Introduction
Anti-N-methyl-D-aspartate receptor (NMDAr) encephalitis is a relatively recent autoimmune entity, as it was first described in 2007. Given that it is a neuropsychiatric condition, inaugural symptoms may well be psychiatric in nature. One of those symptoms may be catatonia. Hence, psychiatrists are often the first physicians to assess these patients.
Even though it has been long established that catatonia may happen in both primary psychiatric disease and organic conditions, there are still no official guidelines on how to manage the patients whose catatonia derives from medical causes, such as anti-NMDAr encephalitis.
In this literature review, we aimed to (1) briefly describe the concept of catatonia and its evolution throughout time, (2) discuss the most important points on anti-NMDAr encephalitis and (3) discuss the management of catatonia secondary to anti-NMDAr encephalitis. Our search was also designed to filter the clinical cases that reported the existence of a catatonic syndrome related to anti-NMDAr encephalitis. These were compiled into a table and the chosen management and recovery outcome were highlighted.
Methods
We performed a PubMed database search with the truncated terms “Catatoni*” and “anti-NMDA receptor encephalitis.” The search was last done on March 19, 2019. As the available literature on the subject of the relationship between catatonia and anti-NMDAr encephalitis is not vast, we considered any relevant studies published in English, regardless of date of publication, sample size, outcomes, comparators, or length of follow-up as potentially eligible for this article. As the potential number of available articles was small, we have also included single case reports. The research yielded 76 results from PubMed. Off-topic papers were excluded, as were case reports that did not feature explicitly identified catatonia. This resulted in the inclusion of 60 papers from the original PubMed research.
Due to our intention to briefly discuss catatonia and anti-NMDAr encephalitis, relevant data from other mentioned in the abovementioned papers but pertaining to other sources were included in this revision and the original papers cited. This led to the inclusion of 107 extra papers. In total, 167 papers remained for inclusion in the review. Figure 1 shows the article selection process for review.

Figure 1. Article selection process for review.

Figure 2. A new area of potential research: catatonia as the tip of the iceberg in various neuropsychiatric disorders (eg, anti-NMDA receptor encephalitis).
All clinical cases resulting from the first PubMed search which reported to patients with catatonia secondary to anti-NMDAr encephalitis were compiled in a table and information on sex, age, management, and recovery outcome was highlighted. Management was defined as the treatment modalities started once anti-NMDAr encephalitis was confirmed or suspected; previous treatments were not mentioned. Outcomes were based on clinical descriptions: expressions equivalent to “return to premorbid state” or descriptions of full disappearance of remaining deficits at follow-up were classified as total remission, whereas expressions like “significant/substantial recovery” or any grade of “improvement” were classified as partial remission.
Results
Catatonia
The term catatonia was first conceptualized and described by the German psychiatrist Karl Ludwig Kahlbaum in 1874, Reference Carroll1, Reference Judd and Burrows2 which is why the term is also recognized by some as “Kahlbaum syndrome.”Reference Peralta, Cuesta, Serrano and Mata3 It describes a complex neuropsychiatric syndrome that combines psychic and motor symptoms (described below), with a characteristic behavioral pattern and autonomic dysregulation:Reference Kaestner, Mostert and Behnken4–Reference Dhossche and Wachtel7
• Catalepsy: postures that are maintained by the patient, including the mundane ones;
• Rigidity: increase in muscle tonicity/resistance to passive movement;
• Waxy flexibility: initial resistance the patient offers during reposturing;
• Stupor: extreme hypoactivity and immobility;
• Motor excitement: extreme hyperactivity and constant motor nonpurposeful unrest;
• Echopraxia and echolalia: mimicry of the examiner’s movements and speech;
• Mitgehen: positivism, passive obedience in response to pressure, despite contrary instructions;
• Gegengreifen: responsive grasping;
• Grimacing: maintenance of odd facial expression;
• Mannerisms: odd, purposeful movements, exaggerated caricatures of mundane movements;
• Stereotypies: repetitive, non-goal-directed motor activity.
• Mutism: verbally unresponsive or minimally responsive;
• Perseveration: repeated return to the same topic or persistence of movements;
• Verbigeration: repetition of phrases or sentences;
Kraepelin later classified catatonia as part of dementia praecox, but noted that it also occurred in manic-depressive illness, Reference Carroll1 whereas Blueler included catatonia in his definition of schizophrenia. The German nosologist Leonhard was the first to identify catatonia as belonging to other disorders, as his definition included catatonia as a part of schizophrenia, affective psychosis, and cycloid psychosis.
Although catatonia was formally associated with schizophrenia throughout the 20th century, Reference Fink8 nowadays it is well established that catatonia is an entity associated with both primary psychiatric and secondary psychiatric (organic) conditions. In regard to psychiatric illnesses, catatonia is often found in schizophrenia, bipolar disorder and major depression, Reference Carroll, Goforth and Thomas9 while neurological illnesses are among the most common causes of organic catatonia.Reference Carroll, Anfinson, Kennedy, Yendrek, Boutros and Bilon10 Because of this duality in etiology, diagnostic mistakes among patients who present with a psychiatric disturbance as the initial feature have frequently been reported, and the differential diagnosis between psychiatric and nonpsychiatric causes is only considered when neurological signs arise.Reference Judd and Burrows2 Hence, catatonia may present itself as a difficult diagnostic dilemma, and a catatonic disorder due to general medical conditions must be considered in every patient with catatonic signs. The fact that catatonia can lead to accelerated medical decompensation requiring rapid and effective treatmentReference Carroll, Goforth and Thomas9 and that these patients are also often treated on hospital medical floors by internists and consultation-liaison psychiatrists only stresses the importance of remembering and excluding organic causes when assessing a catatonic patient.Reference Daniels11
Primary and secondary catatonia epidemiology
Although catatonia appears to be common among psychiatric patients—with reportsReference Lee, Glick, Dinwiddie, Rosa and Marcolin12 suggesting that up to 10% of these inpatients experience catatonic symptoms—it is likely underdiagnosed by psychiatrists and other physicians.Reference van der Heijden, Tuinier, Arts, Hoogendoorn, Kahn and Verhoeven13 A studyReference Daniels11 reported that catatonia was diagnosed clinically in only 1.3% of acute psychiatric inpatients but closer scrutiny showed that actually 18% of patients exhibited 2 or more catatonic signs, highlighting the frequent underdiagnosis of catatonia in routine clinical settings. The prevalence of catatonia is unknown. In acute medical settings, prevalence numbers range from 1.6%Reference Carroll and Spetie14, Reference Cottencin, Warembourg and Lenclave15 to 6.3%Reference Jaimes-Albornoz and Serra-Mestres 16 and although conditions associated with catatonia vary with the clinical setting, it is estimated that 1 in 4 patients with catatonia has it due to a general medical condition (Diagnostic and Statistical Manual of Mental Disorders-5 criteria).Reference Oldham and Lee17 The majority of medical catatonia appears to be due to neurological disease.Reference Oldham and Lee17, Reference Ramírez-Bermúdez, Aguilar-Venegas and Calero-Moscoso 18 In a systematic review on the relationship between catatonia and delirium, Oldham et al.Reference Oldham and Lee17 described other reviews that reported on epidemiologic data of catatonia due to medical causes: a systematic review of medical catatonia cases found that roughly 70% were due to a neurological condition, Reference Carroll, Anfinson, Kennedy, Yendrek, Boutros and Bilon10 30% of which were associated with structural CNS disease, 25% with encephalitis or other CNS infection, and 10% with seizure disorder. Likewise, a retrospective Mayo Clinic chart review identified 95 in-house cases of catatonia per DSM-IV-TR criteria, 20 of whom had medical catatoniaReference Smith, Smith, Philbrick and Kumar19 and among those, 70% had neurological illness: 6 patients suffered from encephalitis, 4 from major neurocognitive disorder, 3 from seizure disorders, and 1 from CNS metastases. Moreover, a 2008 review of pediatric cases of medical catatonia identified 26 cases related to medical conditions and 12 related to medications or toxins.Reference Lahutte, Cornic and Bonnot20 Among the 26 medical catatonias, 10 were related to neurological conditions, 6 genetic conditions (all known to affect brain development), and 4 viral encephalitis, this sum up to 77% causes with cerebral involvement. On a 20-year retrospective cohort analysisReference Smith, Smith, Philbrick and Kumar19 of all patients meeting DSM-IV-TR criteria for catatonic subtypes in a population of individuals with catatonic disorder secondary to a general medical condition, encephalitis was the most common etiologic diagnosis.
