Introduction
Major depression with psychotic features (hereafter psychotic depression, PD) is a severe disorder with a high risk of recurrence and high mortality in both adult samples under 60 years (Lykouras & Gournellis, Reference Lykouras and Gournellis2009) and older people samples (Gournellis et al. Reference Gournellis, Oulis and Howard2014). In spite of the severe course of illness, there seems to be some difficulty identifying the disorder in clinical settings (Rothschild et al. Reference Rothschild, Winer, Flint, Mulsant, Whyte, Heo, Fratoni, Gabriele, Kasapinovic and Meyers2008).
Originally, Kraepelin (Goodwin & Jamison, Reference Goodwin and Jamison2007) considered PD as a type of manic-depressive illness. In the post-kraepelinian era, it has been classified among unipolar major depressive disorders. In International Classification of Diseases, 10th Edition (ICD-10) PD is considered the most severe subtype of major depressive disorder (WHO, 1992), whereas in Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) psychotic features are not an indicator of severity of major depression (APA, 2013). Due to a number of differences between PD and non-psychotic depression (hereafter NPD), it has for long been proposed that PD should be considered a distinct disease entity (Schatzberg & Rothschild, Reference Schatzberg and Rothschild1992; Keller et al. Reference Keller, Schatzberg and Maj2007).
The point prevalence of PD is estimated to be approximately 0.4%, with older adults being in the highest risk (Kivelä & Pahkala, Reference Kivelä and Pahkala1989; Perälä et al. Reference Perälä, Suvisaari, Saarni, Kuoppasalmi, Isometsä, Pirkola, Partonen, Tuulio-Henriksson, Hintikka, Kieseppä, Härkänen, Koskinen and Lönnqvist2007). The prevalence of psychotic features in the adolescent outpatient major depression sample was 18% (Ryan et al. Reference Ryan, Puig-Antich, Ambrosini, Rabinovich, Robinson, Nelson, Iyengar and Twomey1987) while the same figure was 45% in a hospitalized adolescent patient sample (Haley et al. Reference Haley, Fine and Marriage1988). There is a lack of information concerning the risk factors for PD. Previous studies have often studied all affective psychosis, i.e. included bipolar disorder or studied PD as part of all major depressive disorders. Though there are marked similarities in PD and NPD risk factors, some differences are likely to exist. There is also considered a close link between PD and bipolar disorder (Keller et al. Reference Keller, Schatzberg and Maj2007; Østergaard et al. Reference Østergaard, Waltoft, Mortensen and Mors2013). There are no previous systematic reviews on incidence, prevalence, or risk factors of PD.
Clinical course of illness in PD is more severe than in NPD. This applies especially in the short-term outcome, but it has been suggested that in a longer follow-up, the significance of psychotic features might fade (Keller et al. Reference Keller, Schatzberg and Maj2007; Lykouras & Gournellis, Reference Lykouras and Gournellis2009). However, mortality is significantly higher in PD compared with NPD (Vythilingam et al. Reference Vythilingam, Chen, Bremner, Mazure, Maciejewski and Nelson2003), although there are conflicting findings (Suvisaari et al. Reference Suvisaari, Partti, Perälä, Viertio, Saarni, Lönnqvist, Saarni and Härkänen2013). The functional outcome has been suggested to be mostly better in PD compared with schizophrenia (SZ), and differences in outcomes between PD and psychotic bipolar disorder (PBD) have been unclear (Craig et al. Reference Craig, Bromet, Fennig, Tanenberg-Karant, Lavelle and Galambos2000; Jarbin et al. Reference Jarbin, Ott and Von Knorring2003; Keller et al. Reference Keller, Schatzberg and Maj2007).
Huge amount of data and meta-analyses have been published on NPD, SZ, and bipolar disorder, while PD as an own entity has received much smaller attention (Crebbin et al. Reference Crebbin, Mitford, Paxton and Turkington2008). Meanwhile, there has been a concern over the validity of PD diagnosis mainly due to diagnostic instability (Ruggero et al. Reference Ruggero, Kotov, Carlson, Tanenberg-Karant, Gonzalez and Bromet2011). There are some meta-analyses and reviews on pharmacological treatments (Wijkstra et al. Reference Wijkstra, Lijmer, Burger, Cipriani, Geddes and Nolen2015), cognition (Fleming et al. Reference Fleming, Blasey and Schatzberg2004; Zaninotto et al. Reference Zaninotto, Guglielmo, Calati, Ioime, Camardese, Janiri, Bria and Serretti2015), genetics (Domschke, Reference Domschke2013), neuroimaging studies (Busatto, Reference Busatto2013), cortisol non-suppression (Nelson & Davis, Reference Nelson and Davis1997), and PD in old age (Gournellis et al. Reference Gournellis, Oulis and Howard2014). Lykouras & Gournellis (Reference Lykouras and Gournellis2009) present a comprehensive review on neurobiology, treatments, epidemiology, course of illness, and outcomes of PD in comparison to NPD. However, they have not reported their results systematically, and some topics such as risk factors have not been studied. Earlier reviews presenting epidemiology of PD (Schatzberg & Rothschild, Reference Schatzberg and Rothschild1992; Gournellis & Lykouras, Reference Gournellis and Lykouras2006; Lykouras & Gournellis, Reference Lykouras and Gournellis2009) have not combined the data by meta-analytic means and they have compared their findings only to NPD.
Aims
Our aim was to perform a systematic review on epidemiology, especially incidence and prevalence, risk factors, and outcomes of PD. We also aimed to do a meta-analysis on sex differences, onset age, and outcome of PD in comparison to NPD, SZ, PBD, and schizoaffective disorder (SZAFF).
Methods
Data collection
We applied the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines for systematic reviews and meta-analyses (Moher et al. Reference Moher, Liberati, Tetzlaff and Altman2009).
In order to locate potentially suitable studies, we conducted database searches in May 2016 using four electronic databases: PubMed, Scopus, Web of Science, and CINAHL. The used search terms were the following: (‘psychotic depression’ OR ‘delusional depression’ OR ‘depression with psychotic features’) AND (epidemiology OR ‘risk factor’ OR outcome OR employment OR occupational OR progression OR course OR stability OR relapse OR remission OR prevalence OR incidence OR ‘onset age’ OR ‘diagnostic stability’ OR mortality OR suicide OR physical OR somatic OR comorbidity OR ‘early intervention’ OR prevention). No publication date exclusions were used in the search. Articles were also searched manually from the reference lists of the previous reviews.
All abstracts were independently analyzed by two authors (HK and EJ). After the exclusion of irrelevant abstracts, all remaining articles were critically inspected by two authors (EJ or JM). For studies that met inclusion criteria, a third investigator (HL or TJ) independently extracted the data, and the collected data were checked by two authors (JM or EJ). When a disagreement occurred related to data extraction, this was resolved by consensus.
Study selection
Studies on prevalence and incidence were included if these were estimated from population surveys or used both inpatient and outpatient data to estimate prevalence or incidence.
Regarding studies on risk factors and outcomes in PD, to be included in the analyses, the studies had to be characterized by all of the following:
-
(1) Original study included a sample of PD. Also studies including only delusional depression were included, as the early studies on the topic often used only this definition. The sample had to include at least 80% of PD. Studies focusing on psychotic depressive episode of SZ or PBD, or studies with postpartum PD were not included.
-
(2) Diagnostic assessment and diagnostic criteria of PD were based on a commonly used diagnostic system or were otherwise adequately reported.
-
(3) The sample size of PD was at least 15.
-
(4) Studies presented risk or sociodemographic factors, or outcomes of PD.
-
(5) Studies of risk factors and outcomes had to include a comparison group of NPD, PBD, SZ, SZAFF, or healthy controls (HC) without mental disorder. The size of the comparison group had to be at least 15 and the comparison group had to include at least 80% of NPD, PBD, SZ, or SZAFF.
-
(6) The majority of subjects had onset age after 16 years.
Only observational (naturalistic) studies were included, whereas trials and intervention studies were excluded. While many intervention studies report clinical outcomes, the representativeness of these samples may vary widely according to the specific trial inclusion criteria. Thus, a large number of randomized controlled trials were excluded. To the current review, we finally included only studies published in English. In addition, studies analyzing neurobiological risk factors and correlates, treatments, mortality, suicides, and somatic comorbidities were excluded as being either out of the scope of this review (studies on treatments, neurobiology, somatic comorbidities) or being recently studied (mortality and suicides in PD; Lykouras & Gournellis, Reference Lykouras and Gournellis2009; Rothschild, Reference Rothschild2009; Zalpuri & Rothschild, Reference Zalpuri and Rothschild2016).
Incidence, prevalence, and risk factor studies
Studies on incidence and prevalence were reported with a systematic review. Regarding gender distribution and onset age, we pooled studies using meta-analytic methods. Other risk factors were reported only narratively and in a literature table. The included risk factors encompassed both early risk factors and sociodemographic factors, such as marital status and education, collected at study entry.
Outcome studies
Of studies analyzing outcomes of PD, studies analyzing the severity of psychotic symptoms (positive, negative, total symptoms), severity of depression symptoms, number of hospitalizations during prospective follow-up, symptomatic remission, global clinical outcome, global outcome, and occupational functioning were included. Global clinical outcome indicates outcome measured by the presence of clinical symptoms and severity of illness, without a specific instrument for the measurement. Global outcome indicates the outcome measured by Social and Occupational Functioning Assessment Scale (SOFAS), Global Assessment Scale (GAS), or Global Assessment of Functioning scale (GAF). Please see our earlier meta-analyses for the definitions of different outcome dimensions (Käkelä et al. Reference Käkelä, Panula, Oinas, Hirvonen, Jääskeläinen and Miettunen2014; Penttilä et al. Reference Penttilä, Jääskeläinen, Hirvonen, Isohanni and Miettunen2014). Based on the number of studies (at least three studies from different samples per outcome), meta-analysis was performed on depression symptoms, total psychotic symptoms, positive and negative symptoms, global outcome, symptomatic remission, and poor global clinical outcome. For the meta-analysis, we selected symptoms measured at the baseline of studies, since this was the most common time of assessment of symptoms. In meta-analysis, outcomes were compared between PD and NPD, SZ, and PBD when data were available. Systematic review (without meta-analysis) was done for hospitalizations and occupational functioning.
Statistical analyses
Random-effects models were used in order to pool estimates of effect sizes between PD and comparison groups in the meta-analyses of gender, onset age, symptoms, symptomatic remission, global clinical outcome, and hospitalizations, based on the expected heterogeneity of the associations. Meta-analyses were done if at least three studies investigated same outcome. In the random-effects analysis, each study was weighted by the inverse of its variance and the between-studies variance. In continuous variables (onset age and symptoms), the effect size of the standardized mean difference between groups was described with Hedges’ g. Hedges’ g values is comparable with Cohen's d but recommended with small sample sizes. It can be interpreted as small 0.20, moderate 0.50, and large 0.80 effects (Cohen, Reference Cohen1992). In categorical variables, pooled effect size was estimated using Relative Risk (RR) with 95% confidence interval (CI). When the number of studies allowed, we checked the results of meta-analyses in the subgroups of studies based on publication year (1973–1991, 1993–2003, and 2004–2016), mean study age (below 45, 45–55, and above 55 years), or mean age of illness onset (below 45 v. 45 or above). In addition, as a sensitivity analyses, we performed analyses in strata by sample size (studies under 50 cases v. at least 50 cases with PD). In the current study, positive g values indicate that individuals with PD have more symptoms or later onset age than comparison group. Where multiple articles were available on the same or overlapping samples and presenting similar data, we selected one representative paper with the largest sample size or presenting outcomes measured by a more commonly used instrument for the meta-analysis. We assessed the heterogeneity of the studies using I 2 statistics, and the statistical significance in heterogeneity was tested using the χ2 test. Values of I 2 range from 0% to 100%, reflecting the proportion of total variation across studies beyond chance. A value of 25% describes low, 50% moderate, and 75% high heterogeneity (Higgins et al. Reference Higgins, Thompson, Deeks and Altman2003). An α level of 0.05 was used for all statistical tests. The metan command of the Stata version 13 (Sterne, Reference Sterne2009; StataCorp, 2013) was used in all analyses.
Results
Characteristics of the studies
Database searches identified 2926 records, which reduced to 1764 after the removal of duplicates. After analyzing the abstracts, we were left with 279 articles that potentially fulfilled our inclusion criteria. The most common reason for exclusion during abstract screening was that the article did not present results separately to PD. Figure 1 shows the flow diagram that details the exclusion criteria after abstract reading. In total, 99 studies met all of our criteria regarding incidence/prevalence, risk factors or outcome, and were included in the systematic review. The studies included nine studies from manual search.
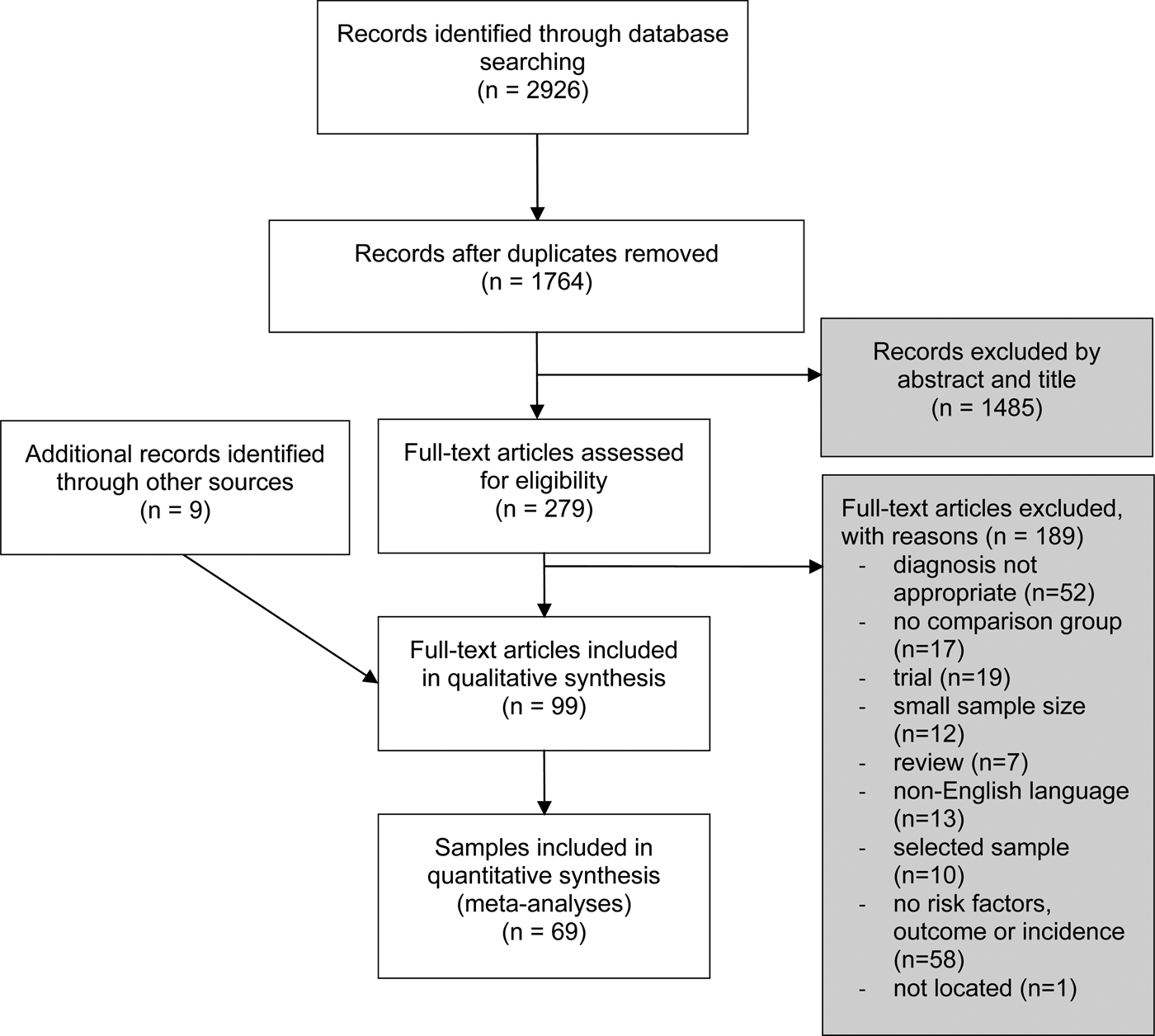
Fig. 1. Flow diagram of the selection of studies.
Incidence and prevalence
Studies reporting prevalence or incidence estimates in community samples and using estimates based on inpatient and outpatient data have been collected into the online Supplementary Table S1.
Only four studies estimated prevalence in community samples, trying also to detect cases not in treatment using different screening methods. In the nationally representative Finnish Health 2000 sample, Perälä et al. (Reference Perälä, Suvisaari, Saarni, Kuoppasalmi, Isometsä, Pirkola, Partonen, Tuulio-Henriksson, Hintikka, Kieseppä, Härkänen, Koskinen and Lönnqvist2007) found a lifetime prevalence of 0.35% for DSM-IV PD. The prevalence was higher among those who were 65 years or more (0.43%) when compared with younger age groups; however, differences were not statistically significant. In the US Epidemiological Catchment Area (ECA) study, Johnson et al. (Reference Johnson, Horwath and Weissman1991) reported a lifetime prevalence of DSM-III PD to be 0.6%. In an older community-based study of those with 60 years or more, the prevalence was 1.0% (0.6% for males, 1.2% for females) (Kivelä & Pahkala, Reference Kivelä and Pahkala1989). In a large European telephone survey in five countries, an overall point prevalence for DSM-IV PD was 0.5%, and significantly higher rates were reported for females (0.6%) than males (0.3%) (Ohayon & Schatzberg, Reference Ohayon and Schatzberg2002).
Five studies used different in- and outpatient admission registers. Estimates for annual incidence (per 100 000 persons) were reported in three studies. In a British study, Farquhar et al. (Reference Farquhar, Le Noury, Tschinkel, Harris, Kurien and Healy2007) reported an incidence of 3.4 in the year 1875–1924 and 3.0 in 1995–1999. In an Irish study, Baldwin et al. (Reference Baldwin, Browne, Scully, Quinn, Morgan, Kinsella, Owens, Russell, O'Callaghan and Waddington2005) found an incidence of 6.4 (males 5.4 and females 7.4), similar estimates (6.0 for those with 16 years and over) were also in a British study by Reay et al. (Reference Reay, Mitford, McCabe, Paxton and Turkington2010). A Finnish study comparing two birth cohorts (from 1966 and 1986) found a substantial increase in cumulative incidence until age 27 years in the later cohort (0.02% v. 0.21%) (Filatova et al. Reference Filatova, Marttila, Koivumaa-Honkanen, Nordström, Veijola, Mäki, Khandaker, Isohanni, Jääskeläinen, Moilanen and Miettunen2017).
Gender differences have been reported in some incidence and prevalence studies. In three studies, females had higher estimates for incidence (Kivelä & Pahkala, Reference Kivelä and Pahkala1989; Ohayon & Schatzberg, Reference Ohayon and Schatzberg2002; Baldwin et al. Reference Baldwin, Browne, Scully, Quinn, Morgan, Kinsella, Owens, Russell, O'Callaghan and Waddington2005); however, in one study, higher lifetime prevalence was reported for males (0.41%) than females (0.29%) (Perälä et al. Reference Perälä, Suvisaari, Saarni, Kuoppasalmi, Isometsä, Pirkola, Partonen, Tuulio-Henriksson, Hintikka, Kieseppä, Härkänen, Koskinen and Lönnqvist2007).
Proportion of psychosis in depression
In studies (n = 43) including both PD and NPD patients, the median proportion of PD patients was 28% of all depressive patients. Median proportion was lower in studies with mean age below 45 years (20%, n = 16) than in the middle age samples (27%, n = 11) or in older samples (34%, n = 10). The median proportion of PD in depression patients was 29% among females and 26% among males. In the studies including only depressive inpatients (n = 22), the median proportion of those with PD was 42%, whereas in the studies including both in- and outpatients or only outpatients (n = 21), corresponding proportion was 19%.
Gender differences in PD when compared with other patient samples
We compared gender distributions in the included studies on PD and patient control groups. In total, 57 studies compared PD with other included patients samples. The median number of PD patients was 45 in these studies, whereas the total number of PD patients was 28 370. In total, 43 studies compared gender distributions between PD and NPD, pooled RR being 1.03 (95% CI 0.97–1.08). Estimates of RR were relatively similar when studies were divided by mean study age or year of publication. The estimated RRs for PD for females are presented in Fig. 2, for the total sample and by mean study age. Studies comparing PD and SZ and PBD found a higher proportion of females in PD than in SZ (14 studies; RR 1.40, 95% CI 1.20–1.71) or in PBD (three studies; RR 1.36, 95% CI 1.01–1.83). The median percentage of females in PD was 65%, in NPD 65%, in SZ 37%, in SZAFF 57%, and in PBD 55%. Proportion of females in PD did not vary significantly when studies were divided by mean study age or year of publication. Proportions of females in different patient groups in the included studies are presented in the online Supplementary Table S2.

Fig. 2. Forest plot for estimated or relative rates (RR) psychotic depression among females in depression patients. Studies are grouped and analyzed separately by mean study age.
Onset age in PD when compared with other patient samples
Eighteen studies compared onset age between PD and NPD in different samples. Based on meta-analysis, there was no significant difference between the groups (Hedges’ g = 0.08, p = 0.44). However, when we divided the studies into three categories based on mean study age, we found conflicting results. In the studies of the youngest subjects (below 45 years, n = 6), PD patients had earlier onset age (g = −0.39, p < 0.001), whereas in the studies among the oldest (above 55 years, n = 7) PD patients had later onset age (g = 0.40, p < 0.001) than NPD patients. The year of publication did not affect the results. A forest plot comparing onset age between PD and NPD by age groups and in the total sample is presented in Fig. 3. In the six studies comparing mean onset age between PD and SZ, five found earlier onset age in SZ, and pooled meta-analysis found significant difference (g = 0.53, p = 0.013). When we compared PD and PBD, PD patients had non-significantly later onset age (g = 0.34, p = 0.069). Mean onset ages in different groups in the included studies are presented in the online Supplementary Table S3.

Fig. 3. Forest plot comparing mean onset age between psychotic depression and non-psychotic depression. Studies are grouped and analyzed separately by mean study age.
Regarding comparison in onset age between PD and NPD, only six studies had sample size of at least 50. There were only two studies from each three age groups; however, all the statistically significant findings remained when compared with the original analyses.
Risk factors and sociodemographic factors
Studies on risk factors and sociodemographic factors in PD are summarized in the online Supplementary Table S4. In total, 36 studies were found.
Studies on early risk factors are rare. The only study analyzing risk factors from birth (place of birth, gestational age, birth weight, small for gestational age, maternal and paternal age at birth) was a large Danish register study that did not find any significant differences in these factors between PD and HC (Østergaard et al. Reference Østergaard, Waltoft, Mortensen and Mors2013). Physical and sexual trauma was more likely in PD than in NPD in one study (84% v. 64%, p = 0.017) (Gaudiano & Zimmerman, Reference Gaudiano and Zimmerman2010), but not in one (Gaudiano et al. Reference Gaudiano, Weinstock, Epstein-Lubow, Uebelacker and Miller2016). Other premorbid factors linked with PD when compared with NPD were rural domicile (Ihezue, Reference Ihezue1985), acute medical problems (Draper & Anstey, Reference Draper and Anstey1996), and poorer social competence score (Sands & Harrow, Reference Sands and Harrow1995). When compared with HC, PD patients differed in the number of physical anomalies (Čulav-Sumić & Jukić, Reference Čulav-Sumić and Jukić2010) and also a loss of mother because of an unnatural cause after age 15 years associated with PD (Østergaard et al. Reference Østergaard, Waltoft, Mortensen and Mors2013). Ethnicity was studied in eight articles. Individuals with PD were less likely to be Caucasian in five different studies (Johnson et al. Reference Johnson, Horwath and Weissman1991; Goldberg & Harrow, Reference Goldberg and Harrow2005; Gaudiano et al. Reference Gaudiano, Dalrymple and Zimmerman2009; Gaudiano & Zimmerman, Reference Gaudiano and Zimmerman2010; Gaudiano et al. Reference Gaudiano, Weinstock, Epstein-Lubow, Uebelacker and Miller2016). The British study by Heslin et al. (Reference Heslin, Desai, Lappin, Donoghue, Lomas, Reininghaus, Onyejiaka, Croudace, Jones, Murray, Fearon, Doody, Dazzan, Fisher, Demjaha, Craig and Morgan2016a ) found that the PD patients had less contact with friends, and they were more likely to have childhood adversity of neurological soft signs when compared with HC.
The family history of different psychiatric illnesses and suicides was analyzed in 14 articles. Most of the associations were non-significant. When PD patients were compared with NPD, they more often had a family history of psychosis (Buoli et al. Reference Buoli, Caldiroli and Altamura2013) and bipolar I disorder (Maj et al. Reference Maj, Pirozzi, Magliano, Fiorillo and Bartoli2007). One study also found a higher likelihood of any mental illness in relatives (Okulate et al. Reference Okulate, Oladapo and Osibogun2001), whereas two other studies did not find differences (Frangos et al. Reference Frangos, Athanassenas, Tsitourides, Psilolignos and Katsanou1983; Nakamura et al. Reference Nakamura, Iga, Matsumoto and Ohmori2015). Studies looking at the family history of affective or depressive disorders did not find differences between PD and NPD (Frangos et al. Reference Frangos, Athanassenas, Tsitourides, Psilolignos and Katsanou1983; Parker et al. Reference Parker, Hadzi-Pavlovic, Hickie, Mitchell, Wilhelm, Brodaty, Boyce, Eyers and Pedic1991; Simpson et al. Reference Simpson, Baldwin, Jackson and Burns1999; Park et al. Reference Park, Choi, Kim, Jun, Lee, Kim, Yim and Park2014). The large Danish register study by Østergaard et al. (Reference Østergaard, Waltoft, Mortensen and Mors2013) found several maternal, paternal, and sibling psychiatric diagnoses to associate significantly with PD when compared with HC, the highest risk (incidence rate ratio of 2.2) being in any maternal mental disorder. A recent study in the UK also found the family history of any mental illness or psychosis to associate with PD (Heslin et al. Reference Heslin, Desai, Lappin, Donoghue, Lomas, Reininghaus, Onyejiaka, Croudace, Jones, Murray, Fearon, Doody, Dazzan, Fisher, Demjaha, Craig and Morgan2016a ).
Educational level or years of education between PD and other patient groups was compared in 18 studies. Differences were mainly non-significant. PD patients had less education when compared with PBD in one study (Breslau & Meltzer, Reference Breslau and Meltzer1988). When PD patients were compared with NPD, they had lower education in six studies (Ihezue, Reference Ihezue1985; Karaaslan et al. Reference Karaaslan, Gonul, Oguz, Erdinc and Esel2003; Goldberg & Harrow, Reference Goldberg and Harrow2005; Gaudiano et al. Reference Gaudiano, Dalrymple and Zimmerman2009; Gaudiano & Zimmerman, Reference Gaudiano and Zimmerman2010; Heslin et al. Reference Heslin, Lappin, Donoghue, Lomas, Reininghaus, Onyejiaka, Croudace, Jones, Murray, Fearon, Doody, Dazzan, Craig and Morgan2016b ) but more years of education in one study (Park et al. Reference Park, Choi, Kim, Jun, Lee, Kim, Yim and Park2014). In the ECA study, PD patients had lower socioeconomic status when compared with NPD (Johnson et al. Reference Johnson, Horwath and Weissman1991). Marital status between PD and other patient groups was compared in 19 studies. Differences were mainly non-significant although two studies found PD patients to be more often single than NPD patients (Baldwin, Reference Baldwin1995; Gaudiano et al. Reference Gaudiano, Weinstock, Epstein-Lubow, Uebelacker and Miller2016) and in one study PD patients were less often single when compared with SZ patients (Heslin et al. Reference Heslin, Lappin, Donoghue, Lomas, Reininghaus, Onyejiaka, Croudace, Jones, Murray, Fearon, Doody, Dazzan, Craig and Morgan2016b ).
Outcomes in PD
Study characteristic and quality
The studies included in outcome review are described in online Supplementary Table S5. We found altogether 44 articles presenting results from 37 separate studies. Several studies did not report characteristics of PD group in detail (online Supplementary Table S5). The sample sizes of PD varied between 16 and 190. Fourteen of the studies had sample size of at least 50. In 25 of the studies, there were more females than males. Twenty-five studies included patients with onset age before 45 years of age (or if age of onset not reported, the sample was under 45 years at the study moment). Most of the studies (n = 16) were cross-sectional, and 13 studies had over 5 years follow-up. Study populations were mostly mixed samples (n = 25), with minority being first episode (n = 10) and consecutive samples (n = 2). Outcomes were most frequently defined using validated scales, but in some studies, the scale or its use was not clearly reported. Most commonly studied outcomes were different symptoms, remission, and global clinical outcome.
Outcome compared with NPD
Based on meta-analysis (online Supplementary Figs S1a–f), compared with NPD, the symptoms of depression were more severe in PD (Hedges’ g = 0.52, p < 0.001). The difference in symptom severity was larger among three samples with onset age 45 years or older (g = 0.84, p = 0.004), but significant also in younger samples (g = 0.40, p = 0.005). Psychosis symptoms were more severe in PD (g = 0.89, p = 0.037). Symptomatic remission tended to be less common in PD though not statistically significantly (RR = 0.82, p = 0.052). There was no significant difference in the global clinical outcome or hospitalizations, though PD patients tended to have poorer outcomes. The global outcome (based on SOFAS, GAS, or GAF score) was somewhat worse in PD, but not statistically significant (g = −0.43, p = 0.065). Sensitivity analyses by sample size were performed for studies comparing depression symptoms, global outcome, symptomatic remission, and poor global clinical outcome in PD v. NPD. The results were mixed. Regarding depression symptoms, the difference in PD v. NPD was not statistically significant in larger samples (50 cases or more), and the results of global outcome remained non-significant. Regarding symptomatic remission and poor clinical outcome, the difference between PD and NPD was statistically significant in large samples (online Supplementary Figs S4a–d).
Based on systematic review (online Supplementary Table S5), the rate of relapses was higher in PD compared with NPD (Baldwin, Reference Baldwin1988; Copeland, Reference Copeland1983). In most of the studies analyzing occupational outcomes, individuals with PD had a somewhat poorer outcome compared with NPD (Coryell et al. Reference Coryell, Lavori, Endicott, Keller and VanEerdewegh1984; Coryell & Tsuang, Reference Coryell and Tsuang1985). However, there were also studies indicating similar occupational outcomes for PD and NPD (Jäger et al. Reference Jäger, Bottlender, Strauss and Möller2005; Rush et al. Reference Rush, Carmody, Ibrahim, Trivedi, Biggs, Shores-Wilson, Crismon, Toprac and Kashner2006; Park et al. Reference Park, Choi, Kim, Jun, Lee, Kim, Yim and Park2014). A good occupational outcome occurred in 60–79% of PD, and 68–78% on NPD (Coryell & Tsuang, Reference Coryell and Tsuang1985; Jäger et al. Reference Jäger, Bottlender, Strauss and Möller2005; Park et al. Reference Park, Choi, Kim, Jun, Lee, Kim, Yim and Park2014), and poor occupational outcome in 28% of PD and 19% of NPD (Coryell & Tsuang, Reference Coryell and Tsuang1985), and unemployment in 90% of PD and 81% of NPD (Rush et al. Reference Rush, Carmody, Ibrahim, Trivedi, Biggs, Shores-Wilson, Crismon, Toprac and Kashner2006). Based on only study analyzing full recovery (both symptomatic and functioning, Coryell et al. Reference Coryell, Tsuang and McDaniel1982), full recovery after 2–3 years of follow-up was more common in NPD (69%) than PD (40%).
Outcome compared with SZ
According to meta-analyses, when compared with SZ, there was no difference in severity of depression symptoms, but total psychosis symptoms (g = −0.77, p = 0.000) and positive (g = −0.81, p = 0.000) and negative symptoms (g = −0.89, p < 0.001) were significantly less severe in PD. Global outcome was better in PD (g = 0.80, p = 0.001) (online Supplementary Figs S2a–e). All but one of the samples in the meta-analyses included patients with mean onset age below 40 years. The rate of relapses was lower in PD (Craig et al. Reference Craig, Bromet, Fennig, Tanenberg-Karant, Lavelle and Galambos2000). Occupational functioning was better in PD, 60–79% of PD and 36–47% of SZ having a good occupational outcome, and 28–29% and 57–88% having poor, respectively (Coryell & Tsuang, Reference Coryell and Tsuang1985; Jarbin et al. Reference Jarbin, Ott and Von Knorring2003; Jäger et al. Reference Jäger, Bottlender, Strauss and Möller2005). Full recovery (both symptomatic and functioning, Coryell et al. Reference Coryell, Tsuang and McDaniel1982) after 2–3 years of follow-up was more common in PD (40%) than SZ (7%) (online Supplementary Table S5).
Outcome compared with PBD
Symptoms of depression did not differ. Negative symptoms (g = 0.65; p = 0.001) were more severe in PD. However, PD had less severe positive symptoms (g = −0.44; p = 0.046). There was no difference in global functioning between PD and PBD (online Supplementary Figs S3a–d). Rehospitalization rates were relatively similar in PD and PBD (Craig et al. Reference Craig, Bromet, Fennig, Tanenberg-Karant, Lavelle and Galambos2000). The unemployment rate was similar in PD and PBD (63% v. 53–69%) (Dell'Osso et al. Reference Dell'Osso, Pini, Cassano, Mastrocinque, Seckinger, Saettoni, Papasogli, Yale and Amador2002), as was functional recovery (32% v. 37%, Tohen et al. Reference Tohen, Strakowski, Zarate, Hennen, Stoll, Suppes, Faedda, Cohen, Gebre-Medhin and Baldessarini2000). Persons with PD were somewhat less often on a disability pension (29% v. 33%), and they were less often unemployed (7% v. 14%) (online Supplementary Table S5).
Outcome compared with SZAFF
Only a few studies comparing PD and SZAFF were found, and no meta-analysis could be performed. Symptomatic remission (Coryell et al. Reference Coryell, Keller, Lavori and Endicott1990; Opjordsmoen, Reference Opjordsmoen1991), and employment (Opjordsmoen, Reference Opjordsmoen1991) were more common in PD, but there was no difference in number of relapses at follow-up (Opjordsmoen, Reference Opjordsmoen1991). In one study, there was no marked difference in syndromatic recovery between PD and SZAFF, but PD subjects had more often functional recovery (Tohen et al. Reference Tohen, Strakowski, Zarate, Hennen, Stoll, Suppes, Faedda, Cohen, Gebre-Medhin and Baldessarini2000).
Discussion
Main results
Based on this systematic review, though not as common as, e.g. SZ, it seems that PD is relatively common, especially in older populations. However, this conclusion is based on a relatively few studies with varying methodology. Within depression, the onset age of PD was earlier than that of NPD in younger samples, but later in older samples. This may be due to PD at first episode being a marker of later bipolar disorder in younger samples. It seems that the proportion of PD is higher in inpatient samples. Based on this review, the median proportion of those with PD was 42% in inpatients, and 19% in outpatients. There was no difference in gender distribution in PD v. NPD, but higher proportion of females was found in PD than in SZ or in PBD. Risk factors have rarely been studied, and most of the findings were statistically non-significant. Family history of psychosis and bipolar I seems to increase the risk of PD.
To our knowledge, this is a first systematic review and meta-analysis comparing the outcomes of PD not only to NPD, but also to SZ, SZAFF, and PBD. Several outcomes of PD were mostly worse when compared with NPD, but better compared with SZ and SZAFF. The outcomes compared with PBD were relatively similar, though there were more negative and less positive symptoms in PD. The number of studies comparing PD to SZ and PBD, and especially SZAFF are very few.
See Table 1 for the summary of main results.
Table 1. Summary of the main results

Diagnoses: PD, psychotic depression; NPD, non-psychotic depression; PD, bipolar disorder; PBD, psychotic bipolar disorder; SZ, schizophrenia; SZAFF, schizoaffective disorder.
Clinical and public health implications
The number of studies on the epidemiology of PD are far from the large amount of studies on SZ (Matheson et al. Reference Matheson, Shepherd and Carr2014) or on unipolar depression in general (Hirschfeld, Reference Hirschfeld2012; Kessler & Bromet, Reference Kessler and Bromet2013) and on bipolar disorder (Sherazi et al. Reference Sherazi, McKeon, McDonough, Daly and Kennedy2006; Benazzi, Reference Benazzi2007; Esan & Esan, Reference Esan and Esan2016). Many of the risk factors reviewed in reviews on NPD and SZ have not been studied on PD at all or only in a few small samples. Based on our review, there is lack of studies on epidemiology, especially risk factors, and longitudinal clinical and functional outcomes in PD. This is in line with the general notion of lack of clinical trials focusing on PD (Wijkstra et al. Reference Wijkstra, Lijmer, Burger, Cipriani, Geddes and Nolen2015). In addition, both treatment algorithms and clinical practice regarding PD are highly heterogeneous. This emphasizes the need for further studies also on the treatment of PD (Leadholm et al. Reference Leadholm, Rothschild, Nolen, Bech, Munk-Jørgensen and Ostergaard2013).
Our review supports the earlier conclusions about more severe depression symptoms in PD compared with NPD, especially in older samples (Lykouras & Gournellis, Reference Lykouras and Gournellis2009). Most of the studies included in the meta-analysis included patients with relatively young age at the study moment, and thus the other results on symptoms and global outcome can be generalized only to this age group.
Our review summarizes the outcomes of PD in comparison to SZ and PBD. After our database searches, very recently, an AESOP study was published, where 10-year outcomes in PD compared with SZ and PBD patients were investigated. The study found only minimal differences in the outcome between PD and PBD. Differences in clinical, social, and service use outcomes between PD and SZ were more substantial with PD patients showing better outcomes on most variables (Heslin et al. Reference Heslin, Desai, Lappin, Donoghue, Lomas, Reininghaus, Onyejiaka, Croudace, Jones, Murray, Fearon, Doody, Dazzan, Fisher, Demjaha, Craig and Morgan2016a ). These results of AESOP seem relatively similar to ours.
The burden of disease of mood disorders to society among EU nations is higher than in any other brain disorders, most of the costs resulting from disability (Olesen et al. Reference Olesen, Gustavsson, Svensson, Wittchen and Jönsson2012). There are not many studies on the disability due to PD. In PD, disability was found to be increased even when compared with severe major depression in all functional dimensions of Short Form-36, there were, moreover, an increased number of absent days and days ill in bed (Kruijshaar et al. Reference Kruijshaar, Hoeymans, Bijl, Spijker and Essink-Bot2003). Severe forms of recurrent depressions, additionally, may have a scar effect in the form of an increase in disability (Ormel et al. Reference Ormel, Oldehinkel, Nolen and Vollebergh2004). Due to earlier age of onset and higher prevalence, the burden of disease on society is likely to be higher in SZ, although self-perceived suffering may be worse due to depression being a robust determinant of quality of life (Saarni et al. Reference Saarni, Viertio, Perälä, Koskinen, Lönnqvist and Suvisaari2010).
Diagnostic instability has been a concern with PD (Bromet et al. Reference Bromet, Kotov, Fochtmann, Carlson, Tanenberg-Karant, Ruggero and Chang2011; Ruggero et al. Reference Ruggero, Kotov, Carlson, Tanenberg-Karant, Gonzalez and Bromet2011). In 10-year follow-up studies of relatively young patient samples, the diagnosis of PD has remained in less than half of the cases (Bromet et al. Reference Bromet, Kotov, Fochtmann, Carlson, Tanenberg-Karant, Ruggero and Chang2011; Ruggero et al. Reference Ruggero, Kotov, Carlson, Tanenberg-Karant, Gonzalez and Bromet2011; Heslin et al. Reference Heslin, Lomas, Lappin, Donoghue, Reininghaus, Onyejiaka, Croudace, Jones, Murray, Fearon, Dazzan, Morgan and Doody2015) and Ruggero et al. (Reference Ruggero, Kotov, Carlson, Tanenberg-Karant, Gonzalez and Bromet2011) have even suggested that PD diagnosis should be considered as a provisional diagnosis. However, in a 2-year follow-up of slightly more aged sample, the stability was 85% (Salvatore et al. Reference Salvatore, Baldessarini, Tohen, Khalsa, Sanchez-Toledo, Zarate, Vieta and Maggini2011). The early onset of PD may well predict conversion to bipolar disorder (Østergaard et al. Reference Østergaard, Straszek, Petrides, Skadhede, Jensen, Munk-Jørgensen and Nielsen2014). Additionally, changes in the symptom presentation seem to explain the instability (Bromet et al. Reference Bromet, Kotov, Fochtmann, Carlson, Tanenberg-Karant, Ruggero and Chang2011). A shift toward SZ has also been found during the course of a decade. Among these cases, poorer functioning and negative symptoms predicted the shift (Bromet et al. Reference Bromet, Kotov, Fochtmann, Carlson, Tanenberg-Karant, Ruggero and Chang2011). Altogether the stability of diagnosis in PD can be highly age-related as especially younger patients are more likely to develop PBD (Lykouras & Gournellis, Reference Lykouras and Gournellis2009). The diagnosis might also be more stable in patients with medical comorbidity (Tohen et al. Reference Tohen, Khalsa, Salvatore, Vieta, Ravichandran and Baldessarini2012). Still, among mood disorders, bipolar disorder has been found to best predict psychosis (Souery et al. Reference Souery, Zaninotto, Calati, Linotte, Sentissi, Amital, Moser, Kasper, Zohar, Mendlewicz and Serretti2011).
Considering the diagnostic validity of PD, it is interesting that gender distribution in PD is similar to NPD, while the proportion of females is lower in SZ and PBD. Meanwhile, the differences between PD and NPD are well documented (Keller et al. Reference Keller, Schatzberg and Maj2007) and our findings are in line with these. The increasing prevalence, though not statistically significant, and proportion of psychosis in depression in older patient samples also contradict with the concept of psychotic illness, e.g. SZ, starting usually at early adulthood. In this systematic review, onset age of PD was earlier than that of NPD in younger samples, but later in older samples. It remains possible that there are two forms of PD. PD in young adulthood may be an etiologically and prognostically different illness than PD in late adulthood and in geriatric populations. Early-onset form of PD may be more unstable, potentially an early expression for some patients of bipolar disorder, and for others perhaps other psychotic conditions. Among older onset cases, it is possible that medical and neurological conditions partly explain the occurrence of PD. Future studies should address these questions and include also late-onset PD patients.
Strengths and limitations
There are several limitations related to this review. We included only articles published in English, meaning that especially older relevant articles on the topic may be missing. It should be acknowledged that the oldest studies in this review were from 1980s. Although we consider our search criteria to be adequate, we may have missed some studies, especially older studies. Because of this, we have also done some manual work to locate these papers, e.g. using the reference lists of previous reviews. It should be noted that we excluded childhood onset samples. The included articles were quite mixed regarding methods, e.g. diagnostic criteria or other inclusion criteria. The original studies on incidence and prevalence rates were few, and they had very heterogeneous methodology. There were four population studies with different design and methods of ascertaining the PD cases, and five registry studies. These two sets of studies produced considerably different estimates of PD. It may be that the available data are too heterogeneous to make precise estimate of incidence and prevalence of PD.
The sample sizes were relatively small, e.g. in risk factors median sample size being 45, and mainly not based on population samples, but comparing clinical samples. Minority of the studies based on first-episode samples. Most of the studies on outcomes had sample size of PD under 50. Due to the low number of studies, it is not possible to have a clear picture on the effect of study quality (e.g. sample size) on the results. However, based on the study characteristic summarized in Results section, many of the original studies have important limitations (e.g. small sample size, short follow-ups, lack of long-term follow-ups in older populations). In outcome analyses, some of the definitions of outcomes were heterogeneous, e.g. definitions of symptomatic remission, global clinical outcome, global outcome varied.
The strength of this review was the comprehensive search strategy, as we searched four electronic databases. We read in detail studies analyzing depression in general, and whenever possible, extracted the data concerning PD as separate group. There was a relatively good amount of data on gender differences, differences in onset age, and differences in some of the outcome measures to also allow new conclusion of the epidemiology of PD.
Conclusions
To our knowledge, this is the first systematic review on different aspects of epidemiology of PD. Based on this review, the amount of research on PD is far from that of NPD, SZ, and bipolar disorder. Based on differences in gender, onset age, and outcomes in PD in comparison to other disorders, PD seems distinguishable from related disorders and needs more scientific attention.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291717002501.
Acknowledgements
This work was supported by grants from the NARSAD: Brain and Behavior Research Fund and the Academy of Finland (#268 336, #278 286). The funders had no role in the manuscript.
Declaration of Interest
None.






