Introduction
Understanding the mechanisms underpinning spatiotemporal diversity patterns of biological communities is a major goal of ecology, with practical and theoretical applications in conservation (Jost et al., Reference Jost, DeVries, Walla, Greeney, Chao and Ricotta2010). The species diversity of a given region (i.e. γ-diversity) can be partitioned into within- and between- components, the so-called α- and β-diversity. The β-diversity was firstly used to describe spatial changes in species identities among sites (Whittaker, Reference Whittaker1960, Reference Whittaker1972), but it can also be used to describe temporal changes in community patterns (Legendre & Gauthier, Reference Legendre and Gauthier2014). In addition, β-diversity can be partitioned into two process-related components: species replacement (turnover) and gain/loss (nestedness) among sites or times (Baselga, Reference Baselga2010, Reference Baselga2012).
In a meta-analysis, Soininen et al. (Reference Soininen, Heino and Wang2018) found that spatial species substitution is the main mechanism driving β-diversity patterns across different organisms and ecosystems. These authors also found that turnover and total β-diversity increased with spatial extent, whereas nestedness was unrelated to scale. It means there is a species substitution related to the geographic distance among sites, in which dispersal limitation and/or environmental filtering may be important drivers (Qian et al., Reference Qian, Ricklefs and White2005). In addition, passively dispersed organisms had lower turnover and total β-diversity than flying organisms, an unexpected result (Soininen et al., Reference Soininen, Heino and Wang2018).
Unlike spatial patterns, temporal patterns in β-diversity components are a little-explored topic (Baselga et al., Reference Baselga, Bonthoux and Balent2015; Shimadzu et al., Reference Shimadzu, Dornelas, Magurran and O'Hara2015). Temporal changes in species composition can be related to both substitution of species from time to time (temporal turnover) and gain or loss of species forming nested subsets from time to time (nestedness-resultant dissimilarity) (Baselga et al., Reference Baselga, Bonthoux and Balent2015). Changes in species composition from time to time are expected to occur due to variation in climatic and environmental conditions, and resource availability. For instance, winter assemblages are expected to be a sub-sample of the summer assemblage in subtropical regions (Hernández & Vaz-de-Mello, Reference Hernández and Vaz-de-Mello2009; da Silva et al., Reference da Silva, Vaz-de-Mello and Di Mare2013). However, there is no way to predict whether there will be a substitution or gain/loss of species over time since the response can be context- and organism-dependent. We, however, can hypothesize mechanisms causing those changes and try to relate them to the components of β-diversity.
Dung beetles (Coleoptera: Scarabaeinae) are a diverse and widely distributed insect group that perform several ecological functions, such as bioturbation, nutrient recycling, secondary seed dispersal, control of dung-dependent parasites, and pollination (Nichols et al., Reference Nichols, Spector, Louzada, Larsen, Amezquita and Favila2008). Several authors have argued about the usefulness of dung beetles in studying theoretical and applied ecological issues (Halffter & Favila, Reference Halffter and Favila1993; Spector, Reference Spector2006; Nichols et al., Reference Nichols, Larsen, Spector, Davis, Escobar, Favila and Vulinec2007; Simmons & Ridsdill-Smith, Reference Simmons and Ridsdill-Smith2011). These beetles show qualitative and quantitative responses to both natural and human-driven environmental changes, being used as good ecological and biodiversity indicators (Barlow et al., Reference Barlow, Gardner, Araujo, Avila-Pires, Bonaldo, Costa, Esposito, Ferreira, Hawes, Hernández, Hoogmoed, Leite, Lo-Man-Hung, Malcolm, Martins, Mestre, Miranda-Santos, Nunes-Gutjahr, Overal, Parry, Peters, Ribeiro-Junior, Silva, Silva Motta and Peres2007; Gardner et al., Reference Gardner, Hernández, Barlow and Peres2008; Culot et al., Reference Culot, Bovy, Vaz-de-Mello, Guevara and Galetti2013; Audino et al., Reference Audino, Louzada and Comita2014; Beiroz et al., Reference Beiroz, Audino, Queiroz, Rabello, Boratto, Silva and Ribas2014; Campos & Hernández, Reference Campos and Hernández2015). In addition, temperature and precipitation influence dung beetle species richness and abundance in temperate and tropical regions, respectively (Davis, Reference Davis1994; Hernández & Vaz-de-Mello, Reference Hernández and Vaz-de-Mello2009; da Silva et al., Reference da Silva, Vaz-de-Mello and Di Mare2013; Batista et al., Reference Batista, Silva Lopes, Marques and Teodoro2016). However, there is a lack of information about the relation between temporal patterns of β-diversity components and climatic variables. In fact, this lack of information can be generalized for several groups and systems.
