Introduction
Iberis pectinata Boiss. & Reuter (Sin. I. crenata Lam., Brassicaceae) and Ziziphora aragonensis Pau (Sin. Z. acinoides L., Labiatae) are winter annual plants with a restricted geographic distribution in the eastern–central Iberian Peninsula (Sainz Ollero and Hernández Bermejo, Reference Sainz Ollero and Hernández Bermejo1981; Moreno, Reference Moreno and Castroviejo1993). Z. aragonensis is less abundant, being included as ‘Rare’ in the Regional Catalogue of Threatened Species in the Aragon region (Sainz Ollero et al., Reference Sainz Ollero, Franco and Arias1996). In the province of Albacete (south-eastern Spain) these species co-occur with the endangered winter annual Sisymbrium cavanillesianum Castrov. & Valdés Berm. (Brassicaceae), which was included both in the Appendix II of the ‘Habitat Directive’ (92/43/CEE) as a priority species, and on the Red List of Spanish Vascular Flora (Domínguez, Reference Domínguez2000) as ‘Vulnerable’. As a consequence, the regional Government of Castilla-La Mancha proposed that the habitat of these species be included in the Nature 2000 network as a Site of Community Importance (SCI) called ‘La Encantada, El Moral y Los Torreones’ (Nature Code ES4210002). The three species are weeds and ruderals. They occur in periodically disturbed fields such as fallow lands, dry cereal fields, roadsides and shrubland gaps nitrified by overgrazing or abundance of wild rabbits. These species are outstanding members of the weed community Ziziphoro aragonensis–Iberidetum crenatae (Gómez-Campo and Herranz, Reference Gómez-Campo and Herranz1993).
A significant area inside the perimeter proposed as SCI was affected by the construction of a wind farm in 2001. The regional Government of Castilla-La Mancha (DOCM, 2002) obliged the company in charge of the project to restore S. cavanillesianum habitat by reinforcing both populations of these threatened species and populations of the most important associated taxa.
Reinforcement of wild plant populations using individuals propagated ex situ may be a valuable tool for reducing the risk of extinction and for conserving endemic and threatened species or populations (Bowes, Reference Bowes1999 Cerabolini et al., Reference Cerabolini, De Andreis, Ceriani, Pierce and Raimondi2004; Giménez-Benavides et al., Reference Giménez-Benavides, Escudero and Pérez-García2005). Thus, data on the environmental conditions required for seed dormancy break and germination are essential to make ex-situ propagation possible, as well as to understand the ecological life cycle of the species and to predict those periods favourable for seedling emergence and establishment in nature (Lentz and Johnson, Reference Lentz and Johnson1998; Baskin and Baskin, Reference Baskin and Baskin2002). These data also help us to assess the potential persistence ability of seeds in the soil (Washitani et al., Reference Washitani, Takenaka, Uramoto and Inoue1997).
Furthermore, a good understanding of the germination capacity and requirements of endemic species is essential for optimal biodiversity conservation and future management (Galmés et al., Reference Galmés, Medrano and Flexas2006). In this way, some endemic plant species grow in frequently disturbed habitats such as cereal fields, roadsides and shrublands that are maintained by human activities: ploughing, mowing and/or grazing. If these activities cease, woody plant species can invade and make these sites unsuitable for those rare species (Buchele et al., Reference Buchele, Baskin and Baskin1989). Preservation of the habitats of some rare species may therefore involve the use of management techniques to prevent the growth of other species (Pavlovic, 1994). The application of these techniques requires knowing at what time of the year it is possible to reduce the cover of undesirable plants without harming the rare species. To achieve this aim, information must be obtained concerning the seed stage in the life cycle of target species (Baskin and Baskin, Reference Baskin and Baskin2000). With this purpose, in addition to optimizing ex-situ plant production techniques, we studied the germination ecology of Z. aragonensis and I. pectinata. Studies related to Sisymbrium cavanillesianum were discussed in a previous paper (Herranz et al., Reference Herranz, Ferrandis and Copete2003).
Optimal germination conditions are normally related to the adaptation of plants to their habitats (Pérez-García et al., Reference Pérez-García, Iriondo, González-Benito, Carnes, Tapia, Prieto, Plaza and Pérez1995). In semiarid Mediterranean environments the temperature requirements for germination might be crucial for plant survival and, thus, adaptive processes tend to favour optimal germination temperature regimes that coincide with the temperatures most likely to occur during the rainfall period (Bell et al., Reference Bell, Plummer and Taylor1993; Gutterman, Reference Gutterman1994; Albert et al., Reference Albert, Iriondo and Pérez-García2002). In consequence, the influence of a wide range of temperature values on the germinative capability of Iberian endemics has been analysed profusely (e.g. Ayerbe and Ceresuela, 1982; Pérez-García et al., Reference Pérez-García, Iriondo, González-Benito, Carnes, Tapia, Prieto, Plaza and Pérez1995; Escudero et al., Reference Escudero, Carnes and Pérez-García1997; Cabello et al., Reference Cabello, Ruiz and Devesa1998; Albert et al., Reference Albert, Iriondo and Pérez-García2002; Herranz et al., Reference Herranz, Ferrandis, Copete and Martínez-Sánchez2002; Galmés et al., Reference Galmés, Medrano and Flexas2006). However, additional aspects of germination ecology included in the present study are not always assessed. These aspects are important to the correct comprehension of adaptations of plants to their habitats: germination phenology, effect of length of dry laboratory storage on germination, dormancy cycles in buried seeds exposed to environmental conditions, and influence of temperature on dormancy loss and induction. In this way, it should be emphasized that the existence of an annual dormancy/non-dormancy cycle in buried seeds of winter annuals can be interpreted as a mechanism that prevents germination during the period of the year (i.e. spring) when seedlings and juveniles are highly likely to die due to summer water stress (Baskin and Baskin, Reference Baskin and Baskin1992, Reference Baskin and Baskin2000). Thus, for the species in this study, it would be of interest to check for the presence of dormancy cycles, since not all seeds that are dormant or conditionally dormant when freshly matured exhibit annual changes in their dormancy states (Baskin and Baskin, Reference Baskin and Baskin1998).
