Published online by Cambridge University Press: 24 May 2005
Aims: We investigated the incidence and outcome of cardiac malformations in 53 patients with Williams syndrome. Methods and results:The mean age, and period of follow-up, were 3.6 and 5.3 years, with standard deviations of 4.0 and 5.6 years, respectively. Of the patients, 45 (85%) had cardiovascular anomalies, often combined. Males presented earlier than females, at the ages of 2.1 years, with standard deviation of 2.8 years, as opposed to 4.5 years, with standard deviation of 4.2 years (p < 0.01). Supravalvar aortic stenosis occurred in 32 patients (71%), pulmonary arterial stenosis in 17 (38%), and mitral valvar prolapse in 12 (27%), 9 of these having regurgitant valves. Pulmonary valvar stenosis, ventricular septal defect, coarctation of the aorta, persistent patency of the arterial duct, hypertrophic cardiomyopathy, and subaortic stenosis all occurred less frequently. In 21 patients (47%), 24 surgical or catheter interventions had been made, most often for repair of supravalvar aortic stenosis, undertaken on 16 occasions with just one recurrence, and in 4 along with surgery to the mitral valve. Other lesions requiring intervention were pulmonary valvar stenosis, pulmonary arterial stenosis, coarctation of the aorta, and subaortic stenosis. We lost 3 patients (7%), with severe supravalvar aortic stenosis and moderate or severe mitral regurgitation, 2 early and one late after surgery. Conclusion: The most frequent cardiovascular anomalies in Williams syndrome were supravalvar aortic stenosis, pulmonary arterial stenosis, and mitral valvar prolapse, which occurred more frequently in our patients than previously observed. Patients with left ventricular pressure and volume overload were at greater risk.
Williams syndrome is an infrequent disorder, usually sporadic, caused by deletion of the elastin gene at 7q11.23.1 Salient clinical features are a friendly personality, characteristic elfin facies, developmental and growth delay, and infantile hypercalcaemia.2–4 Cardiovascular anomalies are present in about four-fifths of the cases, most frequently supravalvar aortic stenosis and pulmonary arterial stenosis.5 We have investigated the incidence and outcome of cardiac malformations in 53 consecutive patients with Williams syndrome, and have found that prolapse of the mitral valve is also a common anomaly.
We included in our cohort all patients with Williams syndrome examined at the Children's and Private Hospitals in Cordoba, Argentina, from 1980 to 2002. Diagnosis of the syndrome was confirmed by genetic means. Deletion of the elastin gene was demonstrated in the last 9 patients subsequent to the availability of fluorescence in-situ hybridization. Cardiovascular defects were diagnosed by clinical examination, conventional ancillary studies, and Doppler echocardiography. Systemic hypertension was considered present if measurements of blood pressure were above the 95th percentile for reported normal values in children. According to the anatomy, supravalvar aortic stenosis was classified as localized, hourglass type, or diffuse. The stenosis was regarded as moderate if the left ventricular-aortic gradient was greater than 50 mmHg, and severe when it reached more than 70 mmHg. Mitral valvar prolapse was diagnosed by echocardiography in the long axis view if any or both leaflets of the valve had greater than 2 mm systolic displacement above the mitral valvar annulus into the left atrium. Mitral regurgitation was assessed by color Doppler echocardiography, and/or left ventricular cineangiography. We performed 30 diagnostic or therapeutic cardiac catheterisations and cineangiographies in 22 patients. The mean age at referral, and the period of follow-up, were 3.6 and 5.3 years, with standard deviations of 4.0 and 5.6 years, respectively. Of the patients, 33 (62%) were male. Two girls were identical twins.
Data were expressed as mean and standard deviations. Univariate comparison of variables, when applicable, was made using Student's t-test. A p value of less than 0.05 was considered significant.
Cardiovascular anomalies, often combined, were found in 45 patients (85%) (Fig. 1). Males presented earlier than females, at the age of 2.1 years, with standard deviation of 2.8 years, as opposed to 4.5 years, with standard deviation of 4.2 years (p < 0.01). Supravalvar aortic stenosis was the most frequent malformation, occurring in 32 patients (71%). Of these, 18 had moderate or severe stenosis, and 11 had diffuse lesions. Pulmonary arterial stenosis was found in 17 patients (38%). Mitral valvar prolapse was found in 12 patients (27%), 9 with mitral regurgitation, which was moderate or severe in 3.
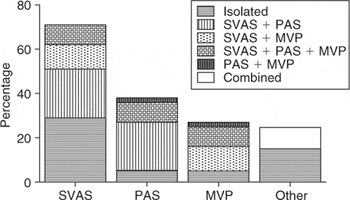
Figure 1. Relative frequency of cardiovascular anomalies in patients with Williams syndrome. SVAS: supravalvar aortic stenosis; PAS: pulmonary arterial stenosis; MVP: mitral valvar prolapse.