In this paper, we aimed to test two ecological hypotheses. First, different from turnover-dominated spatial patterns (Soininen et al., Reference Soininen, Heino and Wang2018), (i) temporal patterns of β-diversity will mostly be driven by nestedness, with a loss of species from summer to winter. Very abundant dung beetle species occur throughout the year with a reduced number of individuals in winter, while some rare or thermal-sensitive species occur only at warm periods (Louzada & Lopes, Reference Louzada and Lopes1997; Errouissi et al., Reference Errouissi, Labidi and Nouira2009; da Silva et al., Reference da Silva, Vaz-de-Mello and Di Mare2013). In addition, climatic features, especially temperature in subtropical and temperate regions, drive the temporal patterns in species richness and abundance of dung beetles, producing nested patterns of community assembly from summer to winter (Hernández & Vaz-de-Mello, Reference Hernández and Vaz-de-Mello2009; da Silva et al., Reference da Silva, Vaz-de-Mello and Di Mare2013). Therefore, due to the increase of species richness in warm conditions, (ii) nestedness values will correlate with climatic variables instead of turnover values, indicating either a loss of species during winter or a gain of species during summer.
Material and methods
Study area
The study was carried out in Atlantic Forest sites in four localities, two of them located at the northwest and two at the central region of the Rio Grande do Sul state, southern Brazil (Supporting Information Figure S1). The sites at northwest region were the Turvo State Park (TSPK; Derrubadas municipality) and Moreno Fortes Biological Reserve (MFBR; Dois Irmãos das Missões municipality), two environmental protection areas with Seasonal Deciduous Forest. The sites at central region were isolated Seasonal Deciduous Forest fragments called ‘Morro do Cerrito’ (MOCE) in Santa Maria municipality and ‘Val Feltrina’ (VAFE) in the Silveira Martins municipality. Both regions (central and northwest) are classified as Cfa (subtropical climate, with hot summers and no rainy season), with an annual precipitation of 1500–1700 mm, and an average annual temperature of 18–19°C (range 0–39°C). Santa Maria and Silveira Martins municipalities belong to the ecotone region between Atlantic Forest and Pampa (grassland-dominated) biomes. The sites at the northwest and central regions are ~275 km apart; the sites at the northwest region are 55 km apart, while sites at the central region are 18 km apart. A detailed description of the sites sampled can be found in Supporting Information Appendix S1.
Dung beetle sampling
Dung beetles were sampled with the use of baited pitfall traps (see da Silva et al., Reference da Silva, Vaz-de-Mello and Di Mare2013 for a detailed trap description) since they are a standard sampling protocol for this group. We used ten traps per forest fragment, 100 m apart from each other along a linear transect (da Silva & Hernández, Reference da Silva and Hernández2015a). Human feces (ca. 15 g) were used as bait to attract dung beetles because several studies have shown it is the most attractive bait for this insect group (Larsen et al., Reference Larsen, Lopera and Forsyth2006; Filgueiras et al., Reference Filgueiras, Liberal, Aguiar, Hernández and Iannuzzi2009; da Silva et al., Reference da Silva, Vaz-de-Mello and Di Mare2012).
Except for TSPK, we sampled dung beetles monthly between May 2016 and July 2017, totaling 12 samples in each site. In TSPK, we were only able to perform seasonal samplings (N = 4, middle of summer, autumn, winter, and spring). Traps remained in the field during 48 h per sampling. We identified dung beetles using dichotomous keys (da Silva et al., Reference da Silva, Vaz-de-Mello and Di Mare2011; Vaz-de-Mello et al., Reference Vaz-de-Mello, Edmonds, Ocampo and Schoolmeesters2011) and vouchers were sent to an expert who confirmed beetle identification. Vouchers were deposited at the Universidade Federal de Santa Maria and Universidade Federal de Mato Grosso.
Climatic variables
Monthly historical values (last 30 years, i.e. 1987–2017) of mean temperature (°C), accumulated precipitation (mm), and relative humidity of air (%) were obtained from the Brazilian National Institute of Meteorology (INMET). We were able to obtain such values for Santa Maria and Dois Irmãos das Missões cities. Although fine scale measures can address micro-environmental and temporal variations in climatic variables truly experienced by organisms (Pincebourde et al., Reference Pincebourde, Murdock, Vickers and Sears2016), in their absence, the use of historical variables has proved useful in investigating the mechanisms underpinning biological patterns (Silva et al., Reference Silva, Souza, Solar and Neves2017). Furthermore, historical data account for variations in climate conditions among years. Thus, only the data from both municipalities (i.e. Santa Maria and Dois Irmãos das Missões) were used to test the relationship between climatic variables and dung beetle assemblage metrics.
Data analysis
The sampling completeness of each site was estimated with the use of a coverage estimator in order to guarantee that compositional comparisons are based on reliable inventories (Chao & Jost, Reference Chao and Jost2012). Sample coverage was calculated using the R package iNEXT with species abundance data (Hsieh et al., Reference Hsieh, Ma, Chao and McInerny2016) in the R software (R Core Team, 2017).
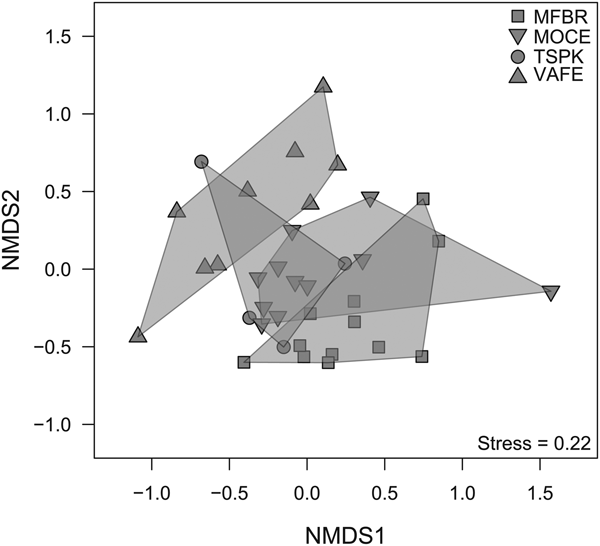
The permutational multivariate analysis of variance (PERMANOVA) (Anderson, Reference Anderson2001) was used to test the compositional differences among sites and regions (‘northwest’ and ‘central’). To avoid bias due to differences in the number of samplings among sites, we performed the analysis using only incidence data (Jaccard coefficient). The adonis function of the R package vegan (Oksanen et al., Reference Oksanen, Blanchet, Friendly, Kindt, Legendre, McGlinn, Minchin, O'Hara, Simpson, Solymus, Stevens, Szoecs and Wagner2016) was used to perform the PERMANOVA analysis, with 999 permutations. We used the betadisper function of the R package vegan to test the multivariate homogeneity of group's dispersions (PERMDISP analysis) (Anderson et al., Reference Anderson, Ellingsen and McArdle2006). The non-metric multidimensional scaling (NMDS) was used to represent graphically the PERMANOVA results.
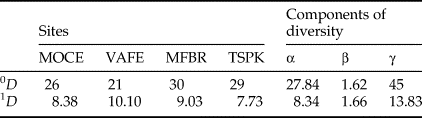
We partitioned species diversity metrics using Hill numbers (Jost, Reference Jost2006) into α, β, and γ in order to investigate species changes and the number of communities among sites sampled. We used the R package entropart (Marcon & Hérault, Reference Marcon and Hérault2014) and multiplicative partitioning because it guarantees the independence (or unrelatedness) between α and β components (Chao et al., Reference Chao, Chiu and Hsieh2012). We used Hill numbers of order 0 (0D, species richness) and order 1 (1D, exponential of Shannon entropy). 0D is not sensitive to the abundance of species, giving too much weight to rare species (Jost, Reference Jost2006). 1D weighs each species according to their abundance in the community, without favoring rare or abundant species and can be interpreted as the number of common species in the community (Jost, Reference Jost2007). β-diversity of Shannon entropy can be considered the effective number of different communities in a given landscape or region (Jost, Reference Jost2007). β-diversity is lower when a community dominates the landscape and higher when all communities share the landscape equally (Jost, Reference Jost2006, Reference Jost2007). We weighed communities by their total abundance when using 1D (Chao et al., Reference Chao, Chiu and Hsieh2012).
We also partitioned incidence-based β-diversity into its turnover and nestedness-related components (Baselga, Reference Baselga2010) in order to verify if temporal patterns of β-diversity are driven by species substitution or species gain/loss. The functions beta.pair and beta.multi of the R package betapart (Baselga & Orme, Reference Baselga and Orme2012) and Jaccard coefficient were used for this purpose. To calculate spatial patterns in β-diversity components, we pooled the data of each site to summarize them in one sample per site. Due to differences in the number of samplings among sites, we selected one sample per season in those sites with 12 samplings in order to make sites to be comparable with the TSPK site. We then selected four samples per site from similar months (i.e. January, April, June, and October).