The classic classification of the catatonic syndrome according to its phenotype is based on behavioral and autonomic activity, and divides it into a withdrawn type (retarded-stuporous or Kahlbaum syndrome), Reference Oldham and Lee17 an excited type (excited-delirious or Bell’s mania)Reference Oldham and Lee17 and a third form, called malignant (lethal) catatonia.Reference Kaestner, Mostert and Behnken4, Reference Daniels11, Reference Oldham and Lee17, Reference Fink and Taylor21–Reference Sahaya and Lardizabal23 The underlying cause does not appear to predict which type is expressed.Reference Daniels11
Despite this division, it is common for patients to exhibit alternance between the first 2 formsReference Kaestner, Mostert and Behnken4 (fluctuating catatonia) and aside from clinical vigilance related to motor consequences, it is unclear whether there is clinical value in differentiating these phenotypical variants.Reference Oldham and Lee17, Reference Taylor and Fink22 Neuroleptic malignant syndrome (NMS) and serotonin syndrome (SS) are often considered variants of malignant (lethal) catatonia. NMS is an idiosyncratic response to dopamine receptor antagonist medications, Reference Kaestner, Mostert and Behnken4 and although malignant catatonia resembles NMS in many ways, it was in fact described long before the introduction of neuroleptics.Reference Philbrick and Rummans24 SS has similar characteristics but is precipitated by serotonergic medicationsReference Looper25 and contrary to NMS, which is not dose-related, Reference Gillman26 SS is a dose-dependent toxic response.Reference Boyer and Shannon27
Catatonia is a syndrome whose etiology has been progressively investigated, leading to several changes in the syndrome’s categorization under the Diagnostic and Statistical Manual of Mental Disorders (DSM). While DSM-III defined it exclusively as a type of schizophrenia, Reference Judd and Burrows2 DSM-IV also listed it for the first time as a modifier of major depression and bipolar disorder and added the concept of catatonic disorder due to a general medical condition.Reference Carroll, Anfinson, Kennedy, Yendrek, Boutros and Bilon10 Nowadays, DSM-5 lists the 12 catatonic symptoms (waxy flexibility, negativism, mutism, catalepsy, stupor, posturing, grimacing, agitation, mannerism, echolalia, echopraxia, and/or stereotypy) and defines a formal diagnosis of catatonia as 3 or more of these. Specifiers include catatonia associated with another mental disorder, catatonia disorder due to another medical condition, and unspecified catatonia.
Due to the potential morbidity and mortality associated with catatonia, the condition should be readily recognized when first assessing a patient. This may be facilitated by the use of rating scales such as the Bush-Francis Catatonia Rating Scale (BFCRS), the Rogers Catatonia and Schizophrenia Scales (RCSS), or the Pediatric Catatonia Rating Scale (PCRS).Reference Benarous, Consoli and Raffin28 BFCRSReference Bush, Fink, Petrides, Dowling, Francis and Catatonia.29 is the most widely used in research studies and case reports.Reference Daniels11, Reference Romanowicz and Sola30 It allows for serial evaluation, but does not provide a clinical cut-off for the diagnosis of catatonia.Reference Oldham and Lee17 This scale has a shorter version, the Bush-Francis Catatonia Screening Instrument (BFCSI), which might be useful to screen for the syndrome. The use of these scales may accelerate the diagnosis in cases where catatonia may be nested within an encephalitis, which in turn may hasten adequate treatment.Reference Ali, Welch and Park31
Catatonia investigation
Medical catatonias can develop secondarily to an illness or exposure to a substance (or both simultaneously) and there seems to be no correlation between the expression of catatonic symptoms and the underlying etiology (psychiatric versus organic).Reference Daniels11 Identification of the catatonic syndrome must be made while simultaneously determining its cause. When first assessing a patient with behavioral symptoms, a thorough neurologic examination and mental status assessment must be made, as certain features can suggest a medical etiology: features regarding orientation, memory or level of consciousness are uncommon in psychiatric disorders, Reference Talbot-Stern , Green and Royle32 as well as abnormal vital signs or other findings on physical examination.Reference Talbot-Stern , Green and Royle32–Reference Marsh36
Various authorsReference Carroll, Anfinson, Kennedy, Yendrek, Boutros and Bilon10, Reference Sahaya and Lardizabal23 have suggested that the differential diagnosis of catatonia due to a medical condition should be divided into simple categories as neurologic, substance-induced, metabolic, infective, and endocrine disorders. Subcategories as encephalitis, seizure disorders, and others should be made, as they allow for a more focused study and practical clinical application. Lumbar puncture with cerebrospinal fluid (CSF) studies is recommended when investigating a patient with catatonia of unclear etiology, Reference Carroll, Anfinson, Kennedy, Yendrek, Boutros and Bilon10 as it seems to be the test most likely to affect acute management.
Regarding encephalitis as the underlying cause, catatonia may be difficult to assess in the context of encephalopathy and this can easily lead to the use of neuroleptics. As previously discussed, the use of catatonia rating scales is encouraged, as to facilitate earlier detection, and neuroleptics should be avoided in patients with catatonic features (and discontinued if malignant catatonia is suspected).Reference Ali, Welch and Park31
Catatonia treatment
As with other entities, treatment aimed at the underlying etiology is always preferred, but when the cause remains unclear, and particularly in the cases of malignant catatonia, symptomatic treatment is required, Reference Romanowicz and Sola30 with stabilization of vegetative parameters and initiation of rehabilitation with physiotherapy.Reference Kaestner, Mostert and Behnken4 Benzodiazepines are considered first-line symptomatic treatment for catatonia (most commonly lorazepam, often producing reduction of symptoms within 24 hours)Reference Babington and Spiegel37 and electroconvulsive therapy (ECT) should be considered when benzodiazepines have failed or are only partially effective.Reference Romanowicz and Sola30, Reference Ali, Welch and Park31 Both of these options have been listed as effective in both acute and chronic catatonia.Reference Falkai, Wobrock and Lieberman38, Reference Dhossche, Carroll and Carroll39 The fact that many catatonic patients respond to benzodiazepines, gamma-amino-butyric acid (GABA) A agonists, is a finding that supports the theory that catatonia results of decreased GABA activity.Reference Babington and Spiegel37 There have been several literature reports on the excelent response and recovery produced by ECT regardless of etiology.Reference Ali, Welch and Park31, Reference Shill and Stacy40–Reference Slooter, Braun, Balk, van Nieuwenhuizen and van der Hoeven43 Interestingly, agents that block dopamine-2 (D2) receptors, such as typical antipsychotics, can induce catatonia in some patientsReference Consoli, Ronen and An-Gourfinkel 44 whereas the exact opposite seems to happen with atypical antipsychotics, such as olanzapine, with scientific literature suggesting that these may be effective in treating catatonia. Similarly, NMDA receptor antagonists, such as ketamine and phencyclidine, Reference Cai and Khawaja45 have been associated with the onset of catatonia, but weaker NMDA receptor antagonists, such as amantadine and memantine, have been reported to improve catatonia.Reference Babington and Spiegel37 A theoryReference Lin, Hung, Tsai and Huang46 that aims to explain these findings is that the NMDA receptor is dysfunctional in catatonia in the striato-cortical or corticocortical pathways, with NMDA hyperactivity appearing to correspond to a loss of GABA A and dopamine activity in these regions, leading to a clinically lorazepam-resistant catatonia.Reference Carroll, Goforth and Thomas9, Reference Northoff47 Thus, the use of NMDA antagonists to improve catatonia seems to be related to the attenuation of glutamatergic hyperactivity and possibly the simultaneous increase of GABA-A and dopamine in previously deficient areas.Reference Carroll, Goforth and Thomas9, Reference Northoff, Eckert and Fritze48, Reference Thomas, Carroll, Maley, Jayanti and Koduri49
Catatonia is a neuropsychiatric syndrome that may respond to one treatment, even after failure to other standard treatments, if treated for a sufficient duration.Reference Carroll, Goforth and Thomas9 Hence, in cases refractory to benzodiazepines, atypical antipsychotics, amantadine and memantine may constitute options worth considering.Reference Babington and Spiegel37
Encephalitis
As mentioned previously, a high percentage of secondary catatonias is caused by neurological disorders, specifically encephalitis. Encephalitis is an acute inflammatory process of the brain parenchyma that generally presents with an altered level of consciousness, disorientation, or behavioral and speech disturbanceReference Talbot-Stern , Green and Royle32, Reference Whitley50 and its clinical findings depend on the location and extent of the particular regions of the brain that are affected.Reference Talbot-Stern , Green and Royle32, Reference Lipkin51–Reference Cummings53 The condition may be life-threatening and as such requires prompt diagnosis and adequate treatment. Etiologies comprise a range of inflammatory disorders, including autoimmune and infectious causes.Reference Schimmel, Bien, Vincent, Schenk and Penzien54, Reference Kruse, Jeffrey, Davis, Dearlove, IsHak and Brooks55 Across the range of etiologies, common symptoms of encephalitis may include headache, confusion, altered level of consciousness, memory disturbances, seizures, and hallucinations. Particular symptom clusters may lead the clinician to consider one etiology over another.Reference Kruse, Jeffrey, Davis, Dearlove, IsHak and Brooks55 StudiesReference Bataller, Kleopa, Wu, Rossi, Rosenfeld and Dalmau56, Reference Ances, Vitaliani and Taylor57 have led to the characterization of autoimmune encephalitis into 2 broad categories: Reference Chapman and Vause58
• Those associated with antibodies to intracellular neuronal antigens (eg, antigens located in the nucleus or cytoplasm, such as Hu, Yo, and Ma2). The more frequently encountered intracellular autoantigens are Hu and Ma2, with CV2/CRMP5 and amphiphysin present less often. This type of immune-mediated encephalitis is more often associated with neoplasms. Since the target epitopes in these disorders are intracellular, and therefore the antibodies have limited accessibility, it has been suggested that many of these antibodies are not pathogenic, but rather reflect a cytotoxic T-cell mediated immune response.Reference Lancaster and Dalmau59 These encephalitis often show limited response to treatment.