Specifically, the aims of the present study on I. pectinata and Z. aragonensis were to: (1) analyse the influence of duration of dry laboratory storage on germination under different light and temperature conditions; (2) characterize the seed germination phenology; (3) determine the response of buried seeds to seasonal temperature changes; and (4) assess temperature requirements for induction and breaking of secondary dormancy.
Material and methods
Seed material
Fully matured seeds of I. pectinata and Z. aragonensis were collected from plants with a vigorous and healthy appearance growing in the Botanical Reserve of La Encantada (south of Villarrobledo, Albacete province; UTM coordinates, 30SWJ2832; altitude 800 m) in winter cereal fields, fallows and roadsides. Seed collection was carried out on 25 June 2000 and 24 June 2001. Seed were dried for 6–7 days at room temperature before germination studies were initiated.
Germination tests
A 100-seed lot was assigned to each temperature-condition test, distributed into four 25-seed replicates. Each replicate was incubated in a 9-cm-diameter Petri dish sealed with Parafilm, on a double layer of filter paper moistened with c. 4 ml of distilled water in germination chambers at 5°C and at 12/12-h daily temperature regimes of 15/4, 20/7, 25/10, 28/14 and 32/18°C. At each temperature regime, seeds were tested for germination in both continuous darkness and a 12-h daily photoperiod which, at the alternating temperatures, coincided with the daily high-temperature period. Darkness treatments were achieved by wrapping Petri dishes in a double layer of aluminium foil. Tests of dormancy cycles and temperature requirements for dormancy break and induction lasted 15 d, as recommended for this type of study (Baskin and Baskin, Reference Baskin and Baskin1990, Reference Baskin and Baskin1992, Reference Baskin and Baskin2000). However, to analyse the influence of duration of laboratory dry storage on germination, tests lasted 30 d, since after 15 d germination had not slowed down for some trials. Protrusion of the radicle was the criterion for successful germination. In treatments with a photoperiod, seeds were checked for germination every 2–3 days. Seeds incubated in darkness were checked only at the end of the test. Ungerminated seeds were checked for viability on the basis of embryo appearance, paying special attention to colour and turgidity. Percentages of germination were computed from viable-seed numbers. Germination chambers (Ibercex F-4 model, Madrid, Spain) were furnished with a digital temperature and light control system [ ± 0.1°C, cool white fluorescent light, ~25 μmol m− 2 s− 1 (1.350 lux)].
The fluctuating temperatures used in tests simulated approximate mean maximum and mean minimum monthly temperatures in the area of Villarrobledo during the growing season. Thus, 15/4°C corresponds to November and March, 20/7°C to October and April, 25/10°C to September and May, 28/14°C to August and June, and 32/18°C to July. The 5°C treatment is close to the mean temperature recorded during winter months (December, January and February).
Greenhouse
The greenhouse used in this study was located on the university campus in Albacete (UTM coordinates, 30SWJ9713; altitude, 690 m; 70 km away from La Encantada Reserve). It was not heated in winter nor air-conditioned in summer. An electric thermograph was used to keep a continuous record of air temperatures.
To simulate soil moisture regimes in the field, pots and trays placed in the greenhouse were watered to field capacity once each month during summer (1 July–30 September) and twice each month during the rest of the year, with the exception of the coldest periods when the substrate was frozen. In this way, substrate was kept moist for periods long enough to ensure uninterrupted seed imbibition, which is essential in the analysis of the effects of warm and cold stratification treatments on breaking and induction of dormancy (Baskin and Baskin, Reference Baskin and Baskin1998).
Influence of seed age on germinability of dry-stored seeds
After seed drying and cleaning, I. pectinata and Z. aragonensis seeds were stored in paper bags under laboratory conditions (18–20°C and 50–60% air humidity) until used in germination tests. Seed lots were tested for germination from 1 July 2000 (age = 0 months) until November 2000 (age = 4 months). Intermediate tests were started the first day of August, September and October 2000 (seed age equal to 1, 2 and 3 months, respectively). Tests lasted 30 d. Temperature and illumination conditions were those described in the ‘Germination tests’ section.
Germination phenology
On 1 July 2000, 5 d after collection, a 500-seed lot of each species was distributed in five 100-seed replicates. Each one was sown at a 1-mm depth in a 40 × 25 × 7 cm plastic seedling tray with drainage holes. Substrate consisted of a sterilized mix of nutrient-enriched peat and sand (2:1). To facilitate counting of emerged seedlings, the trays were placed on tables in the greenhouse. Watering frequency was as described in the ‘Greenhouse’ section. Emergent seedlings were counted and removed weekly until 30 June 2004, taking care not to remove any ungerminated seeds.
Response of buried seeds to seasonal temperature changes
Seeds collected on 25 June 2000 were stored for 6 d at ambient room temperature. Then, 27 lots of 1300 Z. aragonensis seeds each were prepared. In the case of I. pectinata, it was only possible to prepare 22 lots of 1300 seeds and four additional lots of 650 seeds. Every seed lot was homogeneously mixed with a double volume of 0.5 mm sieved sand in order to prevent seeds from sticking to each other when humidity is high, a common phenomenon in seeds with mucilage such as those of I. pectinata. This mixture was placed within nylon bags of 0.1 mm mesh, which were buried to a depth of 7 cm in soil (river sand) in pots (20 cm diameter and 25 cm depth) with drainage holes. Three pots were used per species. Pots were placed in the previously described non-heated greenhouse, where temperatures are near those out-of-doors, and watering regimes were those already described.
Germination tests were conducted in light and darkness on seeds exhumed on the first days of each month from August 2000 to May 2002 (I. pectinata, darkness), to September 2002 (I. pectinata, light) and to October 2002 (Z. aragonensis), depending on the number of available seeds. The exhumation of seeds was always carried out after sunset. Seeds were recovered from bags by pouring the mixture with sand through a 0.5 mm sieve. To prevent light exposure of seeds assigned to germination tests in darkness, both sieving and their handling prior to tests were carried out with a green safe light (Baskin and Baskin, Reference Baskin and Baskin1998).
