlbusch and Mein1990; Garcés et al., Reference Garcés, Cabrera, Agustí and Parés1997). Hipparionine taxa flourished in this continent during Vallesian (European Land Mammal Age, 11.2–8.9 Ma [Hilgen et al., Reference Hilgen, Lourens, Van Dam, Beu, Boyes, Cooper, Krijgsman, Ogg, Piller, Wilson, Gradstein, Ogg, Schmitz and Ogg2012]) and Turolian (European Land Mammal Age, 8.9–5.3 Ma [Hilgen et al., Reference Hilgen, Lourens, Van Dam, Beu, Boyes, Cooper, Krijgsman, Ogg, Piller, Wilson, Gradstein, Ogg, Schmitz and Ogg2012]) periods (Forsten, Reference Forsten1989) and finally became extinct in Europe at the middle Villafranchian (early Pleistocene, 2.6–2.0 Ma [Rook et al., Reference Rook, Bernor, Avilla, Cirilli, Flynn, Jukar, Sanders, Scott and Wang2019]) (Pueyo et al., Reference Pueyo, Muñoz, Laplana and Parés2016). During this latest period, they may have coexisted (Pueyo et al., Reference Pueyo, Muñoz, Laplana and Parés2016; Rook et al., Reference Rook, Cirilli and Bernor2017) with the only Equini representative distributed worldwide (Prado and Alberdi, Reference Prado and Alberdi1996): the genus Equus, which comprises all living and extinct species of monodactyl horses with extremely hypsodont teeth (MacFadden, Reference MacFadden1992). Equus first emerged in North America 4–4.5 Ma (Orlando et al., Reference Orlando, Ginolhac, Zhang, Froese, Albrechtsen, Stiller and Schubert2013), and thereafter they dispersed to Eurasia (Rook et al., Reference Rook, Bernor, Avilla, Cirilli, Flynn, Jukar, Sanders, Scott and Wang2019) in two different migration waves (Alberdi and Bonadonna, Reference Alberdi and Bonadonna1988; Forsten, Reference Forsten1988; Azzaroli, Reference Azzaroli1992; Alberdi and Cerdeño, Reference Alberdi, Cerdeño, Jiménez Fuentes and Civis Llovera2003). A first dispersal event introduced the stenonid horses (zebras and asses) to Eurasia (Alberdi and Bonadonna, Reference Alberdi and Bonadonna1988; Alberdi et al., Reference Alberdi, Ortiz-Jaureguizar and Prado1998) 3.0–2.5 Ma (Equus-Elephant event, Lindsay et al., Reference Lindsay, Opdyke and Johnson1980). Caballoid equids (horses), on the other hand, arrived in Eurasia during a second migration wave that took place in the middle Pleistocene (1–0.8 Ma) (Alberdi and Bonadonna, Reference Alberdi and Bonadonna1988; Forsten, Reference Forsten1988; Orlando, Reference Orlando2015).
Hipparion s.l. (see Material Section for further explanations on hipparionine taxonomy) and Equus are common element faunas in the Neogene and Quaternary of Turkey. From the early Vallesian (MN9 European Land Mammal Age, 11.2–9.9 Ma [Hilgen et al., Reference Hilgen, Lourens, Van Dam, Beu, Boyes, Cooper, Krijgsman, Ogg, Piller, Wilson, Gradstein, Ogg, Schmitz and Ogg2012]) to the early Ruscinian (MN14 European Land Mammal Age, 5.3–5.0 Ma [Hilgen et al., Reference Hilgen, Lourens, Van Dam, Beu, Boyes, Cooper, Krijgsman, Ogg, Piller, Wilson, Gradstein, Ogg, Schmitz and Ogg2012]), Anatolia was inhabited by a high taxonomic diversity of hipparionines, including small-bodied forms (e.g., H. matthewi), medium-sized taxa (e.g., H. dietrichi), and large-bodied species (e.g., H. brachypus) (Tekkaya et al., Reference Tekkaya, Atalay, Gürbüz, Ünay and Ermumcu1975; Koufos and Kostopoulos, Reference Koufos and Kostopoulos1994; Forsten and Kaya, Reference Forsten and Kaya1995; Eisenmann and Sondaar, Reference Eisenmann and Sondaar1998; Kaya and Forsten, Reference Kaya and Forsten1999; Geraads et al., Reference Geraads, Gulec and Kaya2002; Saraç et al., Reference Saraç, Kaya and Geraads2002; Bernor et al., Reference Bernor, Scott, Fortelius, Kappelman, Sen, Fortelius, Kappelman, Sen and Bernor2003; Kaya et al., Reference Kaya, Geraads and Tuna2005a, Reference Kaya, Mayda and Saraç2005b, Reference Kaya, Mayda, Kostopoulos, Alcicek, Merceron, Tan, Karakutuk, Giesler and Scott2012; Koufos and Vlachou, Reference Koufos and Vlachou2005; Mayda et al., Reference Mayda, Sotnikova, Tesakov, Tan and Kaya2015). Quaternary mammals from Turkey are less studied (Erten et al., Reference Erten, Sen and Özkul2005) than late Miocene ones (Demirel and Mayda, Reference Demirel and Mayda2014), but still different stenonid Equus have been described in Plio-Pleistocene and early Pleistocene deposits (Kostopoulos and Sen, Reference Kostopoulos and Sen1999; Alçiçek et al., Reference Alçiçek, Mayda and Alçiçek2012; Mayda et al., Reference Mayda, Titov, Tesakov, Göktaş and Alçiçek2013; Demirel and Mayda, Reference Demirel and Mayda2014; Lebatard et al., Reference Lebatard, Alçiçek, Rochette, Khatib, Vialet, Boulbes and Bourlès2014; Rausch et al., Reference Rausch, Alçiçek, Vialet, Boulbes, Mayda, Titov and Stoica2019).
Studies on bone histology
Since the seminal works of Enlow and Brown (Enlow and Brown, Reference Enlow and Brown1956, Reference Enlow and Brown1957, Reference Enlow and Brown1958) and de Ricqlès (e.g., de Ricqlès, Reference de Ricqlès1975), numerous authors have analysed the bone microstructure of extinct vertebrates to reconstruct different aspects of their life history (e.g., Chinsamy-Turan Reference Chinsamy-Turan2005). These inferences of extinct taxa rely on our understanding of the histology of extant species (e.g., Köhler et al., Reference Köhler, Marín-Moratalla, Jordana and Aanes2012), which has shown that key life history traits like birth, growth rate, age at maturity, and age at death are recorded in the bone microstructure (Amprino, Reference Amprino1947; Chinsamy et al., Reference Chinsamy, Hanrahan, Neto and Seeley1995; de Margerie et al., Reference de Margerie, Cubo and Castanet2002; Castanet et al., Reference Castanet, Croci, Aujard, Perret, Cubo and de Margerie2004; Chinsamy and Valenzuela, Reference Chinsamy and Valenzuela2008; Erismis and Chinsamy, Reference Erismis and Chinsamy2010; Marín-Moratalla et al., Reference Marín-Moratalla, Jordana and Köhler2013; Kolb et al., Reference Kolb, Scheyer, Veitschegger, Forasiepi, Amson, Van der Geer, Van den Hoek Ostende, Hayashi and Sánchez-Villagra2015b; Jordana et al., Reference Jordana, Marín-Moratalla, Moncunill-Solé, Nacarino-Meneses and Köhler2016; Nacarino-Meneses et al., Reference Nacarino-Meneses, Jordana and Köhler2016a; Montoya-Sanhueza and Chinsamy, Reference Montoya-Sanhueza and Chinsamy2017; Nacarino-Meneses and Köhler, Reference Nacarino-Meneses and Köhler2018). In extinct mammals, palaeohistological investigations have focussed on bones of extinct rodents (Geiger et al., Reference Geiger, Wilson, Costeur, Sánchez and Sánchez-Villagra2013; Kolb et al., Reference Kolb, Scheyer, Veitschegger, Forasiepi, Amson, Van der Geer, Van den Hoek Ostende, Hayashi and Sánchez-Villagra2015b; Orlandi-Oliveras et al., Reference Orlandi-Oliveras, Jordana, Moncunill-Solé and Köhler2016; Garrone et al., Reference Garrone, Cerda and Tomassini2019; Miszkiewicz et al., Reference Miszkiewicz, Louys and O'Connor2019, Reference Miszkiewicz, Louys, Beck, Mahoney, Aplin and O'Connor2020), lagomorphs (Kolb et al., Reference Kolb, Scheyer, Veitschegger, Forasiepi, Amson, Van der Geer, Van den Hoek Ostende, Hayashi and Sánchez-Villagra2015b; Moncunill-Solé et al., Reference Moncunill-Solé, Orlandi-Oliveras, Jordana, Rook and Köhler2016), hedgehogs (Kolb et al., Reference Kolb, Scheyer, Veitschegger, Forasiepi, Amson, Van der Geer, Van den Hoek Ostende, Hayashi and Sánchez-Villagra2015b), wombats (Walker et al., Reference Walker, Louys, Herries, Price and Miszkiewicz2020), hippos (Kolb et al., Reference Kolb, Scheyer, Veitschegger, Forasiepi, Amson, Van der Geer, Van den Hoek Ostende, Hayashi and Sánchez-Villagra2015b), seals (Woolley et al., Reference Woolley, Chinsamy, Govender and Bester2019), bovids (Köhler and Moyà-Solà, Reference Köhler and Moyà-Solà2009; Marín-Moratalla et al., Reference Marín-Moratalla, Jordana, García-Martínez and Köhler2011), cervids (Amson et al., Reference Amson, Kolb, Scheyer and Sánchez-Villagra2015; Kolb et al., Reference Kolb, Scheyer, Lister, Azorit, de Vos, Schlingemann, Rössner, Monaghan and Sánchez-Villagra2015a; Lyras et al., Reference Lyras, Giannakopoulou, Lillis, Veis and Papadopoulos2016, Reference Lyras, Giannakopoulou, Lillis and van der Geer2019), ursids (Veitschegger et al., Reference Veitschegger, Kolb, Amson, Scheyer and Sánchez-Villagra2018), and equids (Sander and Andrássy, Reference Sander and Andrássy2006; Martínez-Maza et al., Reference Martínez-Maza, Alberdi, Nieto-Diaz and Prado2014; Orlandi-Oliveras et al., Reference Orlandi-Oliveras, Nacarino-Meneses, Koufos and Köhler2018; Nacarino-Meneses and Orlandi-Oliveras, Reference Nacarino-Meneses and Orlandi-Oliveras2019; Zedda et al., Reference Zedda, Sathe, Chakraborty, Palombo and Farina2020).