• Those associated with antibodies to cell membrane antigens in the neuropil of the hippocampus and cerebellum. Autoimmune limbic encephalitis with antibodies to cell membrane antigens, including NMDA receptors, Reference Dalmau, Gleichman and Hughes60 voltage-gated potassium channels, Reference Gultekin, Rosenfeld, Voltz, Eichen, Posner and Dalmau61 alpha-amino 3-hydroxy 5-methyl 4-isoxazolepropionic acid (AMPA) receptors, Reference Lai, Hughes and Peng62 the GABA-B receptor, Reference Lancaster, Lai and Peng63 the glycine receptor (GlyR), Reference Hutchinson, Waters and McHugh64 and the metabotropic glutamate receptor 5 (mGluR5), Reference Lancaster, Martinez-Hernandez and Titulaer65 are less frequently associated with cancer, have an antibody-mediated pathogenesis, and tend to respond to immunotherapy.Reference Ryan, Costello, Cassidy, Brown, Harrington and Markx66
Anti-NMDAr encephalitis
NMDA receptors are heteromers of NR1 and NR2 subunits (A, B, C, or D) that bind glycine and glutamate, respectively, Reference Lynch, Anegawa, Verdoorn and Pritchett67 and compose ligand-gated cation channels that play an important role in synaptic plasticityReference Lau and Zukin68 and seem to be involved in the physiopathology of neuropsychiatric disorders.Reference Waxman and Lynch69 In order for the NMDA receptor to be functional, both glycine and glutamate must bind to the heteromers.Reference Consoli, Ronen and An-Gourfinkel 44
Anti-N-methyl-D-aspartate receptor (NMDAr) encephalitis is a type of encephalitis characterized by the presence of anti-NMDAr antibodies. These were first described in 2007 by Dalmau et al., Reference Dalmau, Tüzün and Wu70 which coined the term anti-NMDA receptor encephalitis in a patient with an ovarian teratoma. Initially it was thought that the target epitopes were both the NR1/NR2 heteromers, but in 2008 the syndrome was fully described in a case series of 100 patients and the target epitopes were identified only in the NR1 subunit of the NMDAr.Reference Dalmau, Gleichman and Hughes60 The association between subacute encephalitis and a distant tumor was first described by Brierley et al. in 1960Reference Gulyayeva, Massie and Duhamel71 and the clinical syndrome of a paraneoplastic neuropsychiatric disorder associated with ovarian teratoma was primarily described in 2005 by Vitaliani et al. Reference Consoli, Ronen and An-Gourfinkel 44, Reference Vitaliani, Mason, Ances, Zwerdling, Jiang and Dalmau72
The antibodies are detected in the CSF and/or serum of the patient and tend to disappear with clinical improvement, suggesting their pathogenic role. Although recovery occurs without the need for tumor removal, symptoms tend to be more severe and prolonged if it is not excised.Reference Iizuka and Sakai73
Although initial presentation can be variable, the natural history of the disease has now been clearly described and can be predicted, both in adults and teenagers. The disease itself evolves in stages, ultimately culminating in either recovery (limited to full) or death.
• Approximately, 70% of patientsReference Jones, Schwartz, Hermida and Kahn74 report the existence of a prodromal phase, which consists of a brief nonspecific viral-like episode with fever, malaise, headache, fatigue, vomiting, diarrhea, and/or upper respiratory tract symptoms.Reference Dalmau, Lancaster and Martinez-Hernandez 75
This stage is followed by an acute phase that includes neuropsychiatric symptoms such as agitation, psychotic symptoms (eg, delusions or hallucinations), behavioral changes, generalized or partial seizures, and progressive unresponsiveness, shortly followed by abnormal movements (eg, dyskinesia), autonomic instability (eg, paroxysmal hypertension and sinus tachycardia, hypo- or hyperthermia, bradycardia, hypotension, gastrointestinal dysmotility, and sialorrhea)Reference Neyens, Gaskill and Chalela76 and hypoventilation that may require ventilation assistance and intensive care.Reference Consoli, Ronen and An-Gourfinkel 44, Reference Chapman and Vause58 It is in this phase that many patients present to psychiatrists or are admitted to psychiatric units with a diagnosis of acute psychosis or schizophrenia.Reference Chapman and Vause58 A 2018 review of the psychiatric phenotypes seen in anti-NMDAr encephalitis by Sarkis et al., Reference Sarkis, Coffey, Cooper, Hassan and Lennox77 found that 77% of patients with this type of encephalitis presented initially with psychiatric symptoms and of those, catatonia was present in 42% of adult patients and 35% of children. Interestingly, they also found that in many cases, although the signs and symptoms of catatonia were clearly described, the authors did not explicitly identify catatonia.