Temperature requirements for induction and breaking of secondary dormancy
This experiment was designed on the basis of the results obtained in the analysis of seed-dormancy cycles started in July 2000. Seeds of I. pectinata and Z. aragonensis were collected on 24 June 2001 and stored for 7 days at ambient room temperature. For each species, 26 nylon bags containing a 700-seed lot each were prepared as described above. In early July 2001, two bags per pot (13 pots) were buried at 7 cm depth. Pots were placed within the greenhouse and submitted to the same watering regime described previously, until 2 November 2001 (6 pots), and April–May 2002 (7 pots).
On 2 November 2001 one bag of each species was exhumed, and seeds were tested for germination in light at 5°C and at 15/4, 20/7, 25/10, 28/14 and 32/18°C to determine the dormancy stage of seeds. Subsequently, for each species, one pot containing two bags was placed at each of the following temperatures: 5, 15/4, 20/7, 25/10, 28/14 and 32/18°C. After 8 and 16 weeks, seeds from all treatments were exhumed and tested for germination in the light at the six temperature regimes.
The methodological protocol for testing temperature requirements for overcoming secondary dormancy was the same as that described in the above section. Dates of bag exhumation were 1 April and 1 May 2002 for I. pectinata and Z. aragonensis, respectively.
Statistical analysis
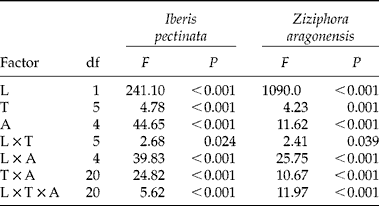
Germination percentage of dry-stored seeds was compared among treatments by multivariate ANOVA. Three factors were considered in the analysis: (1) temperature (six levels); (2) light (two levels); and (3) seed age (five levels). For the comparison of induction and breaking of secondary dormancy a multifactorial ANOVA was performed, including as factors: (1) temperature during seed burial (six levels); (2) temperature for testing germination response (six levels); and (3) the length of stratification (three levels). Treatments responsible for significant main effects were detected by a multiple-comparison Tukey test; significant interactions were explored by contrasting confidence intervals. Prior to analyses, normality (Cochran test) and homoscedasticity (David test) of the data were checked. Values of the final cumulative germination percentage were square-root arcsine transformed.
For each temperature × light treatment on dry-stored seeds, changing trends in germination with seed age were evaluated by a regression analysis. Data were log transformed, and the significant model selected for each treatment was that showing the highest R 2 coefficient. The minimum improvement level for changing the model was established at 10%.
Results
Effect of light, temperature and seed age on seed germination
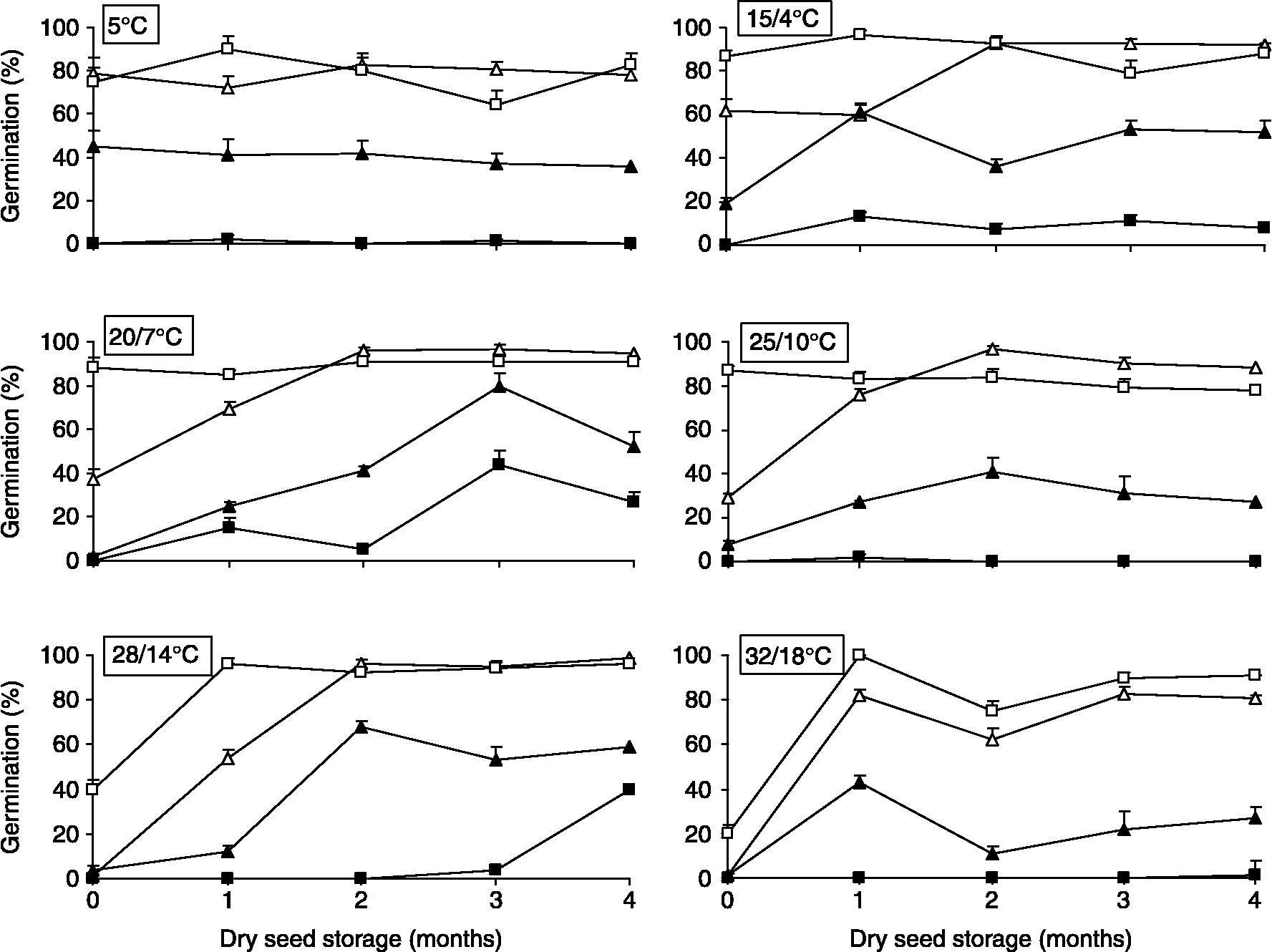
Figure 1 shows the germination response of I. pectinata and Z. aragonensis to light, temperature and seed age. Main effects of the three factors, as well as interactions, were, in general, highly significant (Table 1). In the case of I. pectinata, germination was considerably lower in darkness than with photoperiod at all temperatures analysed. However, at high temperatures (28/14 and 32/18°C) such differences were only manifested after at least 1 month of seed storage. Except for the 5°C treatment, final germination percentages increased with seed age, both in darkness and light. However, such an increase was not constant, showing short oscillations in all thermoperiods. Freshly matured seeds (age = 0 months) reached final germination of 60% only at 5 and 15/4°C with photoperiod, showing low values ( < 4%) at high temperatures (28/14 and 32/18°C). One-month aged seeds reached 60% germination at 20/7 and 25/10°C. At 28/14°C, germination of such magnitude was not achieved until 2 months of seed age.