Bone histology also provides relevant insights into the early taphonomic history of fossil samples (Garland, Reference Garland1989; Child, Reference Child1995; Pfretzschner, Reference Pfretzschner2004; Jans, Reference Jans2005, Reference Jans, Wisshak and Tapanila2008; Turner-Walker, Reference Turner-Walker, Pinhasi and Mays2008; Pfretzschner and Tütken, Reference Pfretzschner and Tütken2011; Kendall et al., Reference Kendall, Eriksen, Kontopoulos, Collins and Turner-Walker2018). In 1864, Wedl described some small tunnels boring the microstructure of ancient bones and tentatively identified fungi, microscopic parasites, and/or parasitic plants as the cause (Turner-Walker, Reference Turner-Walker, Pinhasi and Mays2008, Reference Turner-Walker2019). Since this pioneering work, different experiments have related the presence, shape, and size of these microscopic foci to the diagenetic action of several microorganisms (Marchiafava et al., Reference Marchiafava, Bonucci and Ascenzi1974; Hackett, Reference Hackett1981; Fernández-Jalvo et al., Reference Fernández-Jalvo, Andrews, Pesquero, Smith, Marín-Monfort, Sánchez, Geigl and Alonso2010; Fernández-Jalvo and Andrews, Reference Fernández-Jalvo and Andrews2016; Pesquero et al., Reference Pesquero, Bell and Fernández-Jalvo2018; Turner-Walker, Reference Turner-Walker2019). Today, it is well known that aerobic bacteria (Hackett, Reference Hackett1981; Kendall et al., Reference Kendall, Eriksen, Kontopoulos, Collins and Turner-Walker2018; Turner-Walker, Reference Turner-Walker2019), cyanobacteria (Davis, Reference Davis1997; Turner-Walker and Jans, Reference Turner-Walker and Jans2008; Turner-Walker, Reference Turner-Walker2012), and algae (Davis, Reference Davis1997; Fernández-Jalvo et al., Reference Fernández-Jalvo, Andrews, Pesquero, Smith, Marín-Monfort, Sánchez, Geigl and Alonso2010) are important bioerosion agents that leave permanent marks on the bone tissue (Jans, Reference Jans, Wisshak and Tapanila2008), while the role of fungi as bone tunnellers (Marchiafava et al., Reference Marchiafava, Bonucci and Ascenzi1974) is still under debate (Fernández-Jalvo and Andrews, Reference Fernández-Jalvo and Andrews2016; Turner-Walker, Reference Turner-Walker2019). The action of microorganisms is affected by different environmental factors, including water, soil pH, or temperature (Child, Reference Child1995; Nielsen-Marsh et al., Reference Nielsen-Marsh, Gernaey, Turner-Walker, Hedges, Pike, Collins, Cox and Mays2000; Hedges, Reference Hedges2002; Kendall et al., Reference Kendall, Eriksen, Kontopoulos, Collins and Turner-Walker2018; Turner-Walker, Reference Turner-Walker2019). Some of these abiotic factors, like water or temperature, can further produce microcracks in the bone tissue during fossilization (Pfretzschner, Reference Pfretzschner2000, Reference Pfretzschner2004; Jans, Reference Jans2005; Pfretzschner and Tütken, Reference Pfretzschner and Tütken2011). Thus, the histo-taphonomical analysis of fossil bones can yield important information about the physical factors affecting their depositional environments (Pfretzschner and Tütken, Reference Pfretzschner and Tütken2011; Dal Sasso et al., Reference Dal Sasso, Maritan, Usai, Angelini and Artioli2014). Moreover, microscopic bioerosion influences the long-term survival of bones in the fossil record, as well as the quantity and quality of the biological information recorded on them (Child, Reference Child1995; Trueman and Martill, Reference Trueman and Martill2002; Galligani et al., Reference Galligani, Sartori and Barrientos2019; Turner-Walker, Reference Turner-Walker2019).
MATERIAL AND METHODS
Material
We studied 14 Hipparion and Equus fossil metapodia recovered from various late Miocene, Mio-Pliocene, and early Pleistocene localities spread across the west of Turkey (see Fig. 1, Table 1). Specifically, metatarsi III (henceforth metatarsi) and metacarpi III (henceforth metacarpi) were the bones selected for the study, as these are the most common skeletal elements of equids found in palaeontological sites.
Systematics and phylogeny of Old World hipparionines is still unclear: Woodburne and Bernor (Reference Woodburne and Bernor1980) suggest that, considering the taxonomic variability of this group, all taxa should be grouped at a supra-specific level (e.g., Cremohipparion, Hippotherium), while Alberdi (Reference Alberdi, Prothero and Schoch1989) argues that the taxa should be classified into different morphotypes all belonging to the genus Hipparion s.l. Due to the fragmentary condition of the bones analysed here, we use Hipparion as a generic name for the different hipparionine samples under study. We will only refer to other hipparionine genera (i.e., Cremohipparion, Hippotherium, etc.) to maintain the original taxonomy used in previous investigations (1) when describing the different fossil sites and (2) when making palaeobiological inferences if only one hipparionine species was reported at a specific fossil site.
Çanakkale-Gülpınar
We analysed three metatarsi from the Çanakkale-Gülpınar fossil site (see Table 1). This Turolian locality (MN11-12, 11.2–7.4/6.8 Ma [Hilgen et al., Reference Hilgen, Lourens, Van Dam, Beu, Boyes, Cooper, Krijgsman, Ogg, Piller, Wilson, Gradstein, Ogg, Schmitz and Ogg2012]) (Koufos et al., Reference Koufos, Mayda and Kaya2018) is a seashore cliff 3 km southwest of the city of Gülpınar (Çanakkale province) (Forsten and Kaya, Reference Forsten and Kaya1995) (see Fig. 1). Fossil bones were collected from a fluvial sedimentary sequence that includes brownish sandy and limy mudstones, limestones, sandstones, and conglomerates (Forsten and Kaya, Reference Forsten and Kaya1995). A variety of large mammals were found in this locality, including both carnivores (Koufos et al., Reference Koufos, Mayda and Kaya2018) and herbivores (Forsten and Kaya, Reference Forsten and Kaya1995). Regarding equids, Forsten and Kaya (Reference Forsten and Kaya1995) described three different Hipparion morphotypes: H. cf. matthewi (small-sized), H. sp. medium-sized, H. sp. large-sized.
Izmir-Karaburun
One metatarsus from the early Turolian (MN11-12 11.2–7.4/6.8 Ma [Hilgen et al., Reference Hilgen, Lourens, Van Dam, Beu, Boyes, Cooper, Krijgsman, Ogg, Piller, Wilson, Gradstein, Ogg, Schmitz and Ogg2012]) fossil locality of Izmir-Karaburun (Kaya et al., Reference Kaya, Geraads and Tuna2005a) is included in this study (see Table 1). It belongs to the so-called Esendere fauna from the Esendere locality, in the Karaburun Peninsula, west of Izmir (Kaya et al., Reference Kaya, Geraads and Tuna2005a) (see Fig. 1). Specifically, fossil bones were recovered from the upper part of the Karaburun Formation, a muddy fluvial assemblage grading upwards into lacustrine facies (Kaya, Reference Kaya1981; Kaya et al., Reference Kaya, Geraads and Tuna2005a). The geology of the area mainly consists of massive mudstone and lithic sandstone, with oolitic limestone, claystone, and conglomerate occurring subordinately (Kaya et al., Reference Kaya, Geraads and Tuna2005a). The Esendere fauna is significant because of the description of two new carnivore species (Kaya et al., Reference Kaya, Geraads and Tuna2005a). Other mammalian taxa such as Cremohipparion cf. mediterraneum were also found in this locality (Kaya et al., Reference Kaya, Geraads and Tuna2005a).
Kütahya-Bayat
One metatarsus from Kütahya-Bayat locality was analysed (see Table 1). Fossil mammalian remains at this fossil-bearing site were recovered from the alluvial channel deposits filled with reddish conglomerates, brownish claystone, and mudstone of the Çokköy Formation in Bayat village, 40 km southwest of Kütahya (Kütahya province) (see Fig. 1) (Kaya et al., Reference Kaya, Mayda and Saraç2005b). The Bayat faunal assemblage is characterized by the presence of different herbivores, among which the hipparionine species Cremohipparion cf. matthewi and Cremohipparion cf. mediterraneum are outstanding (Kaya et al., Reference Kaya, Mayda and Saraç2005b). The characteristics of these and other mammalian species indicate a late Miocene age for the site (Turolian, MN11-12, 11.2–7.4/6.8 Ma [Hilgen et al., Reference Hilgen, Lourens, Van Dam, Beu, Boyes, Cooper, Krijgsman, Ogg, Piller, Wilson, Gradstein, Ogg, Schmitz and Ogg2012]) (Kaya et al., Reference Kaya, Mayda and Saraç2005b).
Uşak-Kemiklitepe 2
We analysed one metacarpus from the Uşak-Kemiklitepe 2 Turolian locality (MN11-12, 11.2–7.4/6.8 Ma [Hilgen et al., Reference Hilgen, Lourens, Van Dam, Beu, Boyes, Cooper, Krijgsman, Ogg, Piller, Wilson, Gradstein, Ogg, Schmitz and Ogg2012]) (see Table 1). This fossiliferous site is located 1.5 km south of Karacaahmet village (Uşak province) (Sen et al., Reference Sen, de Bonis, Dalfes, Geraads and Koufos1994) (see Fig. 1) in the Asartepe Formation (Seyitoğlu et al., Reference Seyitoğlu, Alçiçek, Işık, Alçiçek, Mayda, Varol, Yılmaz and Esat2009). Specifically, fossils were collected from a clastic unit composed of massive mudstones that alternate with matrix-supported fine-grained conglomerates representing subaerial deposition in distal alluvial-fan environments (Seyitoğlu et al., Reference Seyitoğlu, Alçiçek, Işık, Alçiçek, Mayda, Varol, Yılmaz and Esat2009). Equidae specimens from this locality were studied by Koufos and Kostopoulos (Reference Koufos and Kostopoulos1994), who described Hipparion mediterraneum, Hipparion matthewi, and a large-size Hipparion sp.