Generalized linear models (GLM) were used to relate monthly climatic variables (i.e. mean temperature, precipitation, and relative humidity) and community metrics (i.e. richness and abundance). We also used GLM to relate β-diversity components of turnover and nestedness and differences of monthly climatic variables between consecutive months. The beta.pair function of the betapart R package was used to calculate β-diversity components between consecutive months. Differences in climatic variables between consecutive months were calculated by subtracting the value of the following month from the previous month. Positive values indicate there was a decrease in the climatic variable, while negative values indicate there was an increase in the climatic variable between consecutive months. Therefore, we had 11 samples for MOCE and MFRB, the unique sites with monthly climatic variables. We ran models searching for the best error distribution for each response variable and correcting for overdispersion or underdispersion when necessary. Therefore, we used quasi-Poisson distribution for species richness, Gaussian distribution for abundance (log10-transformed), and quasi-binomial for turnover and nestedness.
Results
We sampled 5533 dung beetles belonging to 45 species, ranging from 21 (VAFE) to 30 (MFBR) species (Supporting Information Table S1 and S2). Canthidium aff. trinodosum and Canthon rutilans cyanescens were the most abundant species in both MOCE and VAFE (central region), while Scybalocanthon nigriceps and Eurysternus parallelus were the most abundant ones in MFBR and TSPK, respectively. The total abundance ranged between 148 (2.7%; VAFE) and 2178 (39.4%; MOCE) individuals. Only 11 species (24.4%) occurred in all sites sampled, seven species (15.6%) occurred in at least three sites, while 13 species (28.9%) were restricted to one site. Despite having fewer samplings, the TSPK had the second highest total abundance (N = 1901). The sample coverage ranged between 0.967 and 0.999, indicating a good accuracy of inventories.
Dung beetle composition varied among sites (PERMANOVA: R 2 = 16.5%; F = 3.705; P-value = 0.001; fig. 1) and between regions (PERMANOVA: R 2 = 9.7%; F = 4.348; P-value = 0.001). No differences in the variances of groups were found (PERMDISP(sites): F = 2.893; P-value = 0.097; PERMDISP(region): F = 1.486; P-value = 0.231).
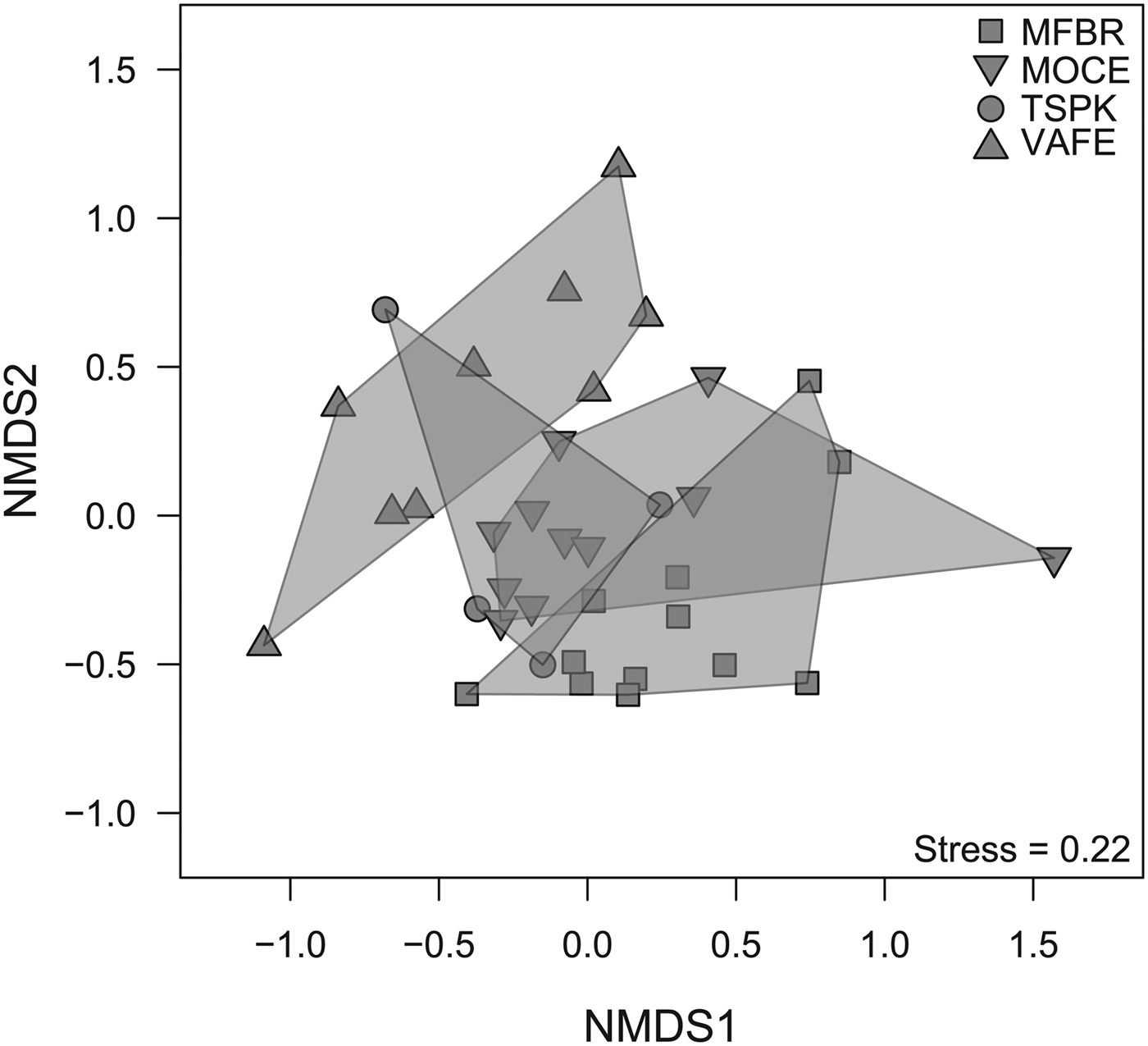
Fig. 1. Non-metric multidimensional scaling of dung beetle community composition (based on incidence) among sites sampled. MFBR, Moreno Fortes Biological Reserve; MOCE, Morro do Cerrito; TSPK, Turvo State Park; VAFE, Val Feltrina.
There was an opposite response pattern between results of the multiplicative partitioning of species richness (0D) and Shannon diversity (1D) (Table 1). The site with lower species richness (VAFE) had the highest Shannon diversity value, while the site with the second highest value of observed richness (TSPK) had the lowest Shannon diversity value. The Shannon β-diversity was 1.66, indicating there were almost one and a half ‘true’ dung beetle assemblages regarding the spatial distribution of species weighed by abundance.
Table 1. Multiplicative partition of dung beetle diversity in the Atlantic Forest sites.
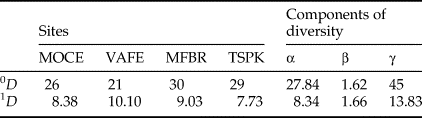
MFBR, Moreno Fortes Biological Reserve; MOCE, Morro do Cerrito; TSPK, Turvo State Park; VAFE, Val Feltrina.
The total spatial dissimilarity was 67.4% among all sites sampled. The turnover component accounted for 90% of the dissimilarity, while nestedness accounted only for 10%. The average seasonal dissimilarity was 77% (±0.06 SD; range 71.3–85.5%) and turnover had higher values (74.8% ± 0.1 SD; range 60.9–85.1%) than nestedness (25.2% ± 0.1 SD; range 14.9–39.1%).
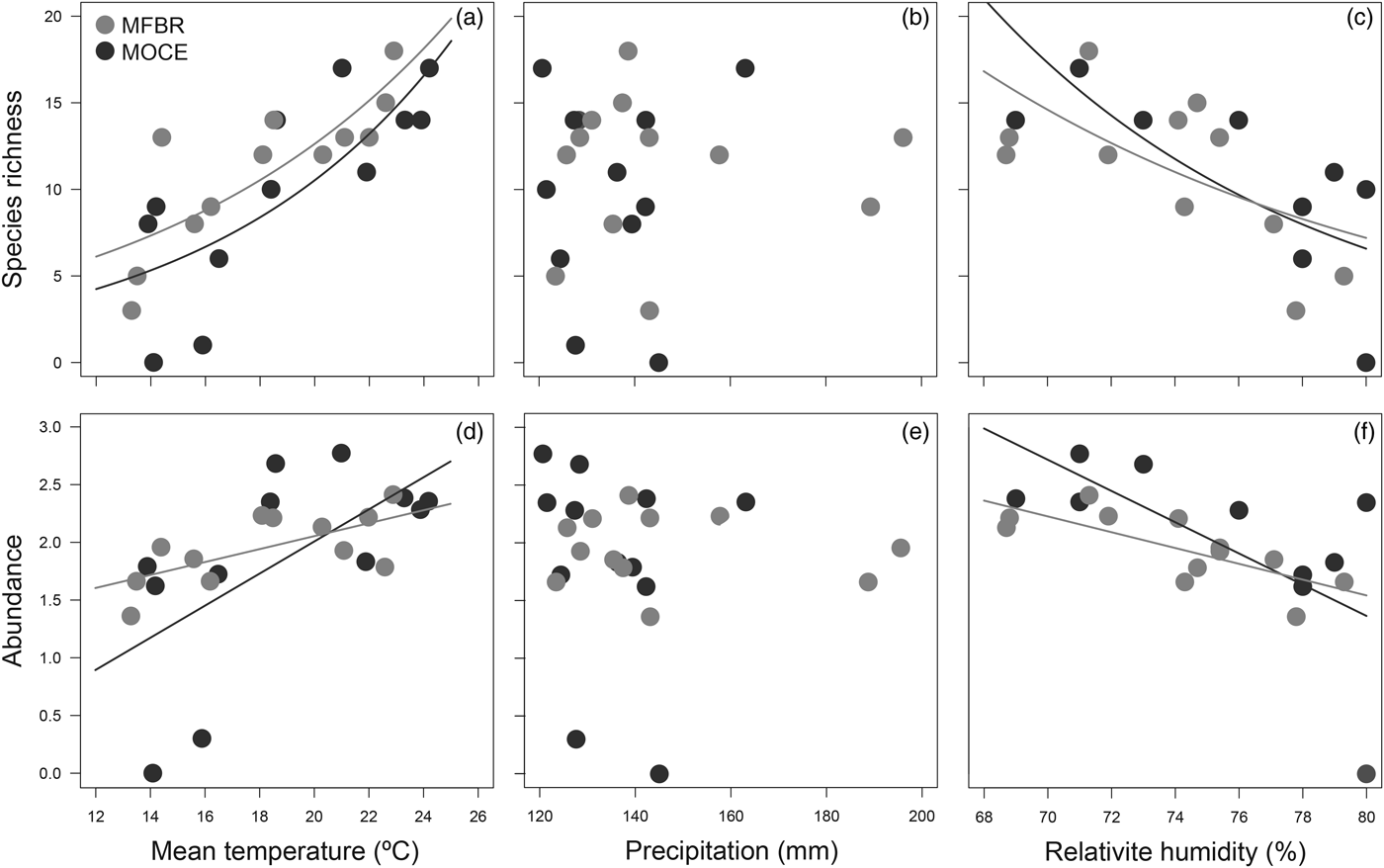
GLM between monthly species richness and abundance of dung beetles and climatic variables revealed a strong influence of mean temperature and relative humidity on species richness and abundance (Table 2). For MOCE and MFBR, there was a positive relationship between community metrics and mean temperature and a negative relationship between community metrics and relative humidity (fig. 2). Precipitation did not affect temporal patterns of dung beetle richness and abundance.

Fig. 2. Relationship between monthly dung beetle community metrics (species richness and abundance) and climatic variables [mean temperature (a, d), precipitation (b, e), and relative humidity (c, f)]. MFBR, Moreno Fortes Biological Reserve; MOCE, Morro do Cerrito.
Table 2. Generalized linear models between dung beetle community metrics (species richness and abundance) and climatic variables (mean temperature, precipitation, and relative humidity).
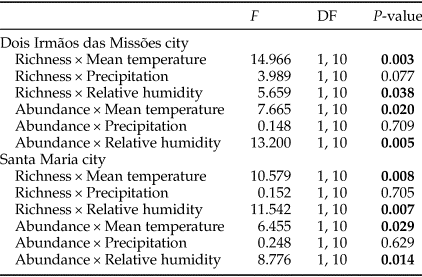
F, test value; DF, degrees of freedom.
P-values <0.05 are in bold.