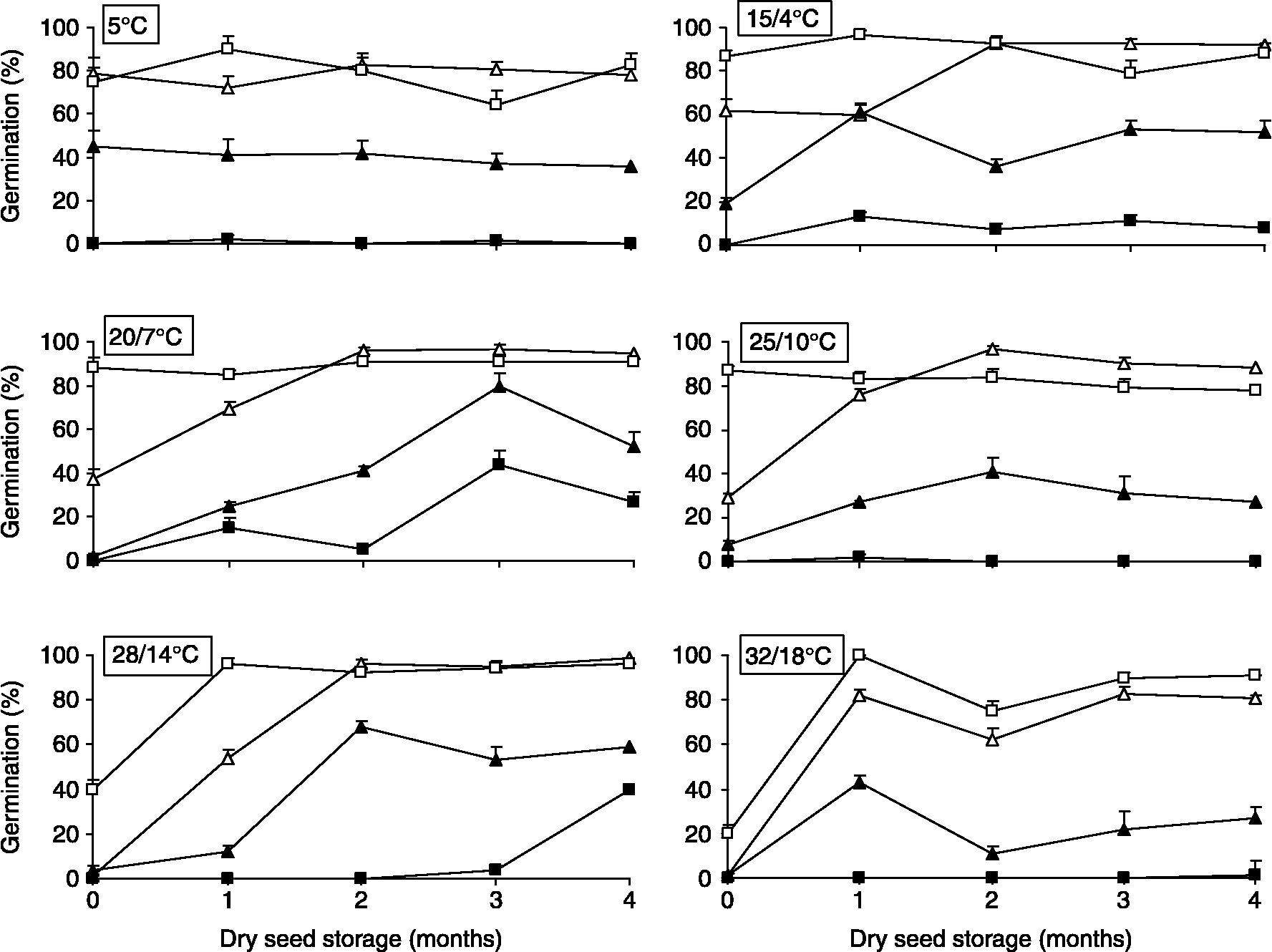
Figure 1 Germination of Ziziphora aragonensis (squares) and Iberis pectinata seeds (triangles) at constant (5°C) and alternating temperatures (15/4, 20/7, 25/10, 28/14 and 32/18°C) after 0–4 months of dry storage both at a 12-h daily photoperiod (open symbols) and in darkness (closed symbols).
Table 1 Effects of light (L), temperature (T) and seed age (A), on I. pectinata and Z. aragonensis seed germination and their interactions analysed by a multifactor ANOVA. The table shows degrees of freedom (df), F-ratio values and the associated probability (P) for main effects and interactions (α=0.05). Residual degrees of freedom: 180
At 28/14°C, there was a positive relation between germination percentage of I. pectinata seeds and seed age, both with light (F 1,18 = 12.78, P = 0.0028, y = 3.26+0.42x, R 2 = 46%) and in darkness (F 1,18 = 34.02, P < 0.0001, y = 2+0.63x, R 2 = 67%). This relation was not significant for the other treatments.
Z. aragonensis seed germination was considerably lower in darkness than with photoperiod in all temperatures and ages of seeds, with differences being more pronounced than those recorded in I. pectinata. Thus, at 5, 25/10 and 32/18°C in darkness, germination was < 2%. With photoperiod, germination at 5, 15/4, 20/7 and 25/10°C was close to 80%, with short oscillations, during the period considered. However, freshly matured seeds only reached 40% and 20% of final germination at 28/14 and 32/18°C, respectively, but exceeded 96% in 1-month aged seeds. In darkness, freshly matured seeds did not germinate at any thermoperiod.
As in the other species, Z. aragonensis showed a positive relation between final germination at 28/14°C and seed age, both with light (F 1,18 = 15.91, P = 0.0009, y = 4.02+0.18x, R 2 = 47%) and in darkness (F 1,18 = 49.80, P = 0.0009, y = − 4.46+2.02x, R 2 = 91%). As occurred with I. pectinata, the relation for Z. aragonensis was not significant in the remaining treatments.
Germination phenology
Iberis pectinata
A high percentage (79.6%) of seeds sown on 1 July 2000 germinated in autumn 2000, with 77% of them germinating between 14 and 25 September, during which time the mean daily minimum and maximum temperatures were 10.9 and 28.2°C, respectively. Mean maximum and minimum temperatures for September were 28.5 and 11.9°C, respectively (Fig. 2). During the two subsequent autumns some seedling emergence was also recorded: 3.8% and 2.4% in 2001 and 2002, respectively. A very low proportion of seeds germinated during winter 2002 (1.2%), winter 2003 (0.8%), and spring 2003 (0.8%). After June 2003 no new emergent seedling was detected. The final cumulative germination was 89%.

Figure 2 Germination phenology of Iberis pectinata and Ziziphora aragonensis (cumulative germination percentage, mean ± SE). Changes in temperature throughout the experiment are also shown (mean daily maximum and minimum monthly air temperatures).
Ziziphora aragonensis
In this species, 41.6% of seeds sown on 1 July 2000 germinated in autumn 2000, with 38.4% of them germinating between 25 September and 20 October; when mean daily minimum and maximum temperatures were 7.6 and 22.0°C. Mean daily maximum and minimum temperatures for October were 20.3 and 6.9°C, respectively (Fig. 2). Some emergence was also recorded during the next three autumns: 3.2% in 2001, 3.6% in 2002, and 1% in 2003. A very small fraction of germination occurred in spring: 0.4% in both April 2001 and April 2002. The final cumulative germination was 50.6%.
Response of buried seeds to seasonal temperature changes: dormancy cycles
Iberis pectinata
Freshly matured seeds of this species were conditionally dormant, as evidenced by 79 and 62% germination in light at 5 and 15/4°C, respectively. Dormancy loss occurred during the summers of 2000 and 2001, and non-dormant seeds re-entered dormancy in the winters of 2000–2001 and 2001–2002 (Fig. 3). During winter 2001–2002, induction of dormancy was not so pronounced as in 2000–2001, and in March 2002 40% of seeds incubated at 5°C germinated. As dormancy was broken during the summer months, high germination percentages (>70%) were first achieved at 5, 15/4 and 20/7°C. This germination magnitude was achieved later at other thermoperiods as well, but not at 28/14 and 32/18°C after summer 2000. When seeds re-entered dormancy in autumn 2001 (November, December), germination was reduced first at 32/18 and 28/14°C; during the following months (January, February and March) germination also decreased at the other temperatures. The highest germination percentages ( ≥ 95%) in October 2000 and 2001 were for seeds incubated at 5, 15/4 and 20/7°C with photoperiod.

Figure 3 Germination percentages of Iberis pectinata seeds incubated (a) at a 12-h daily photoperiod, or (b) in continuous darkness, for 15 d at constant (5°C) and alternating temperatures (15/4, 20/7, 25/10, 28/14 and 32/18°C) following 0–26 (photoperiod) or 0–22 (darkness) months of burial.
Ziziphora aragonensis
Freshly matured seeds did not germinate in darkness at any of the six temperature conditions. In contrast, germination was ≥ 75% at 5, 15/4, 20/7 and 25/10°C with photoperiod; thus, they were conditionally dormant. Germination of seeds exhumed during summer 2000 and incubated with photoperiod decreased progressively until October, when percentages were the lowest. However, in November, germination again showed high values (Fig. 4). In 2001, the highest germination percentages were achieved during summer (July–September) and at the end of autumn (December). In the previous month (November 2001), however, germination was as low as that observed in 2000. In 2002, the highest percentages were achieved in September. As in the case of I. pectinata, the lowest germination values of exhumed seeds were shown after winter: in March 2002 and in May 2001.

Figure 4 Germination percentages of Ziziphora aragonensis seeds incubated (a) at a 12-h daily photoperiod, or (b) in continuous darkness, for 15 d at constant (5°C) and alternating temperatures (15/4, 20/7, 25/10, 28/14 and 32/18°C) following 0–27 months of burial.
Similarly to records with photoperiod, the highest germination percentages in darkness occurred at the end of summer or in autumn: November in 2000, August–October and December in 2001, September in 2002. The months previous to November 2000 and December 2001 showed low values. The lowest germination percentages were achieved after winter: in March 2002 and May 2001.
In the dormancy cycles of both species, the short duration (1 month) of the critical period of the most pronounced conditional dormancy should be noted, as well as the temporal variability of this period from year to year (I. pectinata: April in 2001, March in 2002; Z. aragonensis: May in 2001, March in 2002).
Temperature requirements for induction of secondary dormancy
Iberis pectinata
I. pectinata seeds buried in July 2001 and exhumed in November 2001 were non-dormant: they germinated ≥ 95% when incubated at thermoperiod ≤ 25/10°C (Table 2). When non-dormant seeds were buried at 5, 15/4, 20/7 and 25/10°C for 8 weeks, germination percentages in subsequent tests fell drastically, particularly when incubated at 20/7, 25/10, 28/14 and 32/18°C. In general, germination decreased when temperature of incubation was higher and temperature of burial was lower. After 16 weeks, seeds that had been buried at 32/18 and 28/14 germinated to 68–100% at all temperatures except 32/18°C. On the other hand, seeds buried at 20/7 and 25/10°C germinated to 68–92% only at 5 and 15/4°C. Maximum germination (32%) of seeds buried at 5°C was at 5°C.
Table 2 Germination percentages (mean±SE) of Iberis pectinata and Ziziphora aragonensis seeds incubated at 5°C and various thermoperiods. After 4 months of burial in a pot in the greenhouse, one seed lot was exhumed on 2 November 2001 and tested for germination (control). Six pots were transferred to the laboratory and placed at 5°C and over the range of thermoperiods (burial temperatures) for 8 and 16 weeks before testing germination

Numbers in a column followed by the same lower-case letter are not significantly different from each other, and those in a row followed by the same upper-case letter are not significantly different from each other (P < 0.05).
Ziziphora aragonensis
Z. aragonensis seeds buried in July 2001 and exhumed in November 2001 were non-dormant: they showed germination percentages over 75% at 5, 15/4, 20/7, 25/10 and 28/14°C (Table 2). Seed burial at 5 and 15/4°C for 8 weeks induced seeds into dormancy, as manifested by significant decreases in germination in subsequent tests at all thermoperiods. Such a seed dormancy induction was more pronounced in seeds buried at 15/4°C than at 5°C, except when the subsequent incubation test was at 5°C. If the seed-burial period lasted 16 weeks, dormancy induction occurred at 5, 15/4 and 20/7°C, being more pronounced as temperature was lower. Seed burial at 25/10, 28/14 and 32/18°C only induced dormancy when seeds were subsequently incubated at 5°C.
Temperature requirements for breaking of dormancy
Iberis pectinata
Most of the I. pectinata seeds buried in July 2001 and exhumed in April 2002 were dormant at all thermoperiods except at 5°C; at this temperature, germination was 58% (Table 3). When conditionally dormant seeds were buried for 8 weeks at 5 and 15/4°C, their germination ability increased when incubated at 5°C, but germination was less than 25% for all the other incubation temperatures. In contrast, dormancy was broken in seeds buried at 20/7, 25/10, 28/14 and 32/18°C, with this response being more pronounced at the two higher thermoperiods. When the burial of conditionally dormant seeds lasted 16 weeks, 5 and 15/4°C burial thermoperiods increased the dormancy-induction effect in seeds incubated at ≥ 20/7°C. Other thermoperiods during the burial phase promoted germination. Such a germination-promoting effect was proportional to temperature value, with the exception of seed burial at 20/7°C, which removed dormancy more than at 25/10°C. Seeds buried at 32/18°C for 16 weeks germinated 56% when subsequently incubated at 32/18°C.
Table 3 Germination percentages (mean±SE) of Iberis pectinata and Z. aragonensis seeds incubated at 5°C and various thermoperiods. After 9 (I. pectinata) and 10 months (Z. aragonensis) of burial in a pot in the greenhouse, one seed lot was exhumed on 1 April (I. pectinata) and 1 May 2002 (Z. aragonensis) and tested for germination (control). Six pots were transferred to the laboratory and placed at 5°C and over the range of thermoperiods (burial temperatures) for 8 and 16 weeks before testing germination

Numbers in a column followed by the same lower-case letter are not significantly different from each other, and those in a row followed by the same upper-case letter are not significantly different from each other (P < 0.05).
Ziziphora aragonensis
Most Z. aragonensis seeds buried in July 2001 and exhumed in May 2002 were dormant, since the highest germination was 16%, at 20/7°C (Table 3). Seed burial at 20/7, 25/10, 28/14 and 32/18°C for 8 weeks induced dormancy break, as manifested by germination percentages ( ≥ 67%) achieved in germination tests at 5, 15/4, 20/7 and 25/10°C. Even seed burial at 15/4°C promoted a partial overcoming of seed dormancy, with seeds reaching germination percentages of over 73% at 5, 15/4 and 20/7°C. After 16 weeks of seed burial, the promoting effect of germination detected in the 8-week burial treatments did not intensify, even being lower for most of the temperatures (5, 20/7, 25/10 and 32/10°C).
Discussion
Germinability of dry-stored seeds
Most freshly matured seeds of I. pectinata have conditional physiological dormancy, since they germinated (38–78%) at low and medium temperatures (5, 15/4 and 20/7°C) with photoperiod, but experienced a severe reduction in their germination ( < 5%) at high temperatures (28/14, 32/18°C). A similar dormancy type could be assumed for Z. aragonensis, whose fresh seeds showed high germination values (75–85%) at low and medium temperatures (5, 15/4, 20/7, 25/10°C) with photoperiod, and substantially reduced their response (20%) when incubated at 32/18°C (Fig. 1). In both species, germination at high temperatures (28/14 and 32/18°C) in photoperiod conditions notably increased (>90%) after 1 or 2 months of dry storage at room temperatures (20°C, relative humidity of c. 50–60%), so it may be concluded that they have non-deep physiological dormancy (Baskin and Baskin, Reference Baskin and Baskin1998).
Dry-stored seeds of I. pectinata and Z. aragonensis initially germinated at low and medium temperatures, similar to those recorded in their natural habitat during autumn and winter months. In addition, seeds also germinated at 28/14°C and 32/18°C as they came out of dormancy. Thus, the after-ripening pattern of both species corresponds to Type 1 postulated by Vegis (Reference Vegis1964), which has been described as typical in winter annuals species living under Mediterranean climate conditions (Baskin and Baskin, Reference Baskin and Baskin1998, Reference Baskin, Baskin, McDonald and Kwong2005). Moreover, when exhumed seeds from both taxa came out of dormancy due to exposure to summer temperatures, they then germinated at low and medium temperatures, and even at high temperatures in later phases. When seeds were induced to dormancy by low winter temperatures, they first lost the ability to germinate at high temperatures, while the suppression of germinative response at medium and low temperatures took place in subsequent phases (Figs 1, 3 and 4). All these records are also consistent with Vegis's Type 1 after-ripening pattern of non-deep physiological dormancy.
The inability of I. pectinata and most Z. aragonensis fresh seeds to germinate at high temperatures (28/14°C and/or 32/18°C) represents an effective mechanism to prevent germination in nature in response to occasional summer rainfalls occurring immediately after dispersal, when the probability of seedling survival in dry-summer Mediterranean ecosystems is low. Hence, the non-deep physiological dormancy of Type 1 in both species should be interpreted as a strategy to prevent summer germination and to promote autumn germination. Such a strategy contributes to synchronizing germination to optimize the probability of seedling survival (Bell et al., Reference Bell, Plummer and Taylor1993; Baskin and Baskin, Reference Baskin and Baskin1998; Schütz et al., Reference Schütz, Milberg and Lamont2002).
Dry-stored seeds of both species showed higher germination under photoperiod conditions than in darkness as they overcame dormancy. Such differences, however, were more pronounced in Z. aragonensis. In most studies on germinative models of plant groups coexisting in the same habitat, there are always some species that germinate better under photoperiod conditions than in darkness (Thanos et al., Reference Thanos, Georghiou, Douma and Marangaki1991; Morgan and Lunt, Reference Morgan and Lunt1994; Schütz et al., Reference Schütz, Milberg and Lamont2002; Khan and Gulzar, Reference Khan and Gulzar2003). This light-mediated germination mechanism may favour germination of seeds located near the surface in areas of soil disturbance and may restrict germination of seeds buried too deep in the soil, having very little chance of seedling-emergence success (Grime, Reference Grime1979; Milberg et al., Reference Milberg, Andersson and Thompson2000). In addition, such a mechanism promotes the formation of persistent seed banks (Baskin and Baskin, Reference Baskin and Baskin1998; Schütz et al., Reference Schütz, Milberg and Lamont2002).
Germination phenology
Although most I. pectinata and Z. aragonensis seeds germinated in autumn, a small fraction did this in spring (Fig. 2), demonstrating that the two species are facultative winter annuals. In fact, plants from autumn-germinating seeds behave as winter annuals (the larger part), and those from spring-germinating seeds behave as spring ephemerals, which seldom complete their life cycle in Mediterranean dry-summer environments. Indeed, no surviving plant was detected at the end of summer in the natural habitat during the present study. In facultative winter annuals any non-dormant seeds that do not germinate in autumn are induced back into conditional dormancy by low winter temperatures (i.e. they lose the ability to germinate at high, but not at low temperatures) (Figs 3 and 4) and they can germinate in early spring. In contrast, in strict winter annuals any non-dormant seeds that do not germinate in autumn go into secondary dormancy due to low winter temperatures and cannot germinate in spring (Baskin and Baskin, Reference Baskin and Baskin1989, Reference Baskin and Baskin1992, Reference Baskin and Baskin2000).
The record of seedling emergence during three consecutive phenological cycles after seed sowing (2000–2003) (Fig. 2) demonstrated the ability of both species to form persistent soil seed-banks. This trait would be reinforced by the low ability of seeds of both taxa to germinate in darkness; this pattern is particularly pronounced in Z. aragonensis (Fig. 1). A previous work (Herranz et al., Reference Herranz, Ferrandis and Copete2003), which focused on the soil seed-bank of the entire weed community in the same botanical reserve, detected a short-term persistent seed bank (c. 45 viable seeds per m2) for Z. aragonensis.
In the case of endemic species with a narrow geographical distribution and with short life cycles, such as I. pectinata and Z. aragonensis, the presence of a persistent seed bank in the soil, even though of the short-term type, is essential for ensuring their conservation, since it allows re-establishment and maintenance of populations after disturbances without the necessity of an external seed source, and after years with poor seed set (Baskin and Baskin, Reference Baskin and Baskin1978, Reference Baskin and Baskin2000; Thompson et al., Reference Thompson, Bakker and Bekker1997).
Dormancy cycles in buried seeds and temperature requirements for induction and breaking of conditional dormancy
Buried seeds of I. pectinata and Z. aragonensis exhibited an annual conditional dormancy/non-dormancy cycle, being conditionally dormant at maturity, with dormancy loss occurring in summer (non-dormant seeds in autumn) and conditional dormancy induction in late autumn and winter (Figs 3 and 4). The higher ability detected in both species to germinate at low temperatures (5 and 15/4°C) after winter 2002 when compared to the same period in 2001 may be due to the longer period of burial, or to the inter-annual variability in climatic conditions. A similar trend was detected in Thlaspi arvense (Baskin and Baskin, Reference Baskin and Baskin1989), which showed a similar pattern to that recorded in Z. aragonensis, with months of low germination intercalated in series of high values (Fig. 4).
Seeds exhumed at the end of autumn had high germination ability in darkness at 5 and 15/4°C, temperatures that were similar to temperatures in the greenhouse in this season (Figs 3 and 4). However, few seedlings were detected in the nylon bags containing seeds throughout the study. Other factors, such as low O2 or high CO2 concentration have been suggested as being involved in the restriction of buried-seed germination (Karssen, 1980/Reference Karssen1981).
High temperatures, such as those occurring in the species habitat in summer (e.g. 32/18, 28/14°C), and even mild ones characterizing early autumn (e.g. 25/10°C), promoted loss of seed dormancy (Table 3). Low temperatures, such as those that occur in late autumn and winter (e.g. 15/4 and 5°C), induced seeds into conditional dormancy (Table 2). This high-temperature requirement for loss of dormancy and the low-temperature induction of non-dormant seeds into conditional dormancy have also been found in seeds of other facultative winter annuals, such as Aphanes arvensis (Roberts and Neilson, Reference Roberts and Neilson1982a), Lamium amplexicaule (Baskin and Baskin, Reference Baskin and Baskin1981), Thlaspi arvense (Baskin and Baskin, Reference Baskin and Baskin1989) and Veronica arvensis (Baskin and Baskin, Reference Baskin and Baskin1983).
In the present study, I. pectinata seeds buried in July 2001 were conditionally dormant in April 2002, showing a high ability (58%) to germinate at 5°C. This germination was even increased after 8 and 16 weeks of exposure to 5°C (Table 3), which indicates that cold stratification can cause seeds of I. pectinata to go from dormancy to conditional dormancy. Seeds of Z. aragonensis that were conditionally dormant in early May 2002, also lost dormancy markedly when exposed to 5°C for 8 weeks, increasing germination ability at 5, 15/4 and 20/7°C (Table 3), as recorded for Aphanes arvensis (Roberts and Neilson, Reference Roberts and Neilson1982a) and Veronica hederifolia (Roberts and Neilson, Reference Roberts and Neilson1982b). However, when the time of seed exposure to 5°C was extended to 16 weeks, seeds were induced again to dormancy; this phenomenon also was recorded in Thlaspi arvense (Baskin and Baskin, Reference Baskin and Baskin1989). Dormancy induction mediated by seed exposure to low temperatures was less pronounced in Z. aragonensis than in I. pectinata (Table 2). In this way, Z. aragonensis behaves as other facultative winter annuals, such as Stellaria media, Arenaria serpyllifolia and Cerastium viscosum, which were able to germinate at 5, 15/6 and 20/10°C after a 20-week period of exposure to 5 and 15/6°C (Baskin and Baskin, Reference Baskin and Baskin1986).
Another noteworthy aspect detected in the study was that buried Z. aragonensis seeds, non-dormant in November 2001 and exposed to 5°C for 16 weeks, showed a germinative ability of 22% at 5°C (Table 2), while seeds exposed to ambient conditions during the same period and exhumed in May 2002 were not able to germinate at 5°C (Table 3). It therefore seems clear that moist chilling at constant temperature was much less effective in inducing dormancy than burial outdoors during late autumn–winter. Thus, some other field factor apart from temperature may be partly responsible for governing the dormancy cycle of Z. aragonensis seeds. A similar pattern was recorded by Roberts and Neilson (Reference Roberts and Neilson1982b) in Veronica hederifolia. Those authors suggested that during burial at low temperature in predominantly wet soil, additional dormancy may develop as a result of an inadequate oxygen supply to the embryo. In contrast, I. pectinata seeds exhibited the opposite pattern: autumn moist chilling at 5°C was more effective in inducing dormancy at 5°C than seed burial under field conditions (Tables 2 and 3). Thus, although the major environmental factor causing changes in dormancy states is temperature, other factors, including darkness, desiccation level of the seeds, low oxygen level at high moisture levels, inorganic chemicals such as nitrate and nitrite, and organic chemicals such as ethylene and propylene, may be also important (Bouwmeester and Karssen, Reference Bouwmeester and Karssen1993a, Reference Bouwmeester and Karssenb; Baskin and Baskin, Reference Baskin and Baskin1998).
The present study shows that temperature plays a major role in bringing about the cyclic changes in dormancy of buried seeds of I. pectinata and Z. aragonensis and therefore in determining the time of seedling emergence. Since seeds of both species are conditionally dormant in late winter and early spring with germinative ability at 5°C and 15/4°C, and some dormancy break occurring at 5°C, these seeds might be able to germinate in early spring as well as in autumn. Thus, both species can be considered as facultative winter annuals.
Management recommendations
The reinforcement of wild populations of I. pectinata and Z. aragonensis and the threatened species S. cavanillesianum, all three of which were affected by the installation of the wind farm within the territory of Villarrobledo, was successfully carried out during 2003–2007, making the methodology recommendable for future programmes. We transplanted juveniles produced ex situ to the field and directly sowed seeds in the habitat. Juvenile plants were 3 months old and were produced in a non-heated glasshouse from early September (temperature: 25/10°C), in trays formed by individual 360 cm3 cell-containers. Plantation in the field was carried out in early December and initially watered. Sowing in containers was carried out by burying I. pectinata seeds at 2 mm depth, while Z. aragonensis seeds were laid on the substratum surface since they cannot germinate in darkness at 25/10°C (Fig. 1). For direct sowing in the natural habitat, we prepared mixed seedlots containing 100 seeds per species, which were then spread over ground tilled manually in 1 m2 plots, each in early October, coinciding with the beginning of the autumn rainfall period. Both methods of reinforcement were applied in suitably managed crop edges to increase their effectiveness (Walker et al., Reference Walker, Critchley, Sherwood, Large, Nuttall, Hulmes, Rose and Mountford2007; Fried et al., Reference Fried, Petit, Dessaint and Reboud2009).
Two features of the seed biology of I. pectinata and Z. aragonensis that generally are regarded as characteristics of weed seeds are the existence of a dormancy period, and the light requirement for germination, which prevent germination of seeds buried too deep and promote the formation of persistent soil seed-banks (Holt, Reference Holt, Altieri and Liehman1988; Baskin and Baskin, Reference Baskin and Baskin1990). These two characteristics help to explain how these species can persist in disturbed habitats such as agricultural fields, roadsides and sparse shrublands. Since both species complete their life cycle from October to June (spring-emergent seedlings do not complete their development), the practices for maintaining their periodically disturbed habitat in an early stage of succession (e.g. tillage, mowing, shrub removal) should be concentrated between 1 July and 15 October. However, due to the presence of soil seed-banks, management efforts may not be required annually.
Acknowledgements
This study was funded by the PAI07-0088-0300 (‘Creation of a plant germplasm bank of endangered species in the Botanical Garden of Castilla-La Mancha’), and PREG07-022 (‘Methodology for restoration of endangered plant populations’) projects, supported by the regional Government of Castilla-La Mancha. We also wish to thank the owners of La Encantada reserve for permission to perform the research on their land and K. Walsh for checking the English. The reviewers contributed to enhancing the quality of the manuscript.