Çankırı-Yeniköy
One metatarsus from the fossiliferous locality of Çankırı-Yeniköy was analysed (see Table 1). This fossil-bearing site is located in the red beds around Yeniköy (Çankırı province) (Sen et al., Reference Sen, Seyitoğlu, Karadenizli, Kazanci, Varol and Araz1998) (see Fig. 1). The geology of the area is characterized by the presence of alternating sandstones, siltstones, mudstones, and gypsum that represent an alluvial flood plain depositional environment with braided stream channels (Tekkaya et al., Reference Tekkaya, Atalay, Gürbüz, Ünay and Ermumcu1975). Tekkaya and colleagues (Reference Tekkaya, Atalay, Gürbüz, Ünay and Ermumcu1975) provided a faunal list that includes the presence of Hipparion gracile, which suggests a Turolian age (MN11-12, 11.2–7.4/6.8 Ma [Hilgen et al., Reference Hilgen, Lourens, Van Dam, Beu, Boyes, Cooper, Krijgsman, Ogg, Piller, Wilson, Gradstein, Ogg, Schmitz and Ogg2012]) for this locality.
Kırşehir-Kaman
We studied one metatarsus from the Turolian (MN12, 7.6–7.4/6.8 Ma [Hilgen et al., Reference Hilgen, Lourens, Van Dam, Beu, Boyes, Cooper, Krijgsman, Ogg, Piller, Wilson, Gradstein, Ogg, Schmitz and Ogg2012]) locality of Kırşehir-Kaman (see Table 1). This fossil site, also known as Akkaşdağı, is located in the southern part of the Çankırı-Çorum Basin, 125 km southeast of Ankara (see Fig. 1) (Koufos and Vlachou, Reference Koufos and Vlachou2005). Mudstones, limestones, and sandstones with subordinate conglomerates and tuff compose the sedimentary succession and suggest an alluvial fan as the main depositional environment of the site (Kazanci et al., Reference Kazanci, Karadenizli, Seyitoǧlu, Sen, Alçiçek, Varol, Saraç and Hakyemez2005). The main large mammals (e.g., carnivores, perissodactyls, artiodactyls, suids, and proboscideans) from this locality have been studied in detail by Antoine and Saraç (Reference Antoine and Saraç2005), de Bonis (Reference de Bonis2005), Kostopoulos and Saraç (Reference Kostopoulos and Saraç2005), Koufos and Vlachou (Reference Koufos and Vlachou2005), Liu and colleagues (Reference Liu, Kostopoulos and Fortelius2005), Saraç and Sen (Reference Saraç and Sen2005), and Tassy (Reference Tassy2005). More specifically in terms of equids, Koufos and Vlachou (Reference Koufos and Vlachou2005) described the presence of four different Hipparion species: Hipparion dietrichi, Hipparion moldavicum, Hipparion brachypus, and Hipparion cf. longipes.
Muğla-Yatağan-Salihpaşalar 1
We studied one metatarsus from the Muğla-Yatağan-Salihpaşalar locality (see Table 1). This fossil site, which is found 5 km east of Salihpaşalar village (Muğla province) (see Fig. 1), was first described by Atalay (Reference Atalay1980). The fossil-bearing red-bed unit belongs to the Yatağan Formation (Atalay, Reference Atalay1980; Geraads et al., Reference Geraads, Gulec and Kaya2002) and includes alternating conglomerates, sandstones, siltstones, and mudstones representing an alluvial flood plain depositional environment with braided stream channels (Kaya et al., Reference Kaya, Mayda, Kostopoulos, Alcicek, Merceron, Tan, Karakutuk, Giesler and Scott2012). The Turolian (MN12, 7.6–7.4/6.8 Ma [Hilgen et al., Reference Hilgen, Lourens, Van Dam, Beu, Boyes, Cooper, Krijgsman, Ogg, Piller, Wilson, Gradstein, Ogg, Schmitz and Ogg2012]) ungulate mammalian faunal assemblage found at this fossiliferous locality is characterised by a high diversity of bovids and giraffes (Kaya et al., Reference Kaya, Mayda, Kostopoulos, Alcicek, Merceron, Tan, Karakutuk, Giesler and Scott2012), although it also includes several equid species such as Hipparion mediterraneum and Hipparion matthewi (Geraads et al., Reference Geraads, Gulec and Kaya2002; Saraç et al., Reference Saraç, Kaya and Geraads2002).
Muğla-Yatağan-Şerefköy
Two metatarsi from the Muğla-Yatağan-Şerefköy fossil site were analysed (see Table 1). This Turolian locality (MN12, 7.6–7.4/6.8 Ma [Hilgen et al., Reference Hilgen, Lourens, Van Dam, Beu, Boyes, Cooper, Krijgsman, Ogg, Piller, Wilson, Gradstein, Ogg, Schmitz and Ogg2012]) is situated near the Şerefköy village, 9 km east of Yatağan town (Muğla province) (Kaya et al., Reference Kaya, Mayda, Kostopoulos, Alcicek, Merceron, Tan, Karakutuk, Giesler and Scott2012) (see Fig. 1). Muğla-Yatağan-Şerefköy also belongs to the Yatağan Formation (Kaya et al., Reference Kaya, Mayda, Kostopoulos, Alcicek, Merceron, Tan, Karakutuk, Giesler and Scott2012). Specifically, the fossil-bearing unit at this locality is composed of sandstones, siltstones, and mudstones and alternating fine- to coarse-grained conglomerates with subangular to subrounded gravel of pebble to cobble grade (Kaya et al., Reference Kaya, Mayda, Kostopoulos, Alcicek, Merceron, Tan, Karakutuk, Giesler and Scott2012). The depositional environment described is an alluvial flood plain with braided stream channels (Kaya et al., Reference Kaya, Mayda, Kostopoulos, Alcicek, Merceron, Tan, Karakutuk, Giesler and Scott2012). Hipparionines represent more than 50% of the entire faunal assemblage and can be grouped into five species belonging to three different morphotypes: Cremohipparion sp. type 1 (small-sized), Cremohipparion sp. type 2 (small-sized), “Hipparion” sp. type 1 (medium-sized), “Hipparion” sp. type 2 (medium-sized), and Hippotherium brachypus (large-sized) (Kaya et al., Reference Kaya, Mayda, Kostopoulos, Alcicek, Merceron, Tan, Karakutuk, Giesler and Scott2012).
Manisa-Düzpınar
We studied one metacarpus from the Manisa-Düzpınar fossil site (see Table 1), which is found in the outskirts of Develi village (Manisa province) (see Fig. 1). Fossil material was specifically collected from the light-coloured limestones, mudrocks, and sands that compose the water lacustrine deposits of the Urla Formation (Kaya et al., Reference Kaya, Müller, Rückert-Ülkümen and Kaya1998). Micro- and macromammal fossil remains from this site have recently been reviewed by Mayda and colleagues (Reference Mayda, Sotnikova, Tesakov, Tan and Kaya2015), who assigned the Develi fauna to the Miocene–Pliocene transition (MN13-14, 7.4/6.8–5.0 Ma [Hilgen et al., Reference Hilgen, Lourens, Van Dam, Beu, Boyes, Cooper, Krijgsman, Ogg, Piller, Wilson, Gradstein, Ogg, Schmitz and Ogg2012]). Hipparionine material recovered at Manisa-Düzpınar was tentatively assigned to Cremohipparion cf. matthewi (Mayda et al., Reference Mayda, Sotnikova, Tesakov, Tan and Kaya2015).
Manisa-Aşağıçobanisa
We analysed one metatarsus from the Manisa-Aşağıçobanisa fossil site (see Table 1). This Plio-Pleistocene locality (MN15: 5.0–3.6/3.5 Ma, MN17: 2.5–2.0/1.8 Ma [Hilgen et al., Reference Hilgen, Lourens, Van Dam, Beu, Boyes, Cooper, Krijgsman, Ogg, Piller, Wilson, Gradstein, Ogg, Schmitz and Ogg2012]) is situated in the vicinity of Aşağı Çobanisa village (Manisa province) (see Fig. 1), about 50 km northeast from Izmir (Mayda et al., Reference Mayda, Titov, Tesakov, Göktaş and Alçiçek2013). Fossil remains were recovered from the fluvial deposits of the Turgutlu Formation, which mainly consists of cross-bedded sandstones and less commonly of interclasting mudrocks (Mayda et al., Reference Mayda, Titov, Tesakov, Göktaş and Alçiçek2013). Equid material studied from this locality was assigned to Equus aff. major. It belongs to the mid-early Pleistocene (MN17, 2.5–2.0/1.8 Ma [Hilgen et al., Reference Hilgen, Lourens, Van Dam, Beu, Boyes, Cooper, Krijgsman, Ogg, Piller, Wilson, Gradstein, Ogg, Schmitz and Ogg2012]) faunal association found at this locality, which is dated to 2.1–1.9 Ma (Mayda et al., Reference Mayda, Titov, Tesakov, Göktaş and Alçiçek2013).
Denizli-Pamukkale
One metacarpus from the middle Pleistocene (MN17-18, 2.5–0.6 Ma [Hilgen et al., Reference Hilgen, Lourens, Van Dam, Beu, Boyes, Cooper, Krijgsman, Ogg, Piller, Wilson, Gradstein, Ogg, Schmitz and Ogg2012]) locality of Denizli-Pamukkale (see Fig. 1) was studied (see Table 1). Fossil remains at this site were recovered from the tourist travertine formations known as Pamukkale travertines (Rausch et al., Reference Rausch, Alçiçek, Vialet, Boulbes, Mayda, Titov and Stoica2019). These are freshwater limestones that form when hot ground water rich in calcium and bicarbonate emerges at springs (Guo and Riding, Reference Guo and Riding1998). Specifically, fossils were collected from the 50-m-thick “Upper Travertine” unit (Lebatard et al., Reference Lebatard, Alçiçek, Rochette, Khatib, Vialet, Boulbes and Bourlès2014), which represents travertine depositional environments (Rausch et al., Reference Rausch, Alçiçek, Vialet, Boulbes, Mayda, Titov and Stoica2019). This fossiliferous locality registers the presence of E. cf. altidens s.l. and E. cf. apolloniensis (Boulbes et al., Reference Boulbes, Mayda, Titov and Alçiçek2014; Rausch et al., Reference Rausch, Alçiçek, Vialet, Boulbes, Mayda, Titov and Stoica2019).
Preparation and analysis of histological slides
Bone thin sections of ~100 μm thickness were prepared from each sample following standard procedures that include different steps of embedding, cutting, grinding, and polishing (Chinsamy and Raath, Reference Chinsamy and Raath1992). The obtained histological slides were observed and analysed under transmitted and polarized light to describe the bone histology and preservation. Specifically, we used a Nikon Eclipse E200 and a Zeiss Ax10 Lab.A1 polarising microscopes. High-quality micrographs were taken with the cameras (Canon EAOD 500D and Nikon DS-Fi1, respectively) attached to the different microscopes. Diagenetic alterations of the bone tissue were also studied in a desktop scanning electron microscope Phenom™ ProX.
Bone tissue types and their vascularization were qualitatively studied following classical approaches presented in the literature (e.g., Francillon-Vieillot et al., Reference Francillon-Vieillot, de Buffrénil, Castanet, Géraudie, Meunier, Sire, Zylberberg, de Ricqlès and Carter1990; Chinsamy-Turan, Reference Chinsamy-Turan2005). Generally, bone tissue is classified as primary or secondary based on its origin. Primary bone is usually grouped into different typologies according to the degree of organization of the collagen fibres, the shape and arrangement of bone cells (i.e., osteocytes), and the quantity and organisation of the vascular canals (Francillon-Vieillot et al., Reference Francillon-Vieillot, de Buffrénil, Castanet, Géraudie, Meunier, Sire, Zylberberg, de Ricqlès and Carter1990; Chinsamy-Turan, Reference Chinsamy-Turan2005; Huttenlocker et al., Reference Huttenlocker, Woodward, Hall, Padian and Lamm2013). In large mammals, such as equids, during early ontogenetic stages primary bone is usually fibrolamellar bone tissue (Kolb et al., Reference Kolb, Scheyer, Veitschegger, Forasiepi, Amson, Van der Geer, Van den Hoek Ostende, Hayashi and Sánchez-Villagra2015b). This bone tissue is formed at high rates (Amprino, Reference Amprino1947; de Margerie et al., Reference de Margerie, Cubo and Castanet2002), which results in a highly vascularized bone matrix with multiple primary osteons, many osteocytes, and randomly organized collagen fibres (Francillon-Vieillot et al., Reference Francillon-Vieillot, de Buffrénil, Castanet, Géraudie, Meunier, Sire, Zylberberg, de Ricqlès and Carter1990; Chinsamy-Turan, Reference Chinsamy-Turan2005). When growth rate decreases as the animal approaches adult body size, lamellar bone tissue is deposited. In this case, it is usually called external fundamental system (EFS) or outer circumferential layer (OCL). Lamellar bone tissue is formed at slower rates than fibrolamellar bone (Amprino, Reference Amprino1947; de Margerie et al., Reference de Margerie, Cubo and Castanet2002) and consists of highly organized collagen fibres, few vascular canals and/or primary osteons, and few osteocytes (Francillon-Vieillot et al., Reference Francillon-Vieillot, de Buffrénil, Castanet, Géraudie, Meunier, Sire, Zylberberg, de Ricqlès and Carter1990; Chinsamy-Turan, Reference Chinsamy-Turan2005). Secondary bone, as the name suggests, is deposited after primary bone has been resorbed (Francillon-Vieillot et al., Reference Francillon-Vieillot, de Buffrénil, Castanet, Géraudie, Meunier, Sire, Zylberberg, de Ricqlès and Carter1990; Currey, Reference Currey2002). It results from the joint action of the osteoclasts (i.e., bone-resorbing cells) and the osteoblasts (i.e., bone-forming cells) during a process known as bone remodelling (Currey, Reference Currey2002) or secondary reconstruction (Chinsamy-Turan, Reference Chinsamy-Turan2005). Secondary tissue may take the form of secondary osteons (also named Haversian canals) or can be endosteally formed, such as compacted coarse cancellous bone (Chinsamy-Turan, Reference Chinsamy-Turan2005).
Bone growth marks (BGMs, i.e., lines of arrested growth [LAGs] and annuli) (Castanet et al., Reference Castanet, Francillon-Vieillot, Meunier, de Ricqlès and Hall1993), both cyclical (CGMs) and non-cyclical ones (i.e., neonatal line [NL]) (Nacarino-Meneses and Köhler, Reference Nacarino-Meneses and Köhler2018), were also analysed in the different cross sections. When possible, their perimeter was estimated using Image J software, and the results were plotted to reconstruct the pattern of metapodial growth (Nacarino-Meneses et al., Reference Nacarino-Meneses, Jordana and Köhler2016a). Size variation per year was calculated as a proxy of growth rate by subtracting BGMs’ perimeters of consecutive annual growth cycles (Nacarino-Meneses et al., Reference Nacarino-Meneses, Jordana and Köhler2016a). The non-cyclical BGM described by Nacarino-Meneses and Köhler (Reference Nacarino-Meneses and Köhler2018) as the NL was considered as time zero for growth reconstructions (Woodward et al., Reference Woodward, Padian, Lee, Padian and Lamm2013).
Several diagenetic features were recorded for each specimen, including the general histological index (GHI) (Hollund et al., Reference Hollund, Jans, Collins, Kars, Joosten and Kars2012), the type of bone microscopic focal destruction (MFD) (Hackett, Reference Hackett1981), the presence and appearance of bone microcracks (Jans, Reference Jans2005; Pfretzschner and Tütken, Reference Pfretzschner and Tütken2011), the degree of bone birefringence (Jans, Reference Jans2005), and the presence of inclusions and infiltrations (Garland, Reference Garland1989). The GHI (Hollund et al., Reference Hollund, Jans, Collins, Kars, Joosten and Kars2012) was applied to qualitatively analyse the amount of unaltered microstructure within a bone cross section. Based on previous preservation indexes that only considered microorganisms as the main factors altering bone tissue microstructure (Hedges et al., Reference Hedges, Millard and Pike1995; Millard, Reference Millard, Brothwell and Pollard2001; Haynes et al., Reference Haynes, Searle, Bretman and Dobney2002), the GHI assesses the amount of bone microstructure affected by bioerosion, microcracks, and infiltrations (Hollund et al., Reference Hollund, Jans, Collins, Kars, Joosten and Kars2012). According to this preservation index (Hollund et al., Reference Hollund, Jans, Collins, Kars, Joosten and Kars2012), we classified the microscopic damage into each of the already described six categories that range from GHI 0 (bad preservation) to GHI 5 (excellent preservation). We also examined the presence and type of MFD within each bone slide. These features, also known as tunnels or foci, are changes of the bone tissue caused by the action of microorganisms (Hackett, Reference Hackett1981). MFD is classified into four types on the basis of their size, shape, and the presence of a hypermineralized ring (Hackett, Reference Hackett1981; Jans, Reference Jans, Wisshak and Tapanila2008); the four types are Wedl, linear longitudinal, budded, and lamellate. To simplify our results, linear longitudinal, budded, and lamellate foci where all classified as non-Wedl tunnels following Jans (Reference Jans, Wisshak and Tapanila2008). Furthermore, we preferentially used the term “Wedl-like” instead of “Wedl” because the agent causing this kind of borings in our sample is probably different from that causing the tunnels originally described by Wedl (i.e., cyanobacteria, Turner-Walker, Reference Turner-Walker2019). Bone microcracks or microfissures, on the other hand, tend to appear in bone cross sections as a result of variations in the water content (i.e., wetting and drying cycles that provoke shrinkage and expansion) of the bone matrix collagen during early stages of fossilization (Pfretzschner, Reference Pfretzschner2000, Reference Pfretzschner2004; Pfretzschner and Tütken, Reference Pfretzschner and Tütken2011). Here, we qualitatively describe the presence and extent of bone microcracks for each fossil sample. Bone birefringence is related to the degree of arrangement of the apatite crystals, with ordered apatite crystals showing a high degree of birefringence (Schoeninger et al., Reference Schoeninger, Moore, Murray and Kingston1989). In the present work, bone birefringence was assessed as 1 (perfect), 0.5 (reduced), or 0 (absent) following Jans (Reference Jans2005). Finally, we analysed inclusions and infiltrations within each bone cross section. According to Garland (Reference Garland1989), inclusions appear within bone spaces (e.g., medullary cavity, Haversian canals) while infiltrations appear within the bone substance itself. Inclusions can consist of diverse materials such as sand, minerals, or microorganisms (Jans et al., Reference Jans, Kars, Nielsen-Marsh, Smith, Nord, Arthur and Earl2002). Infiltrations, on the other hand, appear as stains in the bone section (Jans et al., Reference Jans, Kars, Nielsen-Marsh, Smith, Nord, Arthur and Earl2002).
RESULTS
Bone histology and skeletochronology
Primary bone tissue of Turkish hipparionines is characterized by the presence of longitudinal primary osteons (POs) oriented in circumferential rows (see Fig. 2A) throughout the whole bone cortex. In KK-1 we additionally identified compacted coarse cancellous bone (CCCB) composing most of the inner bone cortex (see Fig. 2B). Abundant secondary osteons (SOs) are scattered in the cortex of Turkish Hipparion (see Fig. 2B), which eventually form a well-developed Haversian bone (see Fig. 2C) in some specimens like UEK-1. Secondarily deposited endosteal bone (EB) lines the medullary cavity of several hipparionine samples (e.g., UEK-1, MD-7, CG-1) (see Fig. 2D).

Figure 2. (color online) Bone tissue types in metapodial cortices of extinct equids from Turkey. (A) Hipparion, specimen MD-7. POs oriented in circumferential rows. (B) Hipparion, specimen KK-1. Compacted coarse cancellous bone (CCCB) with scattered secondary osteons (SOs). (C) Hipparion, specimen UEK-1. Well-developed Haversian bone with multiple SOs. (D) Hipparion, specimen UEK-1. Endosteal bone (EB) and SOs; m = medullary cavity. (E) Equus, specimen P-1. Vascular canals present a circumferential orientation (laminar bone, LB) in the most internal half of the cortex but are oriented longitudinally (LVC) in the external half of the cortex. (F) Equus, specimen P-1. Scattered SO found in P-1. Images show bone cross sections observed under polarized light with 1/4λ plate. White scale bars: 250 μm; black scale bars: 500 μm.
Bone tissue types of our Equus sample was only studied in the Denizli-Pamukkale metacarpus (P-1, see Fig. 2E, 2F) since the histology of the other Equus sample was not well preserved (see below). The internal cortex of this specimen consists of a fibrolamellar bone tissue with circumferentially oriented vascular canals (PO), which conforms to the primary bone tissue type known as laminar bone (LB) (see Fig. 2E). Vascular canals within the external half of the cortex predominantly have a longitudinal orientation (see Fig. 2E), resulting in longitudinal POs oriented in circumferential rows (see Fig. 2E). Scattered SOs are also identified in this Equus specimen (see Fig. 2F), mainly in the posterior part of the cortex. EB is found in the innermost cortex.
BGMs, both in the form of LAGs and annuli, were also identified in Hipparion and Equus samples (see Fig. 3). These features were recognized in many of the specimens under analysis (CG-1, CG-2, CG-3, P-1, MD-7, MYSA-1, UEK-1), regardless of the preservation state (see Fig. 3A) or the degree of intracortical remodelling (i.e., presence of multiple SOs) (see Fig. 3B). However, the complete path of the BGMs within the whole cortex could only be followed with confidence in the metacarpus MD7 (see Fig. 3C), which presents one NL and six CGMs (see Fig. 3C). All BGMs in this specimen appear within the fibrolamellar bone tissue, and no EFS/OCL was recognized. The perimeter of each CGM, as well as that of the NL, was measured and plotted to reconstruct the pattern of MD7's growth (see Fig. 3D). As Figure 3D shows, this bone grows faster during the first year of life and then progressively reduces its growth rate. Minimal rates of bone deposition are only attained after the deposition of the fourth CGM (see Fig. 3E). This change in growth rate observed in both graphs (see Fig. 3D, 3E) coincides with a decrease in the spacing between CGMs (see Fig. 3C).

Figure 3. (color online) Bone growth marks (BGMs) in metapodial cortices of extinct equids from Turkey. (A) Hipparion, specimen CG-1. BGMs can still be recognized despite poor histology preservation. (B) Hipparion, specimen UEK-1. BGMs are still identifiable despite a high concentration of secondary osteons (SO). (C) Hipparion, MD-7. Non-cyclical (neonatal line [NL], black arrowhead) and cyclical growth marks (CGMs, white arrowheads) are identified. (D) Growth curve obtained for MD-7 by plotting the perimeter of each BGM (mm, ordinate axis) against the estimated age (years, abscissa axis). (E) Difference in perimeter of consecutive BGM (mm, ordinate axis) is plotted against estimated age (years, abscissa axis) in MD-7, providing a proxy of the growth rate of the bone. Images show bone cross sections observed under polarized light with 1/4λ plate. Scale bars: 500 μm.
Preservation at the histological level
Bone preservation at the histological level varies greatly among the samples studied (see Fig. 4A, 5). Only two of the specimens under analysis (Supplementary Table 1), which represent 14.3% of the sample (see Fig. 4A), present diagenetically unaltered histology (GHI = 5, see Fig. 5A). Bone histological features can also be easily identified in P-1 (see Fig. 5B), which is categorized as GHI = 4 (see Fig. 4A, Supplementary Table 1) due to the high number of bone microcracks that run through its cortex (see Fig. 5B). Bone microstructure is completely altered in four metapodia (28.6% of the sample, see Fig. 4A, Supplementary Table 1), which are scored as GHI = 0 (see Fig. 5E). The remaining specimens under analysis also present altered histological preservation: five (35.7% of the sample, see Fig. 4A, Supplementary Table 1) are classified as GHI = 1 (see Fig. 5D) and two (14.3% of the sample, see Fig. 4A, Supplementary Table 1) as GHI = 2 (see Fig. 5C) according to the amount of bone microstructure preserved in the different cross sections (Hollund et al., Reference Hollund, Jans, Collins, Kars, Joosten and Kars2012). Specimens found within fluvial and alluvial sediments usually present worse histological preservation (lower GHI) than those recovered from lacustrine and travertine environments (Table 2), though some exceptions can be found. UEK-1, for example, was recovered from an alluvial fan (see Table 2) but presents very well-preserved histology (see Fig. 5A, Supplementary Table 1). Thus, there is not a clear trend in the relationship between the degree of bone preservation and its depositional environment.

Figure 4. (color online) Pie charts showing the prevalence of different diagenetic features in the metapodia of extinct equids from Turkey. Percentage values for each group are shown.

Figure 5. (color online) Preservation states (general histological index [GHI]) identified in the metapodia of extinct equids from Turkey. (A) Hipparion, specimen UEK-1. Well-preserved histology, directly comparable to histology of modern bone (GHI = 5). (B) Equus, specimen P-1. Bone tissue is fairly well preserved, although lots of microcracks run through the cortex (GHI = 4). (C) Hipparion, specimen KK-1. Some well-preserved bone microstructure is recognised between altered areas (GHI = 2). (D) Hipparion, specimen MYSA-1. Only small areas of well-preserved bone tissue are present (GHI = 1). (E) Hipparion, specimen MYSE-2. Histology is completely altered with no original histological features identifiable (GHI = 0). Images show bone cross sections observed under transmitted light. Scale bars: 500 μm.
Table 2. Summary of the main preservation results and the depositional environment for the different fossil sites studied. Data on depositional environment from (a) Forsten and Kaya (Reference Forsten and Kaya1995), (b) Kaya (Reference Kaya1981), (c) Kaya and colleagues (Reference Kaya, Mayda and Saraç2005b), (d) Seyitoğlu and colleagues (Reference Seyitoğlu, Alçiçek, Işık, Alçiçek, Mayda, Varol, Yılmaz and Esat2009), (e) Tekkaya and colleagues (Reference Tekkaya, Atalay, Gürbüz, Ünay and Ermumcu1975), (f) Kazanci and colleagues (Reference Kazanci, Karadenizli, Seyitoǧlu, Sen, Alçiçek, Varol, Saraç and Hakyemez2005), (g) Kaya and colleagues (Reference Kaya, Mayda, Kostopoulos, Alcicek, Merceron, Tan, Karakutuk, Giesler and Scott2012), (h) Kaya and colleagues (Reference Kaya, Müller, Rückert-Ülkümen and Kaya1998), (i) Mayda and colleagues (Reference Mayda, Titov, Tesakov, Göktaş and Alçiçek2013), (j) Rausch and colleagues (Reference Rausch, Alçiçek, Vialet, Boulbes, Mayda, Titov and Stoica2019).

MFD (see Fig. 6) is extensive (see Supplementary Table 1), affecting 78.6% of the sample (see Fig. 4B). In very badly preserved specimens (GHI = 0), MFD is widely distributed across the whole cortex (see Fig. 5E), while it is restricted to the internal and/or the external cortex in those samples categorized as GHI = 1 and GHI = 2 (see Fig. 5C, 5D). Non-Wedl tunnels (see Fig. 6A–I) are recognized in 71.4% of the sample (see Fig. 4B, Supplementary Table 1), both in the form of budded (~50 μm diameter, see Fig. 6A–C) and linear longitudinal (~20 μm diameter, see Fig. 6D–F) tunnels. In transmitted light microscopy, both foci present a rim that isolates them from the surrounding alterations (see Fig. 6A, 6D). This rim is not always recognizable, however, when samples are observed under scanning electron microscopy (SEM) (see Fig. 6B, 6C, 6E, 6F). Nonetheless, SEM always allows the identification of the submicron porosity (see Fig. 6C, 6F) characteristic of non-Wedl MFD (Turner-Walker et al., Reference Turner-Walker, Nielsen-Marsh, Syversen, Kars and Collins2002). The generalized damage of the bone tissue in several specimens, such as MYSE-2, greatly hampers the identification of the different types of MFD (Wedl-like vs. non-Wedl tunnels) when analysed under transmitted light (see Fig. 6G). Such specimens examined under SEM show an almost complete destruction of the bone microstructure (see Fig. 6H, 6I) that likely result from the dissolution and re-precipitation of the bone matrix (Turner-Walker, Reference Turner-Walker2012). With SEM, we also identified non-Wedl submicron porosity (see Fig. 6I). Wedl-like tunnels (see Fig. 6J–L), on the other hand, are only found in one sample: KK-1 (see Fig 4B, Supplementary Table 1). In this specimen, branching Wedl-like tunnels seem to start from the lumen of the vascular canal and progress outwards within the cortical bone (see Fig. 6J–L). Interestingly, Wedl-like MFD appears both in the most external cortex and in the middle area of the compacta. When observed under SEM, bone matrix of KK-1 also shows signs of dissolution and precipitation, as well as multiple breakages in disaggregated fragments (see Fig. 6K–L).

Figure 6. (color online) Microscopic focal destruction (MFD) in the metapodia of extinct equids from Turkey. (A) Hipparion, specimen CG-2. Transmitted light micrograph showing multiple non-Wedl tunnels of ~50 μm diameter. (B) Hipparion, specimen CG-2. SEM micrograph of the same area showing bacterial damage. (C) Hipparion, specimen CG-2. Detailed SEM micrograph showing submicron porosity surrounded by a hypermineralized ring around each bacterial colony. (D) Hipparion, specimen KB-1. Transmitted light micrograph showing non-Wedl tunnels of ~20 μm diameter. (E) Hipparion, specimen KB-1. SEM micrograph of the same area showing bacterial damage. (F) Hipparion, specimen KB-1. Detailed SEM micrograph showing the submicron porosity characteristic of bacterial damage. Note that no hypermineralized ring surrounds submicron pores (arrows) in this case. (G) Hipparion, specimen MYSE-2. Transmitted light micrograph showing extensive damage through the cortex. It is difficult to recognize either Wedl-like or non-Wedl tunnels because the overall bone microstructure is destroyed. (H) Hipparion, specimen MYSE-2. SEM micrograph of the same area showing general destruction of the bone microstructure. Bone matrix (right of dashed line) is almost indistinguishable from mineral inclusions (left of dashed lines). (I) Hipparion, specimen MYSE-2. Detailed SEM micrograph showing submicron porosity (arrows) characteristic of bacterial damage. (J) Hipparion, specimen KK-1. Transmitted light micrograph showing Wedl-like tunnels (arrows). (K) Hipparion, specimen KK-1. SEM micrograph of the same area showing microscopic damage. (L) Hipparion, specimen KK-1. Detailed SEM micrograph showing Wedl-like tunnels (arrows) that may have been caused by algae. White dotted rectangles in B, E, H, and K indicate areas of image magnification showed in C, F, I, and L, respectively. White scale bars: 100 μm, black scale bars: 50 μm.
Bone microcracks are only found in two metapodials (14.3% of the sample, see Fig. 4C): P-1 and UEK-1 (see Fig. 7, Supplementary Table 1). In P-1, microfissures extend through the whole cortical bone, from the periosteal to the endosteal surface (see Fig. 5B). Interestingly, they interrupt the primary bone tissue without following any preferential direction. As a result, both radial and circumferential cracks can be detected within the fibrolamellar bone (see Fig. 5B). On the contrary, microcracks within SOs follow the path of the cement line and never trespass it (see Fig. 7A). In UEK-1, bone microcracks are limited to a few SOs (see Fig. 7B). In this case, microfissures appear radial to the Haversian canal crossing the cement line (see Fig. 7B).

Figure 7. (color online) Bone microcracks in the secondary bone tissue of extinct equids from Turkey. (A) Circumferential cracks (arrows) following the cement line of SOs in P-1. (B) Radial fissures (arrows) in the cement lines of SOs in UEK-1. Images show bone cross sections observed under transmitted light. Scale bars: 100 μm.
The medullary cavity of almost all specimens under study, as well as most of the pore spaces and resorption cavities of their cortices, are infilled with mineral sediments (see Supplementary Table 1). These are either a muddy clastic material (see Fig. 2D) or crystals of quartz, depending on the geology of the area. A red-brown staining, which likely results from the infiltration of humic factors (Jans et al., Reference Jans, Kars, Nielsen-Marsh, Smith, Nord, Arthur and Earl2002), is also locally found in most of the bone cortices under analysis (see Fig. 5B, 5D, Supplementary Table 1).
Birefringence is preserved in 85.8% of the sample (see Fig. 4D), but substantially reduced in 6 out of the 12 specimens that still present this histological feature (see Fig. 4D, Supplementary Table 1), indicating a disruption of the collagen-hydroxyapatite bond in these fossil samples (Turner-Walker, Reference Turner-Walker, Pinhasi and Mays2008).
DISCUSSION
Bone histology and life history of Turkish fossil equids
The histology of fossil bone and, in particular, features such as the type of bone tissue, the pattern of vascularization, and the presence of growth marks are useful when undertaking life history studies of extinct taxa (e.g., Chinsamy-Turan, Reference Chinsamy-Turan2005, Reference Chinsamy-Turan2012). The cortices of the metapodia of the Turkish fossil equids consist of a fibrolamellar complex with longitudinal POs oriented in circumferential rows (see Fig. 2A). These histological characteristics have been previously reported in several Hipparion (Martínez-Maza et al., Reference Martínez-Maza, Alberdi, Nieto-Diaz and Prado2014; Orlandi-Oliveras et al., Reference Orlandi-Oliveras, Nacarino-Meneses, Koufos and Köhler2018) and Equus (Nacarino-Meneses et al., Reference Nacarino-Meneses, Jordana and Köhler2016a; Nacarino-Meneses and Orlandi-Oliveras, Reference Nacarino-Meneses and Orlandi-Oliveras2019) species. The presence of CCCB in the Hipparion metatarsus KK-1 is noteworthy since such tissue has not previously been reported in fossil horses (see Fig. 2B). This endosteally formed bone tissue results from the incorporation and compaction of metaphyseal bone trabeculae into the diaphyseal cortex during longitudinal growth (Enlow, Reference Enlow1962; Chinsamy-Turan, Reference Chinsamy-Turan2005). Although it may appear in the mid-diaphysis of long bones (McFarlin et al., Reference McFarlin, Terranova, Zihlman, Enlow and Bromage2008; Geiger et al., Reference Geiger, Wilson, Costeur, Sánchez and Sánchez-Villagra2013; Kolb et al., Reference Kolb, Scheyer, Veitschegger, Forasiepi, Amson, Van der Geer, Van den Hoek Ostende, Hayashi and Sánchez-Villagra2015b; Montoya-Sanhueza and Chinsamy, Reference Montoya-Sanhueza and Chinsamy2017; Legendre and Botha-Brink, Reference Legendre and Botha-Brink2018; Bhat et al., Reference Bhat, Chinsamy and Parkington2019; Garrone et al., Reference Garrone, Cerda and Tomassini2019), CCCB is most commonly found in metaphyseal areas (Enlow, Reference Enlow1962). KK-1 was prepared from the most distal part of the bone shaft (see Supplementary Fig. 1), which explains the presence of this bone tissue type in the cross section.
Another interesting finding was the distinctive stratification present in the proximal section of an Equus metacarpus, P-1 (see Supplementary Fig. 1): multiple circumferentially oriented vascular canals (i.e., LB) occur in the inner half of the cortex while longitudinal POs were present in the outer half of the cross section (see Fig. 2E). Patches of LB have previously been identified in the inner metapodial cortex of hipparionines from Greece and Spain (Orlandi-Oliveras et al., Reference Orlandi-Oliveras, Nacarino-Meneses, Koufos and Köhler2018), as well as in that of Equus hemionus (Nacarino-Meneses et al., Reference Nacarino-Meneses, Jordana and Köhler2016a), but never as extensively developed as in our specimen P-1. The abrupt change in the orientation of the vascular canals in Equus hemionus appears to be linked to birth (Nacarino-Meneses et al., Reference Nacarino-Meneses, Jordana and Köhler2016b), although in the latter instance the vascular canals change from a longitudinal to a circumferential orientation (Nacarino-Meneses et al., Reference Nacarino-Meneses, Jordana and Köhler2016b). We propose that the change in vascular channel orientation observed in P-1 (see Fig. 2E) may also be related to the birth of the animal, as it likely reflects the changes in biomechanics and growth rate associated with the beginning of postnatal life (Nacarino-Meneses and Köhler, Reference Nacarino-Meneses and Köhler2018).
Life history studies on extinct taxa usually involve the analysis of BGMs, as they provide key information about traits such as the age at death, the age at maturity, or the growth rate (Woodward et al., Reference Woodward, Padian, Lee, Padian and Lamm2013; Nacarino-Meneses et al., Reference Nacarino-Meneses, Jordana and Köhler2016a). Unfortunately, the diagenetic alteration of most of our samples greatly hampered the identification of these histological features (see Fig. 3A). Nevertheless, among all our material, BGMs were well preserved in MD-7 (see Fig. 3C), a metacarpal recovered from the Mio-Pliocene fossil locality of Manisa-Düzpınar, which is considered to belong to the medium-sized equid Cremohipparion cf. matthewi—the only equid known from this locality (Mayda et al., Reference Mayda, Sotnikova, Tesakov, Tan and Kaya2015). In this specimen, five CGMs plus one NL were observed in the cross section, and an EFS/OCL was not identified in the most external cortex (see Fig. 3C). This finding agrees with descriptions for the Pleistocene species Equus steinheimensis (Nacarino-Meneses and Orlandi-Oliveras, Reference Nacarino-Meneses and Orlandi-Oliveras2019), but differs from previous findings in Spanish and Greek hipparionines, in which an EFS/OCL usually appears after the second or third year of growth (i.e., second or third CGM) (Martínez-Maza et al., Reference Martínez-Maza, Alberdi, Nieto-Diaz and Prado2014; Orlandi-Oliveras et al., Reference Orlandi-Oliveras, Nacarino-Meneses, Koufos and Köhler2018). The presence of an EFS/OCL in metapodial cross sections of equids indicates the end/decrease of periosteal bone growth (Nacarino-Meneses et al., Reference Nacarino-Meneses, Jordana and Köhler2016a). Hence, the lack of EFS/OCL in Cremohipparion cf. matthewi specimen MD-7 suggests an extended period of periosteal growth (Nacarino-Meneses et al., Reference Nacarino-Meneses, Jordana and Köhler2016a) as compared to other European hipparionines. In these taxa, the EFS/OCL has been correlated with the attainment of skeletal maturity (Martínez-Maza et al., Reference Martínez-Maza, Alberdi, Nieto-Diaz and Prado2014; Orlandi-Oliveras et al., Reference Orlandi-Oliveras, Nacarino-Meneses, Koufos and Köhler2018); therefore, the absence of the EFS/OCL (despite having at least 5–6 years of growth) in Cremohipparion cf. matthewi may indicate extended skeletal maturity. In equids, skeletal maturity of the bone (understood as the time of epiphyseal fusion or the end of longitudinal bone growth) can also be inferred from growth curve reconstructions (Nacarino-Meneses et al., Reference Nacarino-Meneses, Jordana and Köhler2016a). In the histological study of Equus hemionus, Nacarino-Meneses and colleagues (Reference Nacarino-Meneses, Jordana and Köhler2016a) demonstrated that the completion of longitudinal bone growth correlates with a decrease in periosteal growth and that growth rate is minimal after epiphyseal fusion. Although metacarpal MD-7 of Cremohipparion cf. matthewi shows a decrease in growth rate after the deposition of the first CGM (see Fig. 3D), growth rate continues at a similar rate until the fourth CGM (fifth year of life), after which it becomes minimal (see Fig. 3E). This suggests that epiphyses of MD-7 would have fused after the fourth year of life, much later than in similar body-sized Spanish and Greek Hipparion horses (Martínez-Maza et al., Reference Martínez-Maza, Alberdi, Nieto-Diaz and Prado2014; Orlandi-Oliveras et al., Reference Orlandi-Oliveras, Nacarino-Meneses, Koufos and Köhler2018), and also points to a delayed skeletal maturity for the species. These inferences, however, should be considered with caution due to our limited sample size, as they only provide preliminary life history information for this hipparionine that must be tested in future studies.
Histotaphonomy of Turkish fossil equids
Bone degradation is abundant in our sample, affecting almost 80% of the bones studied (see Fig. 4B). Non-Wedl tunnels (see Fig. 6A–I) are the main MFD identified both in Hipparion and Equus specimens (see Fig. 4B, Supplementary Table 1), while Wedl-like tunnelling only affects the hipparionine metatarsal KK-1 (see Fig. 4B, 6J–L, Supplementary Table 1). This contrasts with previous research in fossils reporting a higher or even an exclusive presence of Wedl tunnels (Trueman and Martill, Reference Trueman and Martill2002; Bao et al., Reference Bao, Rachel, Bradley and Curry Rogers2009; Chinsamy-Turan and Ray, Reference Chinsamy-Turan, Ray and Chinsamy-Turan2012), but agrees with the research of Mayer and colleagues (Reference Mayer, Hubbe, Botha-Brink, Ribeiro, Haddad-Martim and Neves2020) and Turner-Walker (Reference Turner-Walker2012), who describe non-Wedl microboring as the primary MFD affecting mammalian bones recovered from the palaeontological fossil record. It should be considered that non-Wedl tunnels are much easier to identify than Wedl ones in poorly preserved samples (Mayer et al., Reference Mayer, Hubbe, Botha-Brink, Ribeiro, Haddad-Martim and Neves2020), so the huge alteration of most of our specimens (see Fig. 4A, 5D–E) might be partially biasing our findings toward the identification of non-Wedl tunnels, as proposed in other investigations (Mayer et al., Reference Mayer, Hubbe, Botha-Brink, Ribeiro, Haddad-Martim and Neves2020).
Wedl and non-Wedl tunnels are traditionally believed to register the action of different microorganisms (Jans, Reference Jans, Wisshak and Tapanila2008). Since Hackett (Reference Hackett1981), non-Wedl MFD is usually attributed to bacteria (Jans, Reference Jans, Wisshak and Tapanila2008), while Wedl foci are thought to be caused by fungi (Marchiafava et al., Reference Marchiafava, Bonucci and Ascenzi1974), algae (Davis, Reference Davis1997; Fernández-Jalvo et al., Reference Fernández-Jalvo, Andrews, Pesquero, Smith, Marín-Monfort, Sánchez, Geigl and Alonso2010), and/or cyanobacteria (Davis, Reference Davis1997; Turner-Walker and Jans, Reference Turner-Walker and Jans2008). Recently, several authors have argued that both kinds of MFD are just the result of specific bacteria boring different tissue types and that tunnelling type cannot be assigned to different decomposition agents (Kendall et al., Reference Kendall, Eriksen, Kontopoulos, Collins and Turner-Walker2018; Turner-Walker, Reference Turner-Walker2019). Here, we follow the classical approach that correlates the MFD observed in our sample with different microbes (e.g., Jans, Reference Jans, Wisshak and Tapanila2008). Thus, the identification of Wedl-like tunnels on the Hipparion specimen KK-1 (see Fig. 4B, Fig. 6J–L, Supplementary Table 1) suggests that it was affected by microorganisms other than bacteria (Jans, Reference Jans, Wisshak and Tapanila2008). This kind of MFD has traditionally been associated with fungi (Marchiafava et al., Reference Marchiafava, Bonucci and Ascenzi1974; Hackett, Reference Hackett1981; Trueman and Martill, Reference Trueman and Martill2002; Jans et al., Reference Jans, Nielsen-Marsh, Smith, Collins and Kars2004; Chinsamy-Turan and Ray, Reference Chinsamy-Turan, Ray and Chinsamy-Turan2012), but, as already stated, it is known that other microbes like cyanobacteria or algae can also leave Wedl-like borings in the bone tissue (Turner-Walker and Jans, Reference Turner-Walker and Jans2008; Fernández-Jalvo et al., Reference Fernández-Jalvo, Andrews, Pesquero, Smith, Marín-Monfort, Sánchez, Geigl and Alonso2010; Turner-Walker, Reference Turner-Walker2019). It has been proposed that the depositional environment of the specimen might be key to establishing the main agent of the Wedl tunnelling, as fungi will only be expected in terrestrial environments, while cyanobacteria and algae occur in aquatic environments (Kendall et al., Reference Kendall, Eriksen, Kontopoulos, Collins and Turner-Walker2018). Metacarpal KK-1 was recovered from an alluvial fan (Kazanci et al., Reference Kazanci, Karadenizli, Seyitoǧlu, Sen, Alçiçek, Varol, Saraç and Hakyemez2005), so aquatic microorganisms cannot be excluded as bioerosion agents. Indeed, the morphology and the specific location of the Wedl-like tunnelling around vascular canals (see Fig. 6J–L) throughout the entire middle and external cortex of KK-1 agrees with previous descriptions of borings caused by algae (Fernández-Jalvo et al., Reference Fernández-Jalvo, Andrews, Pesquero, Smith, Marín-Monfort, Sánchez, Geigl and Alonso2010; Fernández-Jalvo and Andrews, Reference Fernández-Jalvo and Andrews2016). These microorganisms, along with moss and lichen, are known to penetrate the bone through its histological traits (Fernández-Jalvo et al., Reference Fernández-Jalvo, Andrews, Pesquero, Smith, Marín-Monfort, Sánchez, Geigl and Alonso2010), while fungal and cyanobacterial damage usually starts at the periosteal surface and is commonly restricted to the most external part of the bone cortex (Jans, Reference Jans, Wisshak and Tapanila2008; Bao et al., Reference Bao, Rachel, Bradley and Curry Rogers2009). Previous taphonomic research performed at this specific fossil site report macroscopic alterations by roots, fungi, and other microorganisms (Valli, Reference Valli2005), so we also cannot completely discard fungal damage to KK-1. However, the location of the MFD in the section likely points toward freshwater algae (which may be included in “other microorganisms” [Valli, Reference Valli2005]) as the main agent causing the microboring in this sample.
The high presence of non-Wedl tunnels in the fossil Hipparion and Equus metapodia (see Fig. 4B, Fig. 6A–I, Supplementary Table 1), on the other hand, suggests bacterial degradation (Jans, Reference Jans, Wisshak and Tapanila2008). Corpse-decomposing bacteria consists both of exogenous bacteria from the soil (Turner-Walker, Reference Turner-Walker, Pinhasi and Mays2008; Kontopoulos et al., Reference Kontopoulos, Nystrom and White2016; Morales et al., Reference Morales, Catella, Oliva, Sarmiento and Barrientos2018; Galligani et al., Reference Galligani, Sartori and Barrientos2019) and of intrinsic gut microbiota, including bacteria (Child, Reference Child1995; Bell et al., Reference Bell, Skinner and Jones1996; Jans et al., Reference Jans, Nielsen-Marsh, Smith, Collins and Kars2004; Hollund et al., Reference Hollund, Jans, Collins, Kars, Joosten and Kars2012; White and Booth, Reference White and Booth2014; Damann and Jans, Reference Damann, Jans, Carte, Tomberlin, Benbow and Metcalf2017; Brönnimann et al., Reference Brönnimann, Portmann, Pichler, Booth, Röder, Vach, Schibler and Rentzel2018). Currently there is no consensus as to which kind of bacteria has a greater influence on bone bioerosion. Following the “endogenous model,” Jans and colleagues (Reference Jans, Nielsen-Marsh, Smith, Collins and Kars2004) proposed that animal remains from archaeological sites may present low bacterial damage because specimens are usually buried as fragments without flesh (i.e., butchered animals), preventing putrefaction bacteria from spreading from the belly to the limbs (Jans et al., Reference Jans, Nielsen-Marsh, Smith, Collins and Kars2004). The larger prevalence of bacterial attack in our samples (see Fig. 4B, Fig. 6A–I, Supplementary Table 1) may suggest that the Hipparion and Equus fossil specimens analysed here would have entered the fossil record as complete animals, facilitating the invasion of endogenous bacteria throughout the body (Jans et al., Reference Jans, Nielsen-Marsh, Smith, Collins and Kars2004). This hypothesis, however, seems highly improbable, as fossil bones usually enter the soil as fragments because of the previous action of scavengers (Trueman and Martill, Reference Trueman and Martill2002). Indeed, recent studies propose that exogenous bacteria may contribute more to bioerosion (Turner-Walker, Reference Turner-Walker, Pinhasi and Mays2008; Kendall et al., Reference Kendall, Eriksen, Kontopoulos, Collins and Turner-Walker2018) than intrinsic gut microbiota because most decomposing bacteria is already present in the soil (Metcalf et al., Reference Metcalf, Xu, Weiss, Lax, Treuren, Hyde and Song2016). This has been claimed to be particularly true for tropical and subtropical climates (Morales et al., Reference Morales, Catella, Oliva, Sarmiento and Barrientos2018; Galligani et al., Reference Galligani, Sartori and Barrientos2019). Palaeoenvironmental reconstructions for Turkey suggest a dry, warm-temperate climate from the Miocene (Akgün et al., Reference Akgün, Kayseri and Akkiraz2007; Akkiraz et al., Reference Akkiraz, Akgün, Utescher, Bruch and Mosbrugger2011; Kayseri-Özer et al., Reference Kayseri-Özer, Karadenizli, Akgün, Oyal, Saraç, Şen, Tunoğlu and Tuncer2017) to the early Pleistocene (Kahlke et al., Reference Kahlke, García, Kostopoulos, Lacombat, Lister, Mazza, Spassov and Titov2011; Jiménez-Moreno et al., Reference Jiménez-Moreno, Alçiçek, Alçiçek, van den Hoek Ostende and Wesselingh2015), with warmer temperatures than today (Jiménez-Moreno et al., Reference Jiménez-Moreno, Alçiçek, Alçiçek, van den Hoek Ostende and Wesselingh2015). This palaeoclimatic scenario could have led to higher soil bacterial decomposing activity (Galligani et al., Reference Galligani, Sartori and Barrientos2019) that might have been responsible for the large prevalence of bacterial attack observed in fossil Hipparion and Equus samples (see Fig. 4B, Fig. 6A–I, Supplementary Table 1).
In addition to temperature, other physical aspects of the environment, such as oxygen and water, also play a key role in the preservation of the bone microstructure, as they control the action of the different microorganisms (Kendall et al., Reference Kendall, Eriksen, Kontopoulos, Collins and Turner-Walker2018). Bones buried in waterlogged and anoxic conditions, for example, are not usually affected by microbial attack, while soils with periodical or seasonal influx of water and variation in temperature favour microboring (Hackett, Reference Hackett1981). We propose that the greatly degraded samples analysed here (GHI = 0–2, see Fig. 5D, 5E, Supplementary Table 1) may have experienced variation in moisture during early stages of diagenesis. Interestingly, some of the most altered specimens of our sample belong to alluvial flood plain depositional environments (e.g., CY-1, MYSE-1, see Table 2, Supplementary Table 1), which are prone to periodic inundations. These wetting-drying, low-high oxygen cycles may have influenced the quick degradation of the bones. Specimens UEK-1, MD-7, and P-1, on the other hand, were not affected by any kind of MFD (GHI = 4–5, see Fig. 5A, 5B, Supplementary Table 1), so we propose that they were probably buried in anoxic waterlogged soils. Indeed, in UEK-1 the occurrence of radial microcracks that cross the cement lines of SOs (see Fig. 7B) also suggest that fossilization occurred in an aquatic environment (Pfretzschner, Reference Pfretzschner2004; Pfretzschner and Tütken, Reference Pfretzschner and Tütken2011; Tomassini et al., Reference Tomassini, Miño-Boilini, Zurita, Montalvo and Cesaretti2015). According to Pfretzschner (Reference Pfretzschner2004), these radial microfissures appear in response to the swelling of bone collagen by water during the late stage of early diagenesis, which creates a tension stress in the cement line that triggers breakage. Variations in the water content of the collagen may also be responsible for the microscopic breakages of the bone matrix observed in SEM (see Fig. 6H, 6I, 6K, 6L). Bone microcracks were also identified in P-1 (see Supplementary Table 1), both within the fibrolamellar bone (see Fig. 5B) and within areas of secondary bone tissue (see Fig. 7A). However, unlike in UEK-1 (see Fig. 7B), bone microcracks in the areas of SOs in P-1 follow the cement lines and never cross them (see Fig. 7A).
Interestingly, P-1 was recovered from an exceptional depositional environment: the Pamukkale travertines (Rausch et al., Reference Rausch, Alçiçek, Vialet, Boulbes, Mayda, Titov and Stoica2019). These are sedimentary rocks formed by the precipitation of carbonates in geothermally heated hot-springs (Guo and Riding, Reference Guo and Riding1998). Since intense heat is known to provoke fractures in the bone tissue (Hanson and Cain, Reference Hanson and Cain2007), the microcracks observed in P-1 may be the result of its fossilization in a hot environment. When temperatures exceed 100°C the heat can cause the denaturation of collagen in mineralized bones (Bonar and Glimcher, Reference Bonar and Glimcher1970) as a result of the breakage of the triple-helical chains, the disruption of intermolecular cross-links, and the shrinkage of the collagen molecules (Lees, Reference Lees and Hukins1989). Maximum palaeotemperatures inferred for the Quaternary deposits of the Pamukkale travertines are only 70°C (Özkul et al., Reference Özkul, Kele, Gökgöz, Shen, Jones, Baykara, Fórizs, Németh, Chang and Alçiçek2013), so bone collagen would have not completely denatured in this environment. It may have suffered, however, some structural changes that may have led to bone microcracks, including the unravelling of the helical chains, which may occur at lower temperatures (Lees, Reference Lees and Hukins1989). Indeed, heating affects the collagen fibrils differently as compared to variations in water content: while water absorption/adsorption causes changes at right angles (radially) to the collagen fibril direction (Pfretzschner, Reference Pfretzschner2004), thermal shrinkage provokes the longitudinal breakage of the collagen fibrils (Bonar and Glimcher, Reference Bonar and Glimcher1970; Lees, Reference Lees and Hukins1989). This differential rupture of the collagen molecule might explain the different microcrack pattern observed in the SOs of P-1 and UEK-1.
Generally, we could not identify any specific trend relating the depositional environment of the bone and its degree of micropreservation (see Table 2), which might suggest occasional transport and redeposition of bones. Well-preserved specimens of Hipparion and Equus (GHI = 4–5, see Fig. 5A, 5B) were recovered from either alluvial fan, lacustrine, or travertine sediments, while badly preserved metapodia (GHI = 0–2, see Fig. 5C–E) were found within fluvial and alluvial deposits (see Table 2). With the exception of Kırşehir-Kaman (Valli, Reference Valli2005), taphonomy has not been properly studied yet for the different localities under analysis, and only some data about their lithology, stratigraphy, and/or sedimentary environments can be found in the literature (Tekkaya et al., Reference Tekkaya, Atalay, Gürbüz, Ünay and Ermumcu1975; Kaya, Reference Kaya1981; Forsten and Kaya, Reference Forsten and Kaya1995; Kaya et al., Reference Kaya, Müller, Rückert-Ülkümen and Kaya1998, Reference Kaya, Geraads and Tuna2005a, Reference Kaya, Mayda and Saraç2005b, Reference Kaya, Mayda, Kostopoulos, Alcicek, Merceron, Tan, Karakutuk, Giesler and Scott2012; Kazanci et al., Reference Kazanci, Karadenizli, Seyitoǧlu, Sen, Alçiçek, Varol, Saraç and Hakyemez2005; Seyitoğlu et al., Reference Seyitoğlu, Alçiçek, Işık, Alçiçek, Mayda, Varol, Yılmaz and Esat2009; Mayda et al., Reference Mayda, Titov, Tesakov, Göktaş and Alçiçek2013; Lebatard et al., Reference Lebatard, Alçiçek, Rochette, Khatib, Vialet, Boulbes and Bourlès2014; Rausch et al., Reference Rausch, Alçiçek, Vialet, Boulbes, Mayda, Titov and Stoica2019). A complete taphonomical analysis would be useful to fully analyse the relationship between bone histology degradation and depositional environment, but this is beyond the scope of the present investigation. Thus, we encourage future research in this area.
CONCLUSIONS
Among the equid samples studied from Turkey, we found that a few had well-preserved histology, while the majority were diagenetically altered. In the specimens in which the histology was intact they compared favourably with European hipparionines, as well as with extant and extinct Equus. The Hipparion metatarsal (KK-1) permitted the identification of CCCB in the bone cortex, which is likely the result of the more distal location of the histological section on the bone shaft. Regarding vascularity, the proximal cross section of the P-1 Equus metacarpal showed a marked change in the orientation of the vascular canals within the mid-cortex that might be correlated with birth. The well-preserved bone histology and BGMs in MD-7 of Cremohipparion cf. matthewi permitted deductions concerning the later attainment of skeletal maturity as compared to other Mediterranean hipparionines.
The histology of most of the bones we studied was poorly preserved. Most of them were affected by non-Wedl tunnelling, suggesting that most of the Hipparion and Equus fossil metapodia analysed here were degraded by soil bacteria. Wedl-like tunnels were only recognized in KK-1, and their specific position and morphology agree with the damage caused by algae. Many of our specimens presented low histological preservation indexes (GHI = 0–2), so they likely experienced differences in moisture content during diagenesis. The well-preserved UEK-1, MD-7, and P-1 present higher preservation indices (GHI = 4–5) without signs of bioerosion, and we propose that they were initially buried in anoxic waterlogged conditions. UEK-1 and P-1 showed microcracks, which in the case of UEK-1 were likely formed in a water environment, and in P-1 (from the Pamukkale travertines) were most likely formed in response to intense heat and associated collagen denaturation. Given our small sample size, we did not identify a specific correlation between the degree of bone preservation and the sedimentary environment in which the different bones were found (i.e., alluvial fan, lacustrine, etc.).
Our analysis of the bone histology and the diagenetic alterations evident in the metapodia of late Miocene, Mio-Pleistocene, and early Pleistocene equids from Turkey provides a glimpse into the palaeobiology and early taphonomy of these extinct horses. Future research is encouraged to corroborate these initial findings. Furthermore, we note that histological research aiming at obtaining palaeobiological information should focus on the localities Manisa-Düzpınar and Uşak-Kemiklitepe 2 since bones from these sites presented excellent micropreservation. Indeed, increasing the sampling of Cremohipparion cf. matthewi from Manisa-Düzpınar would complement our interesting preliminary skeletochronological findings. Further taphonomical investigations at most of the fossil sites studied here will greatly enable a better understanding of bone preservation and the agents of bioerosion.
ACKNOWLEDGMENTS
We thank Petra Muller (University of Cape Town, South Africa) for guidance and assistance with SEM microscopy and Dr. Alberto Valenciano (Universidad de Zaragoza, Spain) for discussion and advice on several palaeontological aspects of the work. We are also grateful to the managing editor (Karin Perring), the senior editor (Prof. Nicholas Lancaster), the associate editor (Dr. Curtis W. Marean), and two anonymous reviewers, whose comments greatly improved an earlier version of this manuscript. Reviewer 1 is particularly acknowledged for suggesting that collagen thermal denaturation may be responsible for the microcracking observed in the SOs of P-1.
This work was supported by the Scientific and Technical Research Council of Turkey (TUBITAK), project number 110Y154, and the National Research Foundation (South Africa), grant number 117716 to AC. CN-M is supported by a postdoctoral research fellowship from the DST-NRF Centre of Excellence in Palaeosciences (CoE, South Africa), grants COE2019-PD03 and COEPD2020-39. Opinions expressed and conclusions arrived at are those of the authors and are not necessarily attributed to the CoE. SM and TK are grateful to Ege University Scientific Research Center for TTM/2016/002 and TTM/2013/001 research grants.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/qua.2020.87