Less frequent anomalies were pulmonary valvar stenosis in 5 (11%), a small ventricular septal defect 2 (4%), coarctation of the aorta in 2 (4%), one being abdominal, and persistent patency of the arterial duct, hypertrophic obstructive cardiomyopathy, and fibromuscular subaortic stenosis each seen in one patient. Aside from patients with obstructive aortic lesions, hypertension in all limbs was found in only 2 patients, one child and one adolescent. The Wolff–Parkinson–White pattern on the electrocardiogram was present in one patient.
None of the patients submitted to diagnostic or therapeutic cardiac catheterisation died during the procedure. Only one patient had a serious complication with no sequel, namely bradycardia and severe hypotension requiring prolonged resuscitation.
We performed 24 surgical or catheter interventions in 21 patients. Of the 18 patients with moderate or severe supravalvar aortic stenosis, 16 underwent surgery. The left ventricular-aortic gradient was 81 mmHg, with standard deviation of 20 mmHg (Table 1). In 15 patients, we performed different types of patch plasty, and in one, an aorto-aortic bypass was carried out for late recurrence of the stenosis. All the aortic plasties were performed using preclotted or collagen impregnated Dacron, or autologous pericardium. An inverted bifurcated patch plasty was performed in 6 patients with diffuse, and in 2 with localized, obstruction, while the aorta was enlarged with a diamond shaped patch in 3 with diffuse and 4 with localized stenosis. Additional procedures were repair or replacement of the mitral valve in 2 patients each, and pulmonary valvotomy and patch angioplasty of the left pulmonary artery. Other surgical procedures performed in two patients were a pulmonary valvotomy in one, and bypass of an abdominal aortic coarctation along with resection of fibromuscular subaortic stenosis in the other.
Table 1. Patients with supravalvar aortic stenosis undergoing surgery.

Catheter interventions included pulmonary valvotomy, angioplasty of diffuse pulmonary arterial stenosis, and dilation of classical and abdominal coarctation of the aorta.
Of the patients undergoing interventions, 3 (7%) died after surgery, 2 in the early postoperative period after reconstruction of the ascending aorta, giving mortality of 12.5% for the subgroup of patients with supravalvar aortic stenosis. The other died late and suddenly 6 years after surgery. All 3 patients had had attempted relief of supravalvar aortic stenosis combined with additional surgery on the mitral valve because of moderate or severe regurgitation. Only one of the 13 survivors with supravalvar aortic stenosis had recurrence of the obstruction. In this patient, 8 years following relief of diffuse stenosis using a diamond shaped patch, he was admitted with infective endocarditis on a prolapsing mitral valve. A gradient assessed by Doppler of 62 mmHg was found across the stenosis. Following successful medical treatment for the endocarditis, he remains awaiting surgery. The postoperative gradient for all other patients but three was 23 mmHg, with standard deviation of 17 mmHg (p < 0.0001) after a follow-up of 6.0 years, with standard deviation of 4.5 years. One child with severe supravalvar aortic stenosis was lost to follow-up prior to any attempted surgery, and another, with moderate obstruction, refused surgery. All patients undergoing surgery for other lesions, or submitted to interventional catheterization, are alive and well.
The excised mitral valves in both patients who underwent replacement were available for pathological examination. The leaflets were glossy white with yellowish areas. Microscopy showed increased collagen fibers, myxomatous transformation, and neovascularization. There were no inflammatory infiltrates. Focal calcification was found in one of the patients.
Deletion of the elastin gene on chromosome 7q11.23, leading to deficiency of elastin throughout cardiovascular development, is the likely cause for the widespread cardiovascular abnormalities seen in Williams syndrome.6 As a result, medial hypertrophy and subintimal fibrosis of the arteries produce increased mural thickness and secondary luminal narrowing, as shown by anatomic and intravascular ultrasonic studies.7, 8 Though the occurrence of systemic hypertension has been reported with relative frequency, we found it only in 2 instances, possibly because our patients were younger than those collected in other series.9–11 The role of the compliance of the elastic arteries as a pathogenic mechanism for hypertension is still controversial.12, 13
In our series, we found an incidence of 85% for all cardiac malformations, a figure very similar to the reported incidence of four-fifths in other studies.5 The majority of our patients were referred in childhood. As reported very recently, we also found that affected males had been referred earlier than females, indicating more severe disease in the former.14
As have others, we found the most frequent malformations to be supravalvar aortic stenosis and pulmonary arterial stenosis.4, 7, 9 We then found prolapse of the mitral valve, with an incidence of 27%, to be the next commonest anomaly, at times associated with severe mitral regurgitation.9, 15 Though this association was reported as early as 1972,16 it has been rarely mentioned thereafter, with subsequent investigations suggesting an incidence between 7 and 19% for patients with cardiac involvement,7, 9, 11 which is lower than that found in our series. According to these data, nonetheless, Williams syndrome should be added to the list of conditions associated with mitral valvar prolapse.17
Less frequent congenital cardiac abnormalities encountered included pulmonary valvar stenosis, ventricular septal defect, persistent patency of the arterial duct, coarctation of the aorta, abdominal in one patient, fibromuscular subaortic stenosis, and hypertrophic obstructive cardiomyopathy.
Obstructive arterial lesions are certainly related to the deficient elastin gene causing diffuse fibrodysplasia. A connected collagen defect may also be involved in the pathogenesis of mitral valvar prolapse.9 We do not know if the other congenital anomalies, like septal defects, just occur fortuitously or have a common denominator, since the role of contiguous genes within the critical region has still not been settled.5 With regard to our patient with hypertrophic obstructive cardiomyopathy at birth, which resolved spontaneously, we could speculate that it was related to abnormal calcium metabolism. We cannot exclude the possibility, nonetheless, that there was an associated defect in one of the several genes described in hypertrophic cardiomyopathy.18
Cardiac catheterisation should be performed in any patient in whom an intervention is planned, since this diagnostic modality is the gold standard in these patients with frequent coexistence of associated anomalies. This method, however, is not without risk. We did not have any mortality during the procedure, but there was a major complication that fortunately had a full recovery. Sudden death may occur in patients with Williams syndrome undergoing cardiac catheterisation, especially those with obstruction to both ventricular outflow tracts or myocardial ischaemia. Rigorous monitoring during anaesthesia is recommended in these cases.19
The lesions that most frequently required an intervention were supravalvar aortic stenosis and other aortic obstructions. It is well known that they tend to be progressive, mainly because the aorta does not grow with age.7, 20–23 Surgery is the treatment of choice for supravalvar aortic stenosis. Aortic reconstruction by means of patch enlargement has been proposed, and should be tailored individually according to the type of the stenosis, localized or diffuse, and the degree of involvement of the sinutubular junction, the aortic valve, and the origin and course of the coronary arteries.24, 25 Diamond shaped and inverted bifurcated Dacron and autologous pericardial patches have been most effective in relieving the gradient. In just one patient, who had already presented to us with a recurrence and stenosis up to the aortic arch, we had to perform an aorto-aortic bypass instead of a plasty. In all other patients undergoing patch enlargement, there was only one late recurrence in a child with diffuse stenosis repaired with a diamond shaped patch. It has been reported that there are fewer recurrences when symmetrical reconstructions of the arch are achieved using inverted bifurcated patch plasty as opposed to using diamond-shaped patches.26
The timing of surgery is still a matter of discussion.25 Early surgical relief may prevent left ventricular hypertrophy and its adverse consequences, but recurrence might occur more often. Our policy has been to intervene in any patient if symptomatic, or in those with a gradient greater than 50 mmHg between the left ventricle and the aorta regardless of age. Pulmonary arterial stenosis and mitral valvar prolapse with regurgitation necessitated treatment less often. Pulmonary arterial stenosis may improve spontaneously, as shown by serial studies.19–21 Treatment is advised in symptomatic patients, or if after a reasonable period of follow-up, the right ventricular pressure is close to systemic levels. Proximal localized lesions are usually resistant to balloon angioplasty, with or without stenting, so surgery with patch augmentation is required. In contrast, multiple distal stenosis are better addressed by balloon dilation.27
Like in other settings of prolapsing mitral valvar leaflets, mitral insufficiency may be severe enough to require repair or replacement of the valve.15 In our series, moderate or severe regurgitation occurred in a third of the patients with prolapse, and these were always associated with severe supravalvar aortic stenosis, which might have aggravated the mitral incompetence. Whenever this association is present, early surgical treatment should be advised. It is likely that late referral might have contributed to the death of our patients who died immediately after surgery. Severe isolated mitral insufficiency in Williams syndrome may also be rarely found.28 It is as well worth mentioning that the only patient who had subacute bacterial endocarditis in our series had vegetations on a prolapsing mitral valve.
Pulmonary valvar stenosis, and other more uncommon anomalies such as ventricular septal defect, coarctation of the aorta, persistent patency of the arterial duct, hypertrophic cardiomyopathy, and fibromuscular subaortic stenosis received standard care with no mortality.
In our series, therefore, cardiovascular anomalies occurred in seven-eighths of patients, with a rather good outcome. The most frequent anomaly was supravalvar aortic stenosis, producing severe obstruction in more than half the involved cases. Surgical treatment was effective, carrying a mortality of 12.5% for the whole subgroup. In patients with isolated supravalvar aortic stenosis, there were no deaths. Pulmonary arterial stenosis was the second most frequent anomaly, but tended to be mild, and rarely required intervention. Mitral valvar prolapse followed, and occurred more frequently than in previous studies. Moderate or severe mitral regurgitation was found in a third of these cases. Among the less common malformations, we encountered one patient with hypertrophic obstructive cardiomyopathy, an association just recently reported.11, 29 The patients at greater risk were those with left ventricular pressure and volume overload, as seen in the association of supravalvar aortic stenosis and mitral regurgitation.

Relative frequency of cardiovascular anomalies in patients with Williams syndrome. SVAS: supravalvar aortic stenosis; PAS: pulmonary arterial stenosis; MVP: mitral valvar prolapse.

Table 1.