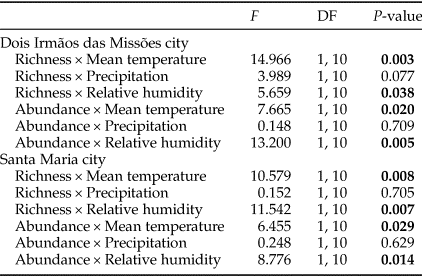
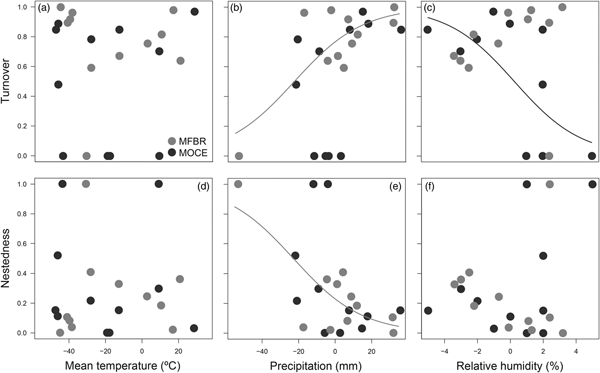
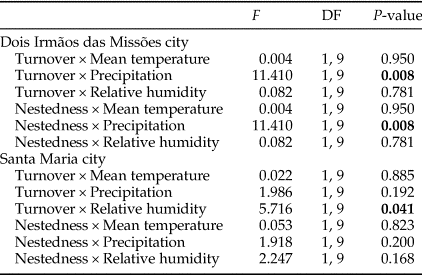
The difference in precipitation and relative humidity values between consecutive months influenced the temporal patterns of turnover and nestedness of dung beetles (Table 3). However, temporal patterns were not consistent between MFRB and MOCE. In MRFB, the turnover increased and nestedness decreased when there was a decrease in precipitation (i.e. positive values) between consecutive months (fig. 3b, e). In MOCE, the turnover decreased with a negative increase (i.e. positive values) in relative humidity between consecutive months. Higher values of turnover were found when there was an increase in relative humidity between consecutive months (fig. 3c). Changes in relative humidity did not affect patterns in nestedness in this site (fig. 3f).

Fig. 3. Relationship between temporal patterns in turnover (upper panels) and nestedness (lower panels) of dung beetles and differences in values of mean temperature (a, d), precipitation (b, e), and relative humidity (c, f). MFBR, Moreno Fortes Biological Reserve; MOCE, Morro do Cerrito. Positive values indicate there was a decrease in the climatic variable, while negative values indicate there was an increase in the climatic variable between consecutive months.
Table 3. Generalized linear models between temporal patterns in turnover and nestedness and differences in climatic variable values (mean temperature, precipitation, and relative humidity) between consecutive months.
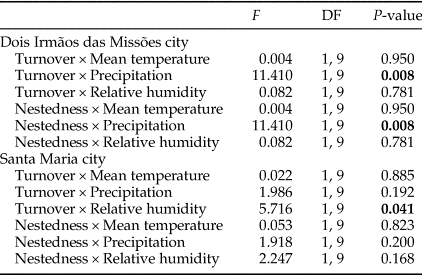
F, test value; DF, degrees of freedom.
The analysis was performed only for MOCE and MFRB.
P-values <0.05 are in bold.
Discussion
Our results highlighted five main findings. First, dung beetle composition varied more among sites than among site geographic position. There were almost one and a half ‘true’ dung beetle assemblages regarding the spatial distribution of species weighed by their abundance. We found a positive influence of mean temperature and a negative influence of relative humidity on both species richness and abundance. Both spatial and temporal dissimilarity among sites were dominated by species replacement; the relative importance of nestedness was higher in temporal than spatial patterns. Finally, there was an effect of precipitation and relative humidity on temporal patterns of β-diversity components, but these effects were site-dependent.
The percentage of explanation of the compositional dissimilarity among sites was higher than that found among regions. The diversity partitioning into spatial scales (α, β, and γ) revealed one and a half ‘true’ dung beetle assemblages, even though both pairs of sites (at the northwest and central regions) were more than 275 km apart. Therefore, there is a set of species shared by all sites, while the remaining dissimilarity is mainly due to species replacement. These results suggest that differences in environmental conditions or resource availability between sites are more important driving the distribution of dung beetle species than spatial effects, even in large spatial extensions (da Silva, Reference da Silva2014; da Silva & Hernández, Reference da Silva and Hernández2014, Reference da Silva and Hernández2015b). Due to high fragmentation and habitat loss of the Atlantic Forest in southern Brazil (Ribeiro et al., Reference Ribeiro, Metzger, Martensen, Ponzoni and Hirota2009), only a small part of the dung beetle assemblage remains in all sites sampled. Our results reveal that less than 25% of the species occur in all sites and several studies highlighted similar patterns (da Silva & Hernández, Reference da Silva and Hernández2014; Bitencourt & da Silva, Reference Bitencourt and da Silva2016; da Silva & Hernández, Reference da Silva and Hernández2016). Since the investigation of the factors underlying the spatial distribution of dung beetles is not the scope of this study, this topic remains important to be inquired to elucidate the high spatial substitution of species even at relatively close sites.
Climatic conditions play a major role in driving the spatiotemporal distribution of dung beetles. Several studies have found strong correlations between dung beetle community metrics and both temperature and relative humidity (Hernández & Vaz-de-Mello, Reference Hernández and Vaz-de-Mello2009; da Silva et al., Reference da Silva, Vaz-de-Mello and Di Mare2013; Nunes et al., Reference Nunes, Braga, Figueira, Siqueira-Neves and Fernandes2016). Temporal changes in temperature are associated with vegetation structure changes and resource availability, and its effects are especially stronger in poikilothermic and heterothermic insects, such as dung beetles (Schowalter, Reference Schowalter2011). Very low temperatures (<10°C) impair dung beetle flight and activity since several species require a minimum temperature for the flight of around 25°C (Verdú et al., Reference Verdú, Arellano and Numa2006). On the other hand, the negative relationship found between relative humidity and both species richness and abundance is explained by the negative correlation of this variable with temperature. In subtropical regions such as our study area, higher values of relative humidity are found during winter (mean 78.6 ± 1.5%; summer: mean 71.8 ± 0.3%).
Investigating the dung beetle seasonality in subtropical Brazil, da Silva et al. (Reference da Silva, Vaz-de-Mello and Di Mare2013) also found no effect of precipitation on dung beetle community metrics. However, decreasing precipitation between consecutive months was an important driver of β-diversity components. Although there is no marked dry season in subtropical Brazil, months of heavy rainfall can be followed by months with very low rainfall and vice-versa, regardless of the season. The month-to-month decrease in rainfall causes a high pattern of species replacement, which occurred at the beginning of spring in our region. Contrary, increased values of month-to-month rainfall occurred during winter when there is a poor and nested dung beetle assemblage from winter to spring. The precipitation indeed plays a major role driving the variation in species richness in tropical regions due to the existence of two marked seasons, dry and rainy (Andresen, Reference Andresen2005; Neves et al., Reference Neves, Oliveira, Espírito-Santo, Vaz-de-Mello, Louzada, Sanchez-Azofeifa and Fernandes2010). In subtropical regions, there is no marked dry season. Therefore, there is a change in the relative importance of temperature and precipitation with increasing latitude driving dung beetle community patterns.
Contrary to our hypothesis, the turnover component played a major role than nestedness driving the temporal dung beetle dissimilarity. We expected that most dung beetle species would occur throughout the year, with a nested subsample of summer species occurring during winter. However, on average, there is a high species substitution among seasons than species gain/loss. Our results, therefore, corroborate the findings of Soininen et al. (Reference Soininen, Heino and Wang2018), who have proved that species replacement is the main component of spatial β-diversity. It seems that temporal patterns in β-diversity components are mostly driven by species substitution as well (da Silva, Reference da Silva2018).
Temporal values of turnover and nestedness were influenced by differences in climatic variables from consecutive months, contrary to our second hypothesis. In addition, there was no general pattern of the effect of climatic variables in β-diversity components among sites over time. Although the results obtained with the use of historical climatic variables instead of in situ climatic measures should be taken with caution, these results revealed that monthly differences in values of these variables affect the temporal pattern of β-diversity components. Our results indicate when there is a successional increase in relative humidity, almost no species substitution occurs. When there is a successional decrease in relative humidity, there is an increase in species replacement. For precipitation, there is an opposite pattern; the species substitution is higher when rainfall increases from month to month, while the nestedness pattern decreases in these conditions. When rainfall is decreasing from month to month, there is a strong nested loss of species. The lack of a general pattern in these results may indicate that the effects of climatic variables on components of β-diversity are context-dependent. Our results suggest that such effects exist and this ecological issue deserves further investigation in future studies on mechanisms driving the temporal patterns of β-diversity.
In summary, we found that historical climatic variables (i.e. mean temperature, precipitation, and relative humidity) representing average climatic conditions of the sites sampled affected the monthly species richness, abundance and temporal β-diversity components of turnover and nestedness of subtropical dung beetle assemblages. Temperature and relative humidity drive dung beetle α-diversity, while precipitation and relative humidity drive β-diversity components temporally. Species substitution is the main driver of spatial and temporal β-diversity. Unlike static snapshots of community data (i.e. spatial comparisons based on one or very few temporal samplings), performing samplings over time enables the investigation of ecological processes underpinning the local extinction-colonization dynamics of metacommunities. Therefore, the partitioning of β-diversity into temporal components is a promising approach to unveil patterns of the community dynamics and to produce insights on mechanisms underlying such patterns.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0007485318000676.
Acknowledgements
The authors would like to thank Fernando Vaz-de-Mello (Universidade Federal de Mato Grosso) for dung beetle identification and reviewers for valuable suggestions. They thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the scholarship to SCF and PGdS, and the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) for permission to collect specimens (permit #54137-1).