• Psychiatric symptoms consist of anxiety, mood dysregulation, or depression progressing to severe behavioral and personality disturbance, delusional or disorganized thinking, paranoid ideation, and hallucinations.Reference Chapman and Vause58
• In the unresponsive state, affected individuals have their eyes open but are unresponsive to visual threats. These patients are often mute, or just mumble unintelligible words. Muscle tone is often increased, and catatonia may happen with possible dystonic and/or cataleptic postures.Reference Chapman and Vause58
• Dyskinesias tend to start in the face and/or mouth and include orofacial dyskinesia (described as kidding, chewing, tongue thrusting, lip smacking, facial grimacing, frowning, and fish- or rabbit-like movements). Although associated with rhythmic abdominal contractions or complex movements of the extremities, these orofacial dyskinesias do not have epileptic correlates on EEG.Reference Chapman and Vause58 Other movement disorders include complex and stereotyped movements such as pelvic thrusting, “floating” of the hands into the air, pseudoplaying piano motions and writhing movements of the extremities; limb movements can be independent or synchronous, at times mimicking epileptic seizures; the movements persist despite declining consciousness, often to the point of self-injury. In severe stages, there may be episodic opisthotonus, dystonic posturing, and oculogyric crises, which are associated with tachycardia and hypertension, reminiscent of autonomic storming.Reference Florance-Ryan and Dalmau78
• Hypoventilation of central origin may be missed until extubation is attempted.Reference Chapman and Vause58
• Autonomic instability is not uncommon and is evidenced by blood pressure and temperature fluctuations, tachycardia, bradycardia, and even cardiac pauses.Reference Chapman and Vause58
• The speech disturbance in anti-NMDAr encephalitis manifests as a progressive language disintegration, with the symptomatic spectrum ranging from reduction of verbal output and echolalia (frequently accompanied by echopraxia) to frank mutism.Reference Florance-Ryan and Dalmau78
• Insomnia is often prominent at presentation, and less frequently in the stages of recovery. Patients may not sleep for days regardless of trials of multiple sedating medications. When sleep does occur, sleep–wake cycles are disturbed, and patients wake frequently throughout the night. During recovery, patients may have hypersomnia and other symptoms of hypothalamic dysfunction.Reference Florance-Ryan and Dalmau78
While patients might present with the florid neurological deterioration described above, milder or incomplete forms of anti-NMDAr encephalitis have been observed in a small subset of patients, with apparently isolated psychiatric symptoms. These patients may have prolonged periods without treatment but not necessarily progress to more severe disease because of that.Reference Kayser, Titulaer, Gresa-Arribas and Dalmau79 In a case described by Hermans et al., Reference Hermans, Santens and Matton80 a 25-year-old woman presenting with psychiatric symptoms and later progressing to catatonia and autonomic instability, went 74 days without the correct diagnosis of anti-NMDAr encephalitis and yet had a good prognosis once adequate treatment was initiated, having returned to her premorbid level. Heekin et al. Reference Heekin, Catalano, Frontera and Catalano81 presented the case of a woman who was followed-up for over 14 years for the treatment of multiple neuropsychiatric symptoms. Initially, she presented with paresthesia, memory loss, and manic symptoms; 9 years later, she was once admitted with left sided numbness, left eyelid droop, and word finding difficulties, and 5 years after that, she presented with manic symptoms, hallucinations, and memory impairment, having subsequently developed catatonic symptoms and seizures during her stay at the hospital. She was found to be positive for anti-NMDAr antibodies and her symptoms responded well to immunotherapy. The authors hypothesize that if this patient’s episodes are in fact attributable to pathogenic anti-NMDA receptor antibodies, this would constitute an extensive illustration of the natural history of the disease in a patient not initially treated with immunotherapy or tumor removal, characterized by relapsing and remitting symptoms subsequently progressing to florid disease with seizures, dyskinesias, and autonomic dysfunction, ultimately requiring intubation and mechanical ventilation.
An interesting feature in anti-NMDAr encephalitis is that recovery follows a sequential multistage process that develops in the opposite direction to that of symptom presentation.Reference Florance-Ryan and Dalmau78
In young children, the syndrome is similar, but the presenting symptoms may be different. Studies have shown that children tend to have neurological symptoms more frequentlyReference Titulaer, McCracken and Gabilondo Cuellar82 as opposed to psychiatric ones, and appear to have a higher incidence of movement disorders, with numbers of children noted to have a movement disorder ranging from 60%Reference Mohammad, Ramanathan, Brilot and Dale83 to 90%, Reference Titulaer, McCracken and Gabilondo84, Reference Irani, Bera and Waters85 which included unilateral dystonia, speech disturbance, status epilepticus, Reference Florance-Ryan and Dalmau78 and gait disturbances.Reference Yeshokumar, Sun, Baranano, Klein and Pardo86 Granata et al. Reference Granata, Matricardi and Ragona87 compared the movement disorders in children and teenagers (>12 years of age) with anti-NMDAr encephalitis and found that catatonic symptoms were much more prevalent in adolescents than in children, as other studiesReference Duan, Weng and Lin88 had shown before. In children, especially those under 12, behavioral changes may present themselves with increased temper tantrums, hyperactivity, or irritability as opposed to frank psychosis.Reference Titulaer, McCracken and Gabilondo Cuellar82 Many parents report changes in speech, namely reduced speech, mutism, echolalia, or perseveration.Reference Florance, Davis and Lam89, Reference Luca, Daengsuwan and Dalmau90
Such as psychiatric symptoms, autonomic dysfunction, common in adults, occurs less frequently in children. Although more than 42% of adults develop hypoventilation, this occurred in only 16% of one series of children.Reference Titulaer, McCracken and Gabilondo Cuellar82 When dysautonomy does happen in children, it usually manifests as urinary incontinence and episodes of tachycardia, hypertension, or hyperthermia, with severe cardiac dysrhythmia and other clinically significant cardiac pauses being less frequent in children than in adults.Reference Florance, Davis and Lam89, Reference Armangue, Petit-Pedrol and Dalmau91
Recognizing anti-NMDAr encephalitis as a possible cause for catatonic presenting patients is important for several reasons.
First, many patients may initially present with psychiatric symptoms and catatonic features, Reference Consoli, Ronen and An-Gourfinkel 44, Reference Chatterjee, Ghosal and Mitra92 and can be misdiagnosed with a primary disorder, with the definitive diagnosis and adequate treatment being delayed, as was the case published (and previously mentioned in this article) by Hermans et al., Reference Hermans, Santens and Matton80 in which a 25-year-old woman who presented primarily with psychiatric symptoms was misdiagnosed and treated as a first psychotic episode within a primary psychiatric disorder. Despite having rapidly deteriorated to catatonia followed by autonomic instability, the diagnosis was still delayed by 74 days. Likewise, Jones et al. Reference Jones, Schwartz, Hermida and Kahn74 described the case of a 17-year-old girl who presented with altered mental status, seizures, catatonia, and autonomic disturbances, who was submitted to over 4 weeks of evaluation and work-up by internal medicine, neurology, infectious disease, and psychiatric services at 2 different facilities before an accurate diagnosis was made and a sustainable treatment modality initiated. Both cases highlight the importance of the need for greater recognition of this autoimmune disorder. Then there are cases of patients who may have a history of catatonic episodes throughout several years, who were symptomatically treated, but remained with unclear etiology, like the case described by Tsutsui et al., Reference Tsutsui, Kanbayashi and Takaki93 whose patient was admitted for catatonic symptoms and treated with steroids and antipsychotics and discharged, and only later was diagnosed with anti-NMDAr encephalitis. This patient is interesting because 3 years before he had also presented with an episode of catatonia of unclear etiology, and one can question whether this was also due to anti-NMDAr antibodies.
Secondly, these patients frequently have an increased mortality and morbidity risk if not treated promptly, frequently requiring intensive care assistance.Reference Jones, Schwartz, Hermida and Kahn74 Both anti-NMDAr encephalitis and catatonia have effective treatments availableReference Khadem, Heble, Kumar and White94 and given the possibility of severe neurological sequalae and death, the importance of searching for a medical condition in catatonic syndrome is related to treating appropriately and avoiding such outcomes.Reference Consoli, Ronen and An-Gourfinkel 44, Reference Türkdoğan, Orengul, Zaimoğlu and Ekinci95 Early identification and intervention can shorten the duration of intensive care admission and ventilation, improve the outcome, and protect against relapse.Reference Irani, Bera and Waters85, Reference Tüzün and Dalmau96, Reference Kuo, Tsai, Lai, Lin and Yang97
Anti-NMDAr encephalitis epidemiology
Since 2007, when anti-NMDAr antibodies were first described, various case reports of anti-NMDAr encephalitis have been published, suggesting that the illness is not rare.Reference Consoli, Ronen and An-Gourfinkel 44, Reference Sansing, Tüzün, Ko, Baccon, Lynch and Dalmau98–Reference Parratt, Allan, Lewis, Dalmau, Halmagyi and Spies102 The exact incidence of anti-NMDAr encephalitis is unknown, but it seems to be more frequent than any other known paraneoplastic encephalitisReference Consoli, Ronen and An-Gourfinkel 44, Reference Dalmau, Lancaster and Martinez-Hernandez 75 and even more frequent than any specific viral etiology in young patients, as reported by the California Encephalitis Project.Reference Ryan, Costello, Cassidy, Brown, Harrington and Markx66, Reference Gable, Sheriff, Dalmau, Tilley and Glaser103 In children and adolescents, anti-NMDAr encephalitis has become a leading cause of autoimmune encephalitis, with 40% of patients being younger than age 18 years.Reference Dalmau, Lancaster and Martinez-Hernandez 75
Anti-NMDAr encephalitis was initially described as typically occurring in young women, Reference Khadem, Heble, Kumar and White94 with papers suggesting up to 80%Reference Consoli, Ronen and An-Gourfinkel 44 of patients being females between the ages of 14 and 44.Reference Schimmel, Bien, Vincent, Schenk and Penzien54 However, cases in male patients—both children and adults—began being increasingly and repeatedly described, so as to the extent that the disease is now described as affecting children and young adultsReference Consoli, Ronen and An-Gourfinkel 44 with a median age of onset at 23 years, although it ranges from 3 to 76 years.Reference Dalmau, Gleichman and Hughes60, Reference Verhelst, Verloo and Dhondt104, Reference van de Riet, Esseveld, Cuypers and Schieveld105
In adults, the disorder is often associated with neural tissue within a tumor that can express NMDA receptors, inducing antibody production. The generated autoantibodies then bind to host NMDA receptors located in the brain, leading to receptor internalization by autophagy and consequent marked loss of surface NMDA.Reference Dalmau, Lancaster and Martinez-Hernandez 75, Reference Hughes, Peng and Gleichman106, Reference Parenti, Jardri and Geoffroy107 The classically and most frequently described associated tumors are ovarian teratomas in women and testicular tumors in men, Reference Consoli, Ronen and An-Gourfinkel 44, Reference Florance, Davis and Lam89 but there are literature reports of other forms of cancers, such as small cell lung cancer and neuroblastomas.Reference Florance-Ryan and Dalmau78, Reference Lebas, Husson, Didelot, Honnorat and Tardieu108 The frequency of tumors varies according to age, sex, and ethnicityReference Consoli, Ronen and An-Gourfinkel 44, Reference Dalmau, Gleichman and Hughes60: its presence has been reported to be more frequent in women who are older than 18 years and who are blackReference Consoli, Ronen and An-Gourfinkel 44, Reference Dalmau, Lancaster and Martinez-Hernandez 75 as well as most likely in adolescents and adult females.Reference Mohammad, Ramanathan, Brilot and Dale83 Children and males have a lower incidence of tumors, Reference Chapman and Vause58, Reference Florance-Ryan and Dalmau78 with a neoplasm being found in approximately 6% of girls younger than 12 years and rarely in boys.Reference Mohammad, Ramanathan, Brilot and Dale83, Reference Titulaer, McCracken and Gabilondo84 In a substantial number of patients, no primary tumor is found and the trigger of the immune response is unknown. According to a literature analysis of cases of anti-NMDAr encephalitis by Kruse et al., Reference Kruse, Jeffrey, Davis, Dearlove, IsHak and Brooks55 only 38% of patients have an underlying neoplasm; neither case described by the authors had an underlying malignancy. Although rare, cases occurring during pregnancy have been reported, as well as in the post-partum setting.Reference McCarthy, Dineen and McKenna109, Reference Yu and Moore110 Delay in diagnosis is not uncommon, with a median time from symptom presentation to initial signs of improvement at around 6 weeks.Reference Florance, Davis and Lam89, Reference Wilson, Shuster and Fuchs111 Mortality rates of anti-NMDAr encephalitis have been reported at 8–10%.Reference van de Riet, Esseveld, Cuypers and Schieveld105
Anti-NMDAr encephalitis investigation
Although the constellation of symptoms in anti-NMDAr encephalitis is characteristic, the disease evolves in stages and so certain presentations at certain stages of the disorder may suggest alternative diagnoses, and the list may be vast.
The most frequently considered disorders in the differential diagnosis of anti-NMDAr encephalitis are toxic and metabolic disorders, other causes of autoimmune encephalitis, viral encephalitis, and primary psychiatric disorders.Reference Khadem, Heble, Kumar and White94, Reference Sansing, Tüzün, Ko, Baccon, Lynch and Dalmau98 Kiani et al. Reference Kiani, Lawden and Eames112 described the case of a 32-year-old woman with a diagnosis of mild intellectual disability, autism and Larsen’s syndrome, whose catatonia presented after the initiation of antipsychotics and thus the first diagnosis considered was NMS. However, given the multitude of psychiatric symptoms she also presented with, a diagnosis of functional catatonia was found to be most appropriate and the diagnosis of anti-NMDAr encephalitis was later established.
• Toxic causes include over-the-counter or illicit drugs (eg, phencyclidine), Reference Cai and Khawaja45 as well as carbon monoxide, methanol, and cyanide. Ketamine is a drug that affects the NMDA receptor and thus produces similar symptoms to anti-NMDAr encephalitis.Reference Florance-Ryan and Dalmau78
• Porphyria, mitochondrial disorders, and disorders of amino or organic acid metabolism should also be excluded.Reference Chapman and Vause58
• Infectious causes include viral (herpes simplex virus (HSV), human herpes virus-6 (HHV-6), enteroviruses, arboviruses, mumps, measles, varicella zoster (VZV), cytomegalovirus (CMV), rubella, influenza, human immunodeficiency viruses (HIV), rabies virus), bacterial (Tropheryma whipplei, Mycoplasma pneumonia, Bartonella henselae, Listeria monocytogenes, Borrelia burgdorferi, Treponema pallidum), parasitic (Toxoplasma gondii, malaria, primary amoebic meningoencephalitis) and fungal (Cryptococcus neoformans, Histoplasma capsulatum, Coccidioides).Reference Kruse, Jeffrey, Davis, Dearlove, IsHak and Brooks55 Pediatric Autoimmune Neuropsychiatric Disorder Associated with Streptococcus (PANDAS) is also a differential diagnosis to be taken into account in children.Reference Florance-Ryan and Dalmau78
• Other autoimmune causes include those involving the previously mentioned classic paraneoplastic antigens or cell membrane antigens (with antibodies to Hu, Ma2, AMPA receptors, etc.), acute disseminated encephalomyelitis (ADEM), Reference Chapman and Vause58 systemic lupus erythematosus cerebritis, antiphospholipid antibody syndrome, Sjögren’s syndrome, encephalopathy associated with Hashimoto’s thyroiditis, and angiitis (primary or systemic).Reference Chapman and Vause58
• Diagnoses of primary psychotic disorders, schizophreniform disorder and even schizophrenia, mood dysregulation disorders, disorders of impulse control, and sleep disorders are often considered.Reference Chapman and Vause58 It’s not uncommon that patients, particularly adults, are initially diagnosed with new onset psychosis, when antibodies have not yet been identified and the disease has not progressed in a temporal continuum so as to provide a pattern of recognition. These patients are often treated initially with medications like haloperidol, and when anti-NMDAr encephalitis expected signs and symptoms arise (like rigidity, autonomic instability), sometimes with elevation of muscle enzymes, Reference Chapman and Vause58 they are diagnosed with NMS.Reference Florance-Ryan and Dalmau78 Some features that may help to clinically distinguish patients with anti-NMDAr encephalitis from those with a true psychiatric disorder include anterograde amnesia (impairment of short-term memory), which is usually present in patients with NMDAr antibody-associated encephalitis but frequently overshadowed by the neuropsychiatric symptoms of the disease, the development of dystonia, seizures, decreased level of consciousness, and central hypoventilation requiring mechanical ventilation, all happening in this form of encephalitis and absent in primary psychiatric disease.Reference Khadem, Heble, Kumar and White94 Verfaille et al. Reference Verfaillie, Bissay, Vanderbruggen, Van Eetvelde, Honoré and Spapen113 described the case of an 18-year-old who presented with acute psychosis who later progressed to a rapidly evolving neuropsychiatric syndrome, characterized by severe motor dysfunction (episodes of catatonia and agitation, orofacial dyskinesia, and dystonic posturing with head deviation) and autonomic instability requiring intubation, and was later found to have highly positive anti-NMDA receptor antibodies. In 2018, Warren et al. Reference Warren, Siskind and O’Gorman114 published a systematic review of the psychiatric symptoms of anti-NMDAr encephalitis looking for symptoms able to differentiate the presentation from a primary psychiatric disorder, and found that catatonia, especially when fluctuating (changing from severe agitation to withdrawal and mutism repeatedly), is a key feature that may indicate this type of encephalitis and may be a potentially useful diagnostic tool, yet has been minimally highlighted in medical literature.
Based on literature review and the experience of a team of experts, Graus et al. Reference Graus, Titulaer and Balu115 developed diagnostic criteria for this type of encephalitis, with criteria grouped under a probable diagnosis and a definite diagnosis Table 1. The definite diagnosis of anti-NMDAr receptor encephalitis is made in a clinically suggestive patient with positive IgG antibodies targeting the NR1 subunit of the NMDA receptor (IgG anti-NR1, also known as IgG anti-GluN1), whereas a probable diagnosis encompasses neurological assessment and conventional tests that are accessible to most clinicians (which is not the case with anti-NMDAr encephalitis-associated antibody testing). The probable diagnosis group is particularly important because antibody test results are not available at disease onset and waiting for the tests results often delays diagnosis and, thus, initiation of treatment.
When antibodies against the NMDA receptor were first described in 2007, Reference Dalmau, Tüzün and Wu70 the target epitopes were thought to be the NR1/NR2 heteromers, but later studiesReference Dalmau, Gleichman and Hughes60 demonstrated that the target epitope is the NR1 subunit. Definitive diagnosis is thus established by demonstrating IgG antibodies to the NR1 subunit of the NMDAr in serum or CSF. These antibodies are demonstrated via cell-based assay.Reference Graus, Titulaer and Balu115 Serum and CSF antibody titers appear to be correlated with the course of the diseaseReference Florance-Ryan and Dalmau78 and possibly with the existence of associated tumors, with higher titers of antibodies often being associated with their presence.Reference Zandi, Paterson and Ellul116 As most patients have intrathecal synthesis of anti-NMDAr antibodies, Reference Kruse, Jeffrey, Davis, Dearlove, IsHak and Brooks55, Reference Dalmau, Lancaster and Martinez-Hernandez 75 detection of antibodies in the CSF is more sensitive and often higherReference Dalmau, Gleichman and Hughes60, Reference Hughes, Peng and Gleichman106 than detection in serum, and the latter may not detect titers at all.Reference Khadem, Heble, Kumar and White94 In an observational cohort study, Titulaer et al. Reference Titulaer, McCracken and Gabilondo84 randomized 250 out of 540 patients diagnosed with anti-NMDAr encephalitis and found that 100% had anti-NMDAr detected in the CSF, but only 85% had anti-NMDAr antibodies detected in the serum. As such, antibody testing should include testing of CSF and if only serum is available, confirmatory tests in addition to cell-based assay should be included, with live neurons or tissue immunohistochemistry, for example.Reference Graus, Titulaer and Balu115
It is important to highlight the specific immune profile assumed for anti-NMDAr encephalitis (IgG NR1 antibodies), as antibodies against the NR1/NR2 heteromers have been found to be present in other disorders, as neurodegenerative disorders, Reference Zandi, Paterson and Ellul116 prion disease, Reference Mackay, Ahmad and Stone117 and psychiatric disorders.Reference Steiner, Walter and Glanz118 Steiner et al. Reference Steiner, Walter and Glanz118 aimed to study the prevalence and specificity of serum anti-NMDAr in patients with an initial diagnosis of schizophrenia and concluded that patients with schizophrenia show a less-specific NMDAr immune response by an ample repertoire of immunoglobulins. In their study, they found 9.9% (n = 21/121) of these patients were seropositive for anti-NMDAr antibodies. Among these, 4 patients had IgG antibodies and the rest had either IgA or IgM antibodies. The authors then went on to determine the target epitopes in the seropositive IgG group and found that 2 of them had high titers of anti-NR1a antibodies and the remaining 2 had low titers of anti-NR1a/NR2b. To determine whether these 2 groups had in fact anti-NMDAr encephalitis (as opposed to their initial diagnosis of schizophrenia), their anti-NR1 CSF IgG levels were determined. The results showed that the 2 patients who had high titers of serum anti-NR1a IgG had also positive CSF titers, which led to a change in diagnosis from schizophrenia to anti-NMDAr encephalitis; the other 2 patients (with low serum titers of anti-NR1/NR2 antibodies) did not have CSF detectable antibodies, thus keeping their initial schizophrenia diagnosis. Interestingly, they also analyzed seropositivity in a group of people with an initial diagnosis of major depression (MD), borderline personality disorder (BPD) and healthy controls, and found seropositivity in 2.8% of the MD group (N = 2/70, low titers of anti-NR1a IgA and anti-NR1a/NR2b IgA) and in 0.4% (n = 1/230, low titers of anti-NR1a IgM). No seropositivity was found in the BPD group.
An initial negative test for NMDAr antibodies in the CSF or serum does not exclude a diagnosis and a follow-up test should be done. This may happen because antibody levels may not develop fast enough despite the presence of a clinically active condition. A case described by Gulyayeva et al. Reference Gulyayeva, Massie and Duhamel71 reported on the detection of antibodies against NMDA receptors in the serum and CSF only in the second set specimens (the first set had come back negative) in a 22-year-old woman.
Anti-NMDAr encephalitis is classically described as being associated with a tumor. As such, when the diagnosis is confirmed or at least suspected, a comprehensive search for a neoplasm should be conducted simultaneously to the start of adequate treatment.
• As previously described, most patients have an ovarian teratoma, which often is a benign or mature dermoid cyst. Investigations include a vaginal ultrasound and pelvic computed tomography (CT). Magnetic resonance imaging (MRI) has also been used as it seems to have higher sensibility in the detection of early, small ovarian tumors.Reference Florance-Ryan and Dalmau78 As 70% of the tumors are benign, positron emission tomography (PET) can be negative.Reference Vitaliani, Mason, Ances, Zwerdling, Jiang and Dalmau72 Recurrent and bilateral neoplasms can occur and the teratoma may be located at a different site.Reference Khadem, Heble, Kumar and White94, Reference Muni, Wennberg, Mikulis and Wong119
• Regarding testicular teratomas, ultrasound of the pelvis is an appropriate initial screen measure.Reference Florance-Ryan and Dalmau78
Regarding other exams:
• For patients with anti-NMDAr encephalitis, brain MRI is frequently normal (up to half of affected patientsReference Dalmau, Tüzün and Wu70) or show only minor and non-specific signs, such as T2 or fluid attenuated inversion recovery (FLAIR) signal hyperintensity in various areas of the brain, including the hippocampus, cerebellar or cerebral cortex, basal ganglia, brainstem, and spinal cord, Reference Dalmau, Gleichman and Hughes60, Reference Ryan, Costello, Cassidy, Brown, Harrington and Markx66, Reference Dalmau, Tüzün and Wu70, Reference Irani, Bera and Waters85 sometimes with subtle, transient cortical or meningeal contrast enhancement.Reference Florance-Ryan and Dalmau78 However, although infrequently, the MRI may show intense and extensive FLAIR abnormalities.Reference Florance-Ryan and Dalmau78
• Patients’ CSF may show pleocytosis (with white blood cell counts often elevated but usually lower than 200 cells/mm3)Reference Florance-Ryan and Dalmau78 and an elevated protein concentration. Initially, lymphocytosis, and sparse oligoclonal bands are present, but with evolving disease, lymphocytosis decreases and oligoclonal bands become more prominent.Reference Verfaillie, Bissay, Vanderbruggen, Van Eetvelde, Honoré and Spapen113, Reference Vincent, Bien, Irani and Waters120 Determination of immunoglobulin (IgG) index and oligoclonal bands is useful, particularly in cases with normal cell count and protein concentration because these can be abnormal in such cases.Reference Florance-Ryan and Dalmau78
• Electroencephalogram (EEG) is abnormal in 90% of patients with anti-NMDAr encephalitisReference Titulaer, McCracken and Gabilondo84 but this is not diagnostic.Reference Ryan, Costello, Cassidy, Brown, Harrington and Markx66 Hence, EEG studies are not particularly helpful in making a specific diagnosis of this condition. However, EEG is helpful in differentiating between psychiatric and encephalitic etiologies of psychiatric and behavioral manifestations, because most patients with encephalitis will have EEG abnormalities. Patients with anti-NMDAr encephalitis most frequently have an EEG that exhibits nonspecific, slow, and disorganized activityReference Consoli, Ronen and An-Gourfinkel 44 and occasionally epileptic activity.Reference Kruse, Jeffrey, Davis, Dearlove, IsHak and Brooks55, Reference Verfaillie, Bissay, Vanderbruggen, Van Eetvelde, Honoré and Spapen113 Seizures occur more often at early stages of the disease, but in catatonia slow continuous rhythmic activity happens in the delta–theta range. However, this EEG activity does not correlate with abnormal movements and does not respond to anti-epileptics.Reference Florance-Ryan and Dalmau78 Recently, a unique EEG pattern described as “extreme delta brush”, identified in 30% of adult patients with anti-NDMAR encephalitis, has been described as possibly characteristic of the disorder and associated with more prolonged hospitalization.Reference Ryan, Costello, Cassidy, Brown, Harrington and Markx66, Reference Schmitt, Pargeon, Frechette, Hirsch, Dalmau and Friedman121 EEG abnormalities in anti-NMDAr encephalitis appear to resolve with clinical improvement.Reference Heekin, Catalano, Frontera and Catalano81, Reference Irani, Bera and Waters85, Reference Schmitt, Pargeon, Frechette, Hirsch, Dalmau and Friedman121
Anti-NMDAr encephalitis treatment
The primary objective of therapy is to eliminate or at least reduce the anti-NMDAr antibody levels, with eradication of associated malignancy or suppression of the immune reaction. Thus, the optimal management of anti-NMDAr encephalitis is tumor resection and immunotherapy. When instituted promptly, these interventions have been shown to decrease morbidity and mortality and reduce the risk of irreversible neuronal damage, with evidence suggesting that early use of immunosuppressants may lead to a more rapid recovery and decreased morbidity.Reference Chapman and Vause58, Reference Dalmau, Gleichman and Hughes60, Reference Florance-Ryan and Dalmau78, Reference Irani, Bera and Waters85, Reference Florance, Davis and Lam89, Reference Breese, Dalmau, Lennon, Apiwattanakul and Sokol122 ICU care for ventilatory support, seizures and autonomic instability can delay tumor removal but it’s been shown that in some patients tumor removal results in significant neurological improvement within days to several weeks.Reference Dalmau, Tüzün and Wu70, Reference Khadem, Heble, Kumar and White94 There are, however, reports of patients who presented with intermittent catatonia and who improved without immunotherapy, as 2 cases published by Yoshimura et al., Reference Yoshimura, Yada, Horigome and Kishi123 whose patients were managed with antipsychotics and benzodiazepines.
There are no established guidelines for the treatment of anti-NMDAr encephalitis, but Dalmau and colleaguesReference Dalmau, Lancaster and Martinez-Hernandez 75 proposed an algorithmic strategy to guide treatment based on an extensive literature review (400 patients over a 3-year period). In summary, their proposed pharmacological treatment approach is as follows:
• The first-line of immunotherapy consists of corticosteroids, intravenous immunoglobulins (IVIg) and plasma exchange (alone or in combination):Reference Verfaillie, Bissay, Vanderbruggen, Van Eetvelde, Honoré and Spapen113
• Methylprednisolone 1 g/day for 5 days. Irani et al. Reference Irani and Vincent124 suggested that pulsed intravenous methylprednisolone treatment should be followed by oral prednisolone administration, which is tapered over a period of 6 to 12 months after hospital discharge;
• IVIg 0.4 g/kg/day for 5 days.
The authors have shown that this first-line of treatment may result in partial neurological improvement or stabilization. Titers are effectively reduced by these immunomodulatory treatments.Reference Barry, Byrne, Barrett, Murphy and Cotter125
Voice et al. Reference Voice, Ponterio and Lakhi126 described the case of a 17-year-old with anti-NMDAr encephalitis with catatonic symptoms which resolved when all 3 first-line measures were combined after tumor resection (left ovarian teratoma), as was the case with the 19-year-old woman treated by Gulyayeva et al. Reference Gulyayeva, Massie and Duhamel71 (also an ovarian teratoma). Chatterjee et al. Reference Chatterjee, Ghosal and Mitra92 described a patient who improved, catatonic symptoms included, with methylprednisolone and plasmapheresis, as did the patient described by Mythri et al., Reference Mythri and Mathew127 with immunotherapy only.
In children, plasma exchange is less often used because of the frequent need for central line placement. There is a subset of patients who remain symptomatic despite these therapies. In some of these patients, serum and CSF antibodies remain high, suggesting that additional courses of IVIg, methylprednisolone, or plasmapheresis may be helpful.Reference Florance-Ryan and Dalmau78
Overall, immunotherapy and tumor removal (when appropriate) result in marked improvement or full recovery of 75% of the patients.Reference Dalmau, Gleichman and Hughes60 Patients who have a tumor detected and removed within 4 months of onset have a more complete and fast recovery when compared with those without teratoma.Reference Florance-Ryan and Dalmau78
In refractory cases (patients who do not respond to steroids and IVIg, which comprise approximately 50% of NMDAr encephalitis patients)Reference Titulaer, McCracken and Gabilondo84, particularly those with no response after 10 days or with severe symptoms at 1 month, delayed diagnosis or in the absence of a tumor, additional treatment may be necessary with second-line immunotherapy:Reference Consoli, Ronen and An-Gourfinkel 44, Reference Florance-Ryan and Dalmau78
• Second-line immunotherapy is composed of rituximab, cyclophosphamide, or both.
• Rituximab is given at 375 mg/m2/week for 4 weeks (in adults);
• Cyclophosphamide at a dose of 750 mg/m2 given with the first dose of rituximab, followed by monthly cycles of cyclophosphamide;
• This treatment is discontinued when patients have substantial clinical recovery. The immunosuppressive agent mycophenolate mofetil is also considered as second-line therapy, being recommended for patients with nonparaneoplastic encephalitis for a minimum of 1 year.Reference Kramina, Kevere and Bezborodovs128
• The use of azathioprine (eg, another case published by Kuppuswamy et al. Reference Kuppuswamy, Takala and Sola129), alemtuzumabReference Liba, Sebronova, Komarek, Sediva and Sedlacek130 and bortezomibReference Scheibe, Prüss and Mengel131 has also been described in the neurological literature.
In 2009, Parratt et al. Reference Parratt, Allan, Lewis, Dalmau, Halmagyi and Spies102 published a case regarding a woman whose symptoms did not respond to IVIg, methylprednisolone, plasmapheresis, and bilateral oophorectomy, but did start to improve once second-line therapy was instituted. Similarly, Kramina et al., Reference Kramina, Kevere and Bezborodovs128 described a patient whose catatonia, neurological signs and dysautonomy were refractory to benzodiazepines, ECT, and first-line immunotherapy, but responded to the combination of rituximab and cyclophosphamide. Bowes et al. Reference Bowes, Levy, Lawson, Mandalis, Mohan and Shannon Weickert132 described the case of a 15-year-old girl that not only had refractory symptoms to first-line immunotherapy with corticosteroids and intravenous immunoglobulins, but actually clinically deteriorated, with catatonic symptoms arising after, and only improving with second-line therapy, specifically rituximab. Keller et al. Reference Keller, Roitman, Ben-Hur , Bonne and Lotan133 reported the case of a 32-year-old woman that despite the urgent removal of a 5-cm dermoid cyst followed plasmapheresis, still presented fluctuations between communicable periods, catatonia, and extreme agitation, having only improved with rituximab as well.
Interestingly, Kuppuswamy et al. Reference Kuppuswamy, Takala and Sola129 published the case of a woman whose symptoms only resolved with the combination of IVIg (first-line therapy) and rituximab (second-line therapy).
Rituximab has also been effective in children as young as 20 months.Reference Florance-Ryan and Dalmau78, Reference Wong-Kisiel , Ji and Renaud134 Because of the potential adverse effects of cyclophosphamide (malignancies, infertility, premature gonadal failure) most pediatricians only use it when the above treatments have failed.Reference Titulaer, McCracken and Gabilondo Cuellar82 In such cases, cyclophosphamide is often effective.Reference Armangue, Petit-Pedrol and Dalmau91, Reference Kashyape, Taylor, Ng, Krishnakumar, Kirkham and Whitney135
In pregnancy, immunomodulatory therapy can also be effectively used.Reference Dalmau, Lancaster and Martinez-Hernandez 75 McCarthy et al.Reference McCarthy, Dineen and McKenna109 concluded that plasma exchange can be used safely in pregnancy but, should hypotension occur, it could decrease fetal perfusion.Reference McCarthy, Dineen and McKenna109 Their experience with patients severely affected with anti-NMDAr encephalitis found that treatment with plasma exchange is superior to treatment with IVIg, as none of their patients were rendered antibody-negative when IVIg was used.
However, clinical improvement of symptoms does not seem to be clearly associated with a standard treatment, with the pattern of response to different therapies being quite diverse:
• Khadem et al. Reference Khadem, Heble, Kumar and White94 described the case of a 57-year-old female with anti-NMDAr encephalitis treated with intravenous lorazepam + 12 sessions of ECT + methylprednisolone and without much clinical improvement but with definite improvement in the next 8 months after bilateral removal of macroscopically and histologically normal ovaries + IVIg + cyclophosphamide.
• Schimmel et al. Reference Schimmel, Bien, Vincent, Schenk and Penzien54 described the case of a 12-year-old girl with anti-NMDAr encephalitis and catatonia who started to improve until almost full recovery after the initiation of plasmapheresis (started after the last steroid dose). The authors highlight that despite the improvement being apparently strongly time related to plasma exchange, a causative effect could not be inferred based on this single case, as a delayed steroid effect or simply a favorable natural course of the disease might have had justified the clinical response. This case is particularly interesting as previous literature mainly reported on remission after tumor resection and/or immunotherapy, which was not the case with this patient. The authors concluded that plasmapheresis might have at least accelerated recovery. Interestingly, Kamran Mirza et al. Reference Kamran Mirza, Pogoriler and Paral136 described the case of a 14-year-old with anti-NMDAr encephalitis and catatonia who failed therapy with intravenous steroids, IVIg, one dose of rituximab, 7 sessions of ECT and who presented no improvement with plasmapheresis.
• Ryan et al.Reference Ryan, Costello, Cassidy, Brown, Harrington and Markx66 described the case of a 37-year-old woman with anti-NMDAr encephalitis and catatonia treated with intravenous steroids and immunoglobulins, cyclophosphamide, and rituximab with an almost full recovery after 4 months of treatment, with the authors attributing the favorable response primarily to rituximab.
DeSena et al. Reference DeSena, Greenberg and Graves137 published an interesting approach to treatment response variability in a pediatric population based on their clinical experience with their own patients followed by a literature review of other cases: they classified patients based on the type of predominant symptomatic manifestation and duration of catatonic symptoms, and found that there may be 3 clinical subtypes, with implications to treatment response and prognosis, and which could be used to elaborate a risk stratification regarding immunotherapy decisions in the future:
• Type 1 or classic anti-NMDAr encephalitis: predominantly characterized by a catatonic or stuporous state of less than 60 days. The authors describe this group as the one with “fairly equal representations of periods of altered mental status, behavior problems, and movement disorders” whose prognosis is an intermediate one but will likely require aggressive immunotherapy.
• Type 2 or psychiatric-predominant anti-NMDAr encephalitis: no notable catatonic or stuporous state and predominant behavioral/psychiatric symptoms. This group was found to have excellent responses to plasmapheresis or other immunotherapies and appear to have the least residual deficits at follow-up.
• Type 3 or catatonia-predominant anti-NMDAr encephalitis: predominantly characterized by a catatonic or stuporous state lasting for 60 days or more. The authors found that this group were the poorest responders to treatment, even with aggressive immunotherapies.
Lee et al. Reference Lee, Kang, Oh, Kim, Shin and Kim138 questioned whether metabolic changes observed with 18F-Fluorodeoxyglucose positron-emission tomography (FDG-PET) were correlated with the severity of the catatonic symptoms and clinical course. To investigate this, 3 patients with anti-NMDAr encephalitis showing variable degrees of catatonia were submitted to FDG-PET scans during the acute and recovery phase. The findings of hypermetabolism occurring in the fronto-temporoparietal regions and bilateral basal ganglia in the patient with mild catatonia, but more widespread hypermetabolic regions, including the thalamus and brainstem, in patients with more severe catatonia led to the conclusion that the extent of cerebral hypermetabolic changes correlates with the severity of catatonia accompanied by behavioral, motor, autonomic, and breathing abnormalities in anti-NMDAr encephalitis patients.
Early and aggressive immunotherapy has improved the natural history of the disease and anti-NMDAr encephalitis has a better prognosis than most forms of paraneoplastic encephalitis, Reference Khadem, Heble, Kumar and White94, Reference Graus, Keime-Guibert and Reñe139 although it varies. As previously stated, 75% of cases recover with immunotherapy and tumor ablation (when present), while 25% of cases lead to severe sequelae and even death.Reference Dalmau, Gleichman and Hughes60 In a cohortReference Titulaer, McCracken and Gabilondo84 of 577 patients, including 211 children, the rate of mortality at 24 months was estimated at 7% due to uncontrolled disease progression, infections, or spread of tumor.
The outcome is usually good, with approximately 80% of patients having a substantial or full recovery, despite it being slow (weeks to months and even years)Reference Titulaer, McCracken and Gabilondo Cuellar82 with frequent protracted symptoms of frontal-limbic dysfunction (poor attention and planning, impulsivity, and behavioral disinhibition) and patients reporting amnesia for the entire event once the illness resolves.Reference Florance-Ryan and Dalmau78 However, long-term follow-up shows that overall symptoms tend to improve, with autonomic instability, dyskinesias, level of consciousness, and seizures improving firstReference Armangue, Petit-Pedrol and Dalmau91 and behavioral problems, decrease of verbal output, and social interactions being the last to recover. MRI signs of brain atrophy may also show improvement with long-term follow-up.Reference Florance-Ryan and Dalmau78, Reference Iizuka, Yoshii and Kan140
Clinical outcome in children is similar to that in adults, with about half requiring second-line immune therapy.Reference Mohammad, Ramanathan, Brilot and Dale83 In patients who do not improve with first-line immune therapy within the first week, second-line immune therapy should be instituted as soon as possible as it has been shown to result in better outcomes if administered early.Reference Mohammad, Ramanathan, Brilot and Dale83 Besides early treatment (with approximately 75% of patients progressing to a complete or near-complete recovery with early aggressive therapy), a good outcome was also associated with low severity of disease within 4 weeks of onset and lack of need for ICU admission.Reference Dalmau, Lancaster and Martinez-Hernandez 75 Türkdoğan et al., Reference Türkdoğan, Orengul, Zaimoğlu and Ekinci95 however, report a patient who had a dramatic recovery (in both clinical and laboratory settings) despite the presence of severe and long-lasting clinical symptoms and late onset of immunomodulatory therapy. For that reason, instead of adding one other immunomodulatory agent as a second-line treatment, the authors preferred long-term treatment of oral prednisolone with decreasing doses to prevent relapses.
Relapses are reported to occur in 7–25% of all cases, Reference Consoli, Ronen and An-Gourfinkel 44, Reference Dalmau, Gleichman and Hughes60, Reference Titulaer, McCracken and Gabilondo84, Reference Gabilondo, Saiz and Galan141 with literature suggesting 20% in children.Reference Armangue, Petit-Pedrol and Dalmau91 It is not uncommon to find fully recovered patients who maintain detectable levels of antibodies in serum or CSF fluid, suggesting a potential for reactivation of the immune response.Reference Alexopoulos, Kosmidis, Dalmau and Dalakas142 The efficacy of chronic immunosuppression with azathioprine or mycophenolate mofetil in preventing relapses is unknown.Reference Armangue, Petit-Pedrol and Dalmau91 Identified risk factors for relapse include lack of immunotherapy at disease onset, delayed tumor removal and failure to identify a tumor.Reference Dalmau, Lancaster and Martinez-Hernandez 75, Reference Irani, Bera and Waters85, Reference Gabilondo, Saiz and Galan141, Reference Tan, Shuey and Bladin143 There should be surveillance for occult neoplasm together with observation for recurrence or deterioration of psychiatric symptoms, which may signal relapse of encephalitis:
• In females of all ages previously diagnosed with anti-NMDAr encephalitis, recommendations include periodic surveillance for at least 2 years using MRI and ultrasound of the abdomen and pelvis.Reference Florance, Davis and Lam89 Some papers recommend continued yearly screening for tumor, particularly if the patient has a recurrence or remains symptomatic.Reference Florance-Ryan and Dalmau78
• Formal information on tumor surveillance in males is not available. In one of the cases described by DeSena et al., Reference DeSena, Greenberg and Graves137 a testicular tumor was noted 4 years after the diagnosis of anti-NMDAr encephalitis, and in a case described by Chapman et al., Reference Chapman and Vause58 a testicular ultrasound and full-body PET were scheduled at 6-months intervals.
• Due to the cases reported—relapses at 6 and 15 years post-first presentation—Ramanathan et al.Reference Ramanathