Published online by Cambridge University Press: 10 June 2004
Knowledge about how parasites choose their hosts is scarce and incomplete. Recent work has primarily focused on host health (i.e. immunocompetence) whereas ecological factors have been largely neglected. Here we investigate whether the immunocompetence, the nutritional condition or body size of nestling European bee-eaters Merops apiaster are used as parameters for habitat choice of the haematophagous fly Carnus hemapterus. We found that (i) flies consistently and non-randomly preferred larger nestlings, even after controlling for differences in habitat availability (host surface), (ii) in the presence of similar-sized hosts, parasites' choice for an individual was less likely than if hosts differed in size, (iii) the more the hosts differed in size, the more the parasites aggregated on the larger nestling and (iv) parasites changed their preference according to size criteria regardless of the identity of the larger host. Neither immunocompetence nor host body condition could account for parasites' preference. Our results do not support the prediction of the Tasty Chick Hypothesis, namely that the poor immunocompetence ability of junior chicks makes them more attractive to parasites. We conclude that basic ecological factors (e.g. body size) can be essential for parasites when choosing a host.
Studies of avian host–parasite interactions have mainly focused on how parasites influence host fitness and behaviour (Møller, Allander & Dafva, 1990; Loye & Zuk, 1991; Møller, 1997). In turn, less attention has been paid to proximate mechanisms regulating host–parasite interactions such as how parasites find and select among hosts (e.g. Krasnov, Khokhlova & Shenbrot, 2003) or parasites' preference for certain host types (e.g. in relation to sex – Poulin, 1996; Schalk & Forbes, 1997). Moreover, recent work on the criteria used by parasites to choose hosts has focused on host health (see, for instance, Møller, Erritzoe & Saino, 2003; Schmid-Hempel & Ebert, 2003) whereas ecological factors (e.g. sociality or body size) influencing habitat choice by parasites have been largely neglected (but see, for instance, Rózsa, 1997a,b). However, comprehensive knowledge of how parasites choose their hosts could be particularly fruitful for understanding host–parasite relationships. For instance, definite and consistent parasites' preferences for certain host types could result in selective forces acting in favour or against specific features of the host important for surviving or mate choice. Christe, Møller & de Lope (1998) studied parasites' preferences within avian broods focusing on host immunocompetence and proposed the Tasty Chick hypothesis (TCH), whose main assumption is that the youngest and smallest chicks in a brood are of poorer quality (less immunocompetent) than their senior siblings and thus, more attractive to ectoparasites. This hypothesis predicts that parasites will aggregate (i.e. prefer) on such ‘tasty chicks’, which could be beneficial for the parent birds and siblings and concludes that parasites' preference for the last-hatched chicks in a brood could be a key factor in the evolution and maintenance of hatching asynchrony.
In contrast to the TCH, other studies suggest that aggregated distribution of parasites within broods reflects parasites' preference for larger hosts or individuals in good condition since these host types might be a better food source than smaller hosts or individuals in weak condition (Lee & Clayton, 1995; Darolová, Hoi & Schleicher, 1997; Dawson & Bortolotti, 1997). This hypothesis predicts that parasites prefer the fittest chick in a brood (which is usually the oldest and biggest one). Both hypotheses, more or less implicitly, assume that the most important factor determining host attractiveness for the parasite is the ability of the host to offer resistance to parasitism. However, morphology, life-history and ecological requirements of parasites can play a critical role in the determination of host preference (Roulin et al. 2003). For instance, body size by itself is a basic ecological factor that has been shown to determine the abundance of ectoparasites at an interspecific (e.g. Marshall, 1981; Poulin & Rohde, 1997; Rózsa, 1997a,b) and intraspecific level (see, for instance, Grutter & Poulin, 1998). Host individuals can be seen as habitat islands for parasites and the larger an island is, the more abundant ectoparasite assemblage is expected to inhabit it. Moreover, ectoparasites could select hosts on the basis of other factors not related to feeding, like body temperature (that can be important for parasite thermoregulation) or availability of refugia (e.g. larger birds provide more types of refugia used by ectoparasites to avoid host defences (Rózsa, 1997a,b)). Ideally, parasites should choose the host providing the best set of resources but it is also possible that ectoparasites move from one host to another to exploit different resources from each. In other words, ‘tastiness’ of hosts (sensu Christe et al. 1998) can change according to the different needs of the parasite.
Here we examine whether the immunocompetence, the nutritional condition or body size of the host is used for habitat choice by the haematophagous fly Carnus hemapterus (Nitzsch 1818, Diptera: Carnidae), an ectoparasite of European bee-eaters (Merops apiaster). We performed a ‘host-preference test’ exposing 2 individuals to a given number of parasites to answer the following questions. (i) Do parasites prefer specific chicks? (ii) Is the aggregation of parasites on particular nestlings a size related effect? (iii) Are parasites' preferences related to host immunocompetence (measured as T-cell mediated immune response) or nutritional condition? Indeed we test the prediction of the TCH that the youngest and smallest chicks in a brood are more attractive to ectoparasites and thus will be preferred by the latter. To our knowledge no study has tested the TCH including host features ecologically relevant for parasites and host ability to resist parasite infection. Finally, we discuss the consequences of host preferences in this host–parasite system for both the parasite and the host species.
The European bee-eater is a burrow-nesting, colonial breeding migratory species. A clutch of 4–7 eggs is laid at approximately 2-day intervals and there is a progressive increase in incubation during laying, which produces a high degree of hatching asynchrony. Size hierarchy represents the hatching sequence and it is maintained throughout the nestling period (Lessells & Avery, 1989).
The ectoparasite Carnus hemapterus is a 2 mm long fly that parasitizes nestling birds (Kirkpatrick & Colvin, 1989; Dawson & Bortolotti, 1997; Roulin, 1998). Winged flies are more common at the beginning of the season whereas the abundance of wingless forms increases during the season (Roulin, 1998). Carnus hemapterus is haematophagous (Kirkpatrick & Colvin, 1989; Dawson & Bortolotti, 1997) and therefore is capable of having detrimental effects on nestlings. However, published evidence has been equivocal. Some studies (Walter & Hudde, 1987; Kirkpatrick & Colvin, 1989; Dawson & Bortolotti, 1997; Liker et al. 2001) could find no evidence that infestations adversely affected hosts. In contrast, other works described detrimental effects, from lower haematocrit and haemoglobin concentrations (Whitworth, 1976; Schulz, 1986, 1990), to lower mass growth rate (Lacina, 1999), blood loss (Soler et al. 1999) and even death (Cannings, 1986). Concerning the bee-eater, Hoi et al. (manuscript in preparation) found an association between parasitism by carnid flies and lower nestling weight in an adverse year but not in a favourable one. In our study area, parasite prevalence is high (about 80% of the nestlings being infested) and parasitic load can reach up to more than 40 flies per nestling (Kristofík, Masán & Sustek, 1996). At the age of maximal parasite intensity (ca. 15 days old) we found a mean parasitic load of 31·9 flies ±10·2 (S.E., n=37 nestlings) (Hoi et al. manuscript in preparation).
Three characteristics make this host–parasite system suitable for this study. (i) This fly parasitizes nestlings only (Kristofík et al. 1996). (ii) Carnid flies do not need the host for transmission since flies colonize nest hosts actively during the winged phase of their life-cycle (Grimaldi, 1997; Roulin, 1998, 1999). Therefore, it is unlikely that the need for successful transmission influences C. hemapterus choice of hosts. (iii) Bee-eaters show large intrabrood differences in nestling size and age as a consequence of a marked hatching asynchrony (Lessells & Avery, 1989).
The study took place during the 2000 breeding season, when 38 bee-eater nestlings were taken from several breeding colonies located in southern Slovakia. Parents were left with at least 2 chicks in the nest and after the test chicks were returned to burrows. This procedure is not pernicious either for chicks or for adults (Krebs & Avery, 1984).
It has been described for many hosts of C. hemapterus that infestations diminish as birds age because the increased density and layering of feathers make nestlings an inhospitable environment (Kirkpatrick & Colvin, 1989; Dawson & Bortolotti, 1997; Roulin, 1998; Liker et al. 2001). Since we wanted to highlight preference patterns of the parasite, we focused on the age classes subjected to parasitism, i.e. non-feathered nestlings. Thus, collected nestlings ranged between ca. 5 and 15 days old, therefore excluding feathered ones (22-day-old birds appear fully feathered, Cramp, 1985).
Four morphological parameters of nestlings were measured: tarsus length, wing length (without quills), outermost primary length and mass. For the purposes of this work, body surface is the most important morphological parameter as parasites can distribute among nestmates proportionally to the availability of habitat. We therefore calculated the surface area of each individual by using the proposed exponent of the intraspecific allometric relationship between body surface and mass (surface=mass0·67) (McMahon & Bonner, 1983; Heusner, 1985).
For the study of the relationship between body size and body temperature we used 23 bee-eater nestlings collected from different colonies during the same breeding season in the framework of a different research. The nestlings were within the same age range (ca. 5–15 days old) as the ones used for the parasite choice test. Shortly after their capture (maximum 3 h), the nestlings were measured (wing and tarsus length) and their body temperature registered to the nearest 0·01 °C by placing a thermometer in the cloaca until a constant value was displayed. The nestlings were collected within a week, kept individually in containers, and body temperature was taken at a similar hour of the day (12.00 a.m.–16.00 p.m.).
The experiment consisted of a ‘host-preference’ test in which 2 nestlings were exposed to a given number of carnid flies. We chose our experimental pairs randomly but trying to have a gradient of size (i.e. surface) differences (Table 1). Therefore, members of some pairs had a similar size, widely overlapping in their morphological measurements, whereas members of some other pairs differed in all morphological measurements taken. The median body surface ratio was 1·33 (range=1·008–2·16, lower and upper quartile=1·13, 1·76 respectively). Parasites were collected from many different bee-eater nests and temporarily kept in dark boxes with sand until added to the experimental pairs.
Table 1. Summary statistics for the factors under study: body size (wing length), body mass and T-cell immune response (wing web index) (Mean values (±S.E.) and minimum/maximum values (in parentheses) are shown for experimental individuals and for the differences between members of the experimental pairs.)

Every pair was housed in cardboard boxes measuring approximately 25×25×20 cm, with an environment simulating natural conditions (dark place with sandy ground). Twenty wingless adult parasites were placed into the box with the experimental pair, in the opposite corner to where the nestlings stayed. Such numbers of ectoparasites are below those typical of the age range of our nestlings (Hoi et al. unpublished data), thus preventing misleading results due to different degrees of intraspecific competition among parasites according to host size (Kristofík et al. 1996). We recorded the number of ectoparasites on each nestling 45 min after adding the flies by screening the whole body of both birds. This visual census method of assessing ectoparasite intensity has been found to be reliable (Roulin, 1998; Roulin et al. 2001). To assess how the effect of the treatment (size difference) varied with time (i.e. consistency in host preference) we conducted 2 more successive trials with an interval of 45 min each. In other words, after counting the number of flies on each nestling, flies were removed and added again to the same experimental pair. Forty-five min later we checked the number of flies in each nestling again. This was repeated a third time. Thus, during each test, and with an interval of 45 min, we added 20 parasites 3 times, and counted the flies in each nestling, collected the flies and added them again 3 times. As a rule the same flies were added to each pair during the 3 successive trials but in some cases not all flies could be recovered for the next trial and, thus, new flies had to be added to make up the full quota of 20 individuals (see below for statistical considerations). Although flies probably expend much time on the hosts they also move around under natural conditions (flies are frequently found in the sand when sampling nests) and, in our case, some flies probably escaped from the box. On average we counted 62·22% of the flies on the nestlings (n=26 pairs, S.E.=3·39, upper and lower quartile: 47·85%, 78·33% respectively). It could be argued that non-recovered parasites could be hidden on the birds. If so, we would expect a negative relationship between the percentage of recovered parasites and the size of the nestling (larger birds provide more refugia). We did not find such a relationship (r=0·06, P=0·75, n=26). Every pair was tested again (i.e. subjected to 3 successive trials) the next day. Parasites used for this replica were not the ones used in the previous one. Tests were run at ambient temperature (mean daily temperature was ca. 21 °C).
Our experimental design included the repeated use of some randomly selected nestlings in different pairings as an additional way to test if size differences were more important for parasites than other variables not related to size. If size is a factor ruling parasites' decisions, we would predict that changing the size rank of a given nestling in different experimental pairs should influence parasites' preferences. For instance, let us assume that parasites prefer larger chicks. Then, if a specific nestling were larger in a first experimental pair but smaller when compared to a second, different individual, we would expect that parasites should prefer that specific individual in the first combination but not in the second. Similarly, if a given individual was larger in a first pairing but of similar size to the partner (mass differences lower than 4 g) in a second, different combination we would expect parasites to choose that particular individual in the first case whereas no selection should occur in the second pairing.
Thirty-six nestlings were used to form 26 pairs, therefore 11 individuals were used in more than 1 test. Concerning the sample size for the experiment based on the repeated use of individuals, our data set consisted of 7 nestlings whose size ranking varied in 2 different pairings.
We consider that the repeated use of hosts and parasites does not bias our results for the following reasons. (i) We work on the basis of ‘experimental pair’ and not ‘individual’ given that parasites had to compare and choose between 2 nestlings. Thus, for our analyses we did not use the values (body size, immunocompetence, body mass) of each nestling in a pair but the differences in such variables between members of each pair. Moreover, data from the repeated use of specific individuals show that size rank of the host is more important than other individual variables non-related to size (see Results section). (ii) It could be argued that if the immune system of nestlings used in multiple pairings responded to increased parasite exposure, the results could be biased. Exposure to a low number of ectoparasites during a short period probably does not bias the results. Moreover, all nestlings had been parasitized by carnid flies prior to our research and multiple pairings occurred at maximum during an interval of 2 days, one of them used for assessment of immune response.
To assess the robustness of our results, we repeated our analyses by using each nestling once. The results did not differ from the ones obtained when using some nestlings to form several different pairs. Therefore, we here report on the results obtained with our complete data set (i.e. 26 pairs formed with 36 nestlings).
Re-use of flies within an experiment was a way to measure consistency in the selection of the parasites. Such consistency is, in fact, a verification that parasites are certainly choosing and that their choice is not provisional. Including new flies in successive trials reinforces our results, since new flies could need some time to compare and select the best host. In turn, we found a high consistency in the selection within each experiment (see below).
For the purpose of determining the relative health of each nestling (i.e. immunocompetent status) we measured the intensity of T-lymphocyte response, a cell-mediated in vivo immune response to an injection of 0·1 mg of phytohaemagglutinin (PHA-P, Sigma Chemical Co., St Louis, USA) in 0·02 ml of phosphate-buffered saline (PBS) in the middle of the wing web. Before injection the thickness of the right and left wing web was measured with a digital ruling calliper to the nearest 0·01 mm. In the other wing we injected the same volume of PBS. Twenty-four hours later the thickness of the wing web at the inoculation sites was measured. The change in thickness in the wing where PHA was injected minus the change in the control wing was used as a measure of T cell immune response (wing web index) (McCorkle, Olah & Glick, 1980). Immune response was not measured in 1 nestling, which accounts for differences in sample size when comparing immunological data with other variables (Table 1).
The use of T-lymphocyte response to assess immunocompetence is a well-established method (McCorkle et al. 1980) and is an adequate technique for our purposes since the intensity of the T-cell response is known to be important for resistance against ectoparasites: the antigens released from the feeding parasites are processed and presented to T cells, which initiate inflammatory and antibody responses (Wakelin, 1984). Similarly, PHA has a mitogenic effect on T-lymphocites, and the injection stimulates macrophage infiltration and perivascular accumulation of lymphocytes (McCorkle et al. 1980). This method has been used in a similar context to the one here considered (see Christe et al. 1998; Roulin et al. 2003).
Observed frequencies of ectoparasites were tested against expected ones by using the Heterogeneity chi-square test (Zar, 1984). In the absence of size differences (i.e. nestlings of the same size) the expected frequencies should logically respond to the ratio 1[ratio ]1. However, the surface of a larger nestling is a larger patch of space and therefore may harbour more flies even if flies distribute randomly (Rózsa, 1997a). We therefore calculated the ratio of surface areas between members of each pair and calculated the expected number of ectoparasites accordingly. Our null hypothesis is that parasites distribute between members of a pair of nestlings randomly, and thus proportionally to their body surface. The heterogeneity chi-square test also allows us to examine if relative parasite numbers for each trial come from the same population, therefore informing about consistency or homogeneity in parasites' preferences (see Zar, 1984).
For every trial we calculated the percentage of parasites per nestling in relation to the total number of parasites found on both nestlings. In a few cases less than the half of the parasites was found on the nestlings. These cases were not included in the analysis. Given that parasites showed high consistency in the selection of the host both within each replica (i.e. during the 3 successive trials) as well as between both replicas (see results), we averaged the percentages obtained in all trials to get an overall parasitic load/individual (mean parasitic load hereafter).
Body condition has usually been calculated from the residuals of body mass against a body size indicator (e.g. wing length) (see, for instance, Ranta, Laurila & Elmberg, 1994). However, this method has been recently criticized (García-Berthou, 2001; Freckleton, 2002). Thus, to estimate the effect of body condition on parasite choice we used body mass after removing the effect of body size (i.e. wing length) by means of ANCOVAs and multiple regression techniques, as recommended by García-Berthou (2001) and Freckleton (2002).
We used regression analyses to highlight the relationships between the pattern of host selection by the carnid flies and immunocompetence, size and body condition of potential hosts. In a first analysis our dependent variable had a binary nature: ‘pairs where parasites significantly chose an individual’ versus ‘pairs where parasites were distributed randomly’. We used a generalized linear model (McCullagh & Nelder, 1989) as a mathematical description of the relationship between the pattern of host selection by the carnid flies and within-pair differences in immune response, surface ratio, wing length and mass. For our dependent variable we used a binomial function for the error and a logistic link. A forward stepwise procedure was used entering predictor variables one by one and excluding variables not significantly contributing to the model.
Additionally we used a multiple regression to examine whether the variation in strength of the choice among pairs (i.e. the difference in parasite load between pair members) is related to differences in body size, immunocompetence and body condition. We used the mean parasitic load of the larger bird as the dependent variable, and within-pair differences in immune response, within-pair surface ratio, and differences in wing length and mass between pair members as independent variables. Obviously, some of our independent variables (the last 3) were intercorrelated. Each of them contributes some different piece of information: area ratio reports about differences in habitat availability (surface) between potential hosts. This measurement of size is more meaningful for parasites than a linear measurement of body size like wing length. Weight reports about the importance of condition on parasite choice after control for the effect of a body size indicator (wing length). Following Freckleton (2002), we report about semi-partial correlations to highlight how much variation each variable explains independently of each other. Semi-partial correlations measure the unique contribution of each variable relative to the total variability in the dependent variable and, therefore, are a good indicator of the practical relevance of a predictor (Freckleton, 2002).
Parametric tests were used where the assumptions for normality were met. In some cases logarithmic or arcsine transformations were used to meet the requirements for normality. Unless otherwise stated mean and standard errors are provided and two-tailed tests used. Statistical analyses were carried out with the STATISTICA 6.0 package (StatSoft, Inc 2001).
Parasites' choice was highly consistent: in 24 out of 26 pairs parasite distribution was homogeneous both when comparing the successive trials within a replica and when comparing the 2 successive replicas (heterogeneity chi-square test, P>0·1 in all cases). In the 2 remaining pairs parasites' preferences differed between the first and the second replica, and therefore, these pairs were not included in further analyses.
Sorting the birds of each pair into ‘larger’ and ‘smaller’ (Fig. 1) we found that host size influenced parasites' distribution given that parasite burdens were significantly high on the larger chick (t-test, t=10·0, D.F.=46, P<0·001) (Fig. 1). In fact we found that in 21 out of these 24 pairs, flies were more abundant on the larger chick and in no case were parasites significantly more abundant on the smaller nestling of the pair (heterogeneity chi-square test, P>0·1 in all cases). That larger chicks may harbour more flies than smaller hosts is not surprising. However, in 16 (66·7%) out of 24 pairs (in 12 cases P<0·001, in 2 pairs P<0·01 in 1 pair P<0·05 and only in 1 case P=0·06) the parasite load of the larger chick was significantly larger than expected according to body surface differences. In these 16 pairs, flies overwhelmingly preferred the larger chick (Fig. 2). Within-pair differences in body condition (i.e. differences in body mass after removing the effect of body size) did not vary between pairs where parasites significantly chose a host and pairs where parasites did not choose (ANCOVA, F=2·1, D.F.=1,21, P=0·15).

Fig. 1. Mean parasitic load (+S.E.) of each pair member after sorting the birds of each pair according to body surface and immunocompetence (wing web index) (figures on the top of the bars are number of individuals used).

Fig. 2. Mean parasitic load (+S.E.) of the larger chick in each pair (figures on the top of the bars) for pairs where parasites significantly choose a host and for pairs where parasites do not choose.
In contrast, sorting the pair members in ‘lower’ and ‘higher’ immunocompetence revealed that immunocompetence had no effect on parasites' distribution (t-test, P>0·1) (Fig. 1). Differences in body surface between pair members did not influence the former analysis as evidenced by the fact that in 8 out of 15 cases where parasites significantly selected a nestling (after correcting for size differences) the preferred host was of lower immunocompetence whereas in the remaining 7 cases parasites chose the nestling of higher immunocompetence (one-sample binomial test, z=0·26, P=0·79).
A logistic regression analysis provided a model where only area ratio (Wald's χ2=4·64, P=0·03) explained the pattern of host selection by C. hemapterus (i.e. pairs where parasites exhibited a significant preference versus pairs were parasites were distributed randomly) (deviance of the null model=29·72, D.F.=22; deviance of the final model=22·25, D.F.=21). This result is further supported when examining the factors accounting for variation in parasitic load among pairs. A multiple regression analysis provided a significant model explaining variation in the mean parasitic load of the larger bird (F=5·40, D.F.=4,18, P=0·005, R2=0·54). Again, only area ratio entered the model (β=0·82, P=0·016, semi-partial correlation=0·42), therefore suggesting that the larger the differences in body surface between the pair members, the more the parasites aggregated on the larger one (Fig. 3). Neither within-pair variation in immunocompetence nor differences in body mass seemed to have any obvious effect on parasites' preferences.

Fig. 3. Relationship between body size differences between pair members (area ratio) and mean parasitic load of the larger nestling in each experimental pair (n=24 pairs).
Data from our repeated use of particular nestlings support that size was the factor ruling parasites' decision and that carnid flies preferred larger hosts. Changing the size rank of 7 individuals in 2 successive pairings resulted in 6 cases where parasites behaved as predicted in both combinations, i.e. choosing the larger individual regardless of the identity or not choosing in the absence of size differences. This observed probability differs from that expected by chance (one sample binomial test, one-tailed, z=1·89, P=0·029). The only case failing came from an experiment where the parasites significantly preferred a given individual when faced with a smaller one, but the parasites' preference did not reach significance when the same nestling was faced with a larger one.
Body size and temperature of nestling bee-eaters was positively related (wing length: r=0·49, P=0·017; tarsus length: r=0·61, P=0·002, n=23 in both cases), so that younger, smaller birds had a lower body temperature than older, larger ones.
Our host-preference experiments show that C. hemapterus flies consistently aggregated on the larger individual since (i) in most pairs more flies were found in the larger nestling and (ii) such preference is maintained with time (i.e. during the approximately 2 h that each experimental session lasted). Aggregation of C. hemapterus on larger, non-feathered bee-eater nestlings has also been found in a field study (Hoi et al. manuscript in preparation). Similarly, studies on starlings Sturnus vulgaris (Walter & Hudde, 1987; Liker et al. 2001), American kestrels Falco sparverius (Dawson & Bortolotti, 1997) and European barn owls Tyto alba (Roulin, 1998) report that C. hemapterus flies were more likely to infest the largest nestlings (before developing feathers) in a nest. Roulin et al. (2003) reported that both in the barn owl and Eurasian kestrel (Falco tinnunculus) carnid flies were more abundant on junior chicks. This seeming contradiction can be explained by the fact that these authors worked with feathered and non-feathered chicks; it is well known that feathers make nestlings an inhospitable environment for this parasite (Kirkpatrick & Colvin, 1989; Dawson & Bortolotti, 1997; Roulin, 1998; Liker et al. 2001).
That more flies are found on the larger nestling can be easily explained by a simple reasoning, namely that larger hosts offer more space for parasites (Rózsa, 1997a). None of the above-cited works corrected for size. After controlling for habitat availability (i.e. differences in body surface) we still found that parasitic load in larger nestlings was much higher than expected in a considerable percentage of cases (67%). Moreover, our results suggest that the key variable followed by parasites for choosing a host was size differences between experimental individuals since (i) in the presence of hosts of similar size, parasites' choice for a given individual was less likely than if hosts differed in size, (ii) the larger the within-pair differences in body size, the more the parasites aggregated on the larger nestling. This, in fact, suggests that the more asynchronous the brood the more parasites gather on the oldest nestlings, what contradicts the proposal of the TCH, (iii) using nestlings in successive pairings that modified the size rank of the hosts revealed that parasites changed their preference according to size criteria regardless of the identity of the larger host.
What benefits may carnid flies get by choosing large nestlings? Several authors (see, for instance, Blanco, Tella & Potti, 1997; Darolová, Hoi & Schleicher, 1997) have described a positive relationship between body condition and parasitic load, therefore supporting the idea that parasites were choosing good-quality hosts. A similar reasoning has been specifically exposed by Dawson & Bortolotti (1997), who found that nestling American kestrels infested with C. hemapterus within a nest were heavier than uninfected chicks whereas there were no differences in wing length. These authors argue that since C. hemapterus procures resources from their hosts, it would seem wise for them to choose the healthiest host. Our data do not support a direct effect of body condition on parasites' choice, which agrees with the results obtained by Roulin (1998, 1999) and Roulin et al. (2001), who found that C. hemapterus abundance and fecundity were not related to physical condition of young barn owls. However, there could still be a role for condition. Physical and mechanical factors can be important for ectoparasites when choosing a host. For instance, biting insects are limited by the skin thickness/composition of their host to extract large amounts of blood (Lehane, 1991). It seems commonplace for researchers used to extracting blood from nestlings that it is easier to collect blood from healthy, warm and smooth-skinned ones with fluid blood circulation than from sick, cold, wrinkled-skinned nestlings. We also found a positive relationship between body temperature and body size so that younger birds had a lower, and probably less stable body temperature than older ones (see also Bezzel & Prinzinger, 1990). Flies are ectothermic, thus those on a warmer host will become warmer themselves, and consequently can speed up their metabolism and breeding as compared to conspecific flies on colder chicks. Parasites' preferences for larger chicks would render them an adaptive value and therefore healthy rather than poor condition chicks would be ‘tasty’ chicks (sensu Christe et al. 1998). Carnid flies could assess host quality indirectly, following a general rule of thumb, larger chicks are in better condition, which could work in most cases.
Alternatively it could be that other factors apart from immunocompetence and body condition are more important for parasites when choosing a host. Body size can be a preferred feature per se. Flies could choose larger individuals because they provide more diverse types of the refugia used by ectoparasites to avoid host defences (Rózsa, 1997a,b).
An alternative way to study host preference is to examine the costs of a ‘wrong’ choice for the parasites. From the point of view of parasites, host selection should try to reduce the risk of moving from host to host. Carnid flies probably move from one nestling to another as the elders grow feathers (Kirkpatrick & Colvin, 1989; Roulin, 1998). The risk of losing a host is probably lower for carnid flies if they move from the older nestling (as it becomes feathered) to the next old one than if they choose the weakest nestling (with higher probabilities of dying) and have to move to older nestlings, which can be already feathered and therefore inhospitable. To select a large nestling ensures an appropriate habitat for the present and the future (the second, third … nestlings in the clutch that still have no feathers). Thus, in the case of C. hemapterus, preference for larger hosts can also be age related.
In the light of the TCH Christe et al. (1998) predicted an increase of the frequency of hatching asynchrony in colonial breeding and hole nesting bird species, 2 groups of birds particularly subject to virulent parasitism, as a way to partially circumvent the detrimental consequences of parasites. Evidence in favour of the TCH comes from observational studies on ticks (Ixodes lividus) parasitizing Sand martins (Riparia riparia) (Szep & Møller, 1999, 2000). The results of the 3 studies that, to our knowledge and to date, have explicitly addressed the predictions derived from the TCH are contradictory. Roulin et al. (2003) found that some ectoparasite species preferred junior chicks whereas some others were randomly distributed or preferred senior chicks. Descamps et al. (2002) found no evidence for aggregation of parasites (Protocalliphora spp.) on the last-hatched chick of Blue tits (Parus caeruleus) whereas Simon et al. (2003), working on the same species and population, found that low ranking chicks lost more blood. Our results show that C. hemapterus flies consistently aggregated on the larger individual. If we assume that the host on which a parasite is found equals the host that is parasitized (an assumption supported by the association between presence of the fly and occurrence of scabs, Kirkpatrick & Colvin (1989), Fitzner & Woodley in Grimaldi (1997); personal observations) our results would not support the TCH. An alternative interpretation of our findings is that parasites do not feed on the chick where they are found and that flies choose larger hosts for other purposes (e.g. thermoregulation) than feeding. If so, our study would describe a different perspective of host–parasite interactions: the ‘tastiness’ of hosts can change according to the different needs of the parasite.
Our test of the TCH with the bee-eater (a colonial hole nester with a relatively extreme degree of hatching asynchrony) suggests that Christe et al.'s predictions cannot be generalized mainly because immunocompetence is not the only factor determining host choice by parasites. Roulin et al. (2003) concluded that the within brood distribution of parasites is not generally ruled by rank-related variation in host defence of chicks and that other factors could equally determine the former. We found that a basic ecological factor like host body size seems to be more significant for explaining within-brood distribution of parasites in bee-eater than the quality of the host.
We thank L. Rózsa and A. Roulin for reviewing and greatly improving earlier versions of this manuscript. B. Walther gave valuable suggestions and A. Roulin also shared with us unpublished information. R. Cannings kindly provided useful literature. Two anonymous referees made valuable criticisms. Christine Hoi was of a great help during fieldwork. F.V. was supported by a DGES contract from the project PB97-1233-C02-01 and by an l3P contract funded by the European Union. A.D and J.K. were funded by VEGA project 7170/00.

Table 1. Summary statistics for the factors under study: body size (wing length), body mass and T-cell immune response (wing web index)
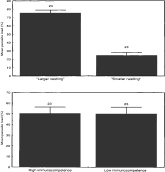
Fig. 1. Mean parasitic load (+S.E.) of each pair member after sorting the birds of each pair according to body surface and immunocompetence (wing web index) (figures on the top of the bars are number of individuals used).

Fig. 2. Mean parasitic load (+S.E.) of the larger chick in each pair (figures on the top of the bars) for pairs where parasites significantly choose a host and for pairs where parasites do not choose.

Fig. 3. Relationship between body size differences between pair members (area ratio) and mean parasitic load of the larger nestling in each experimental pair (n=24 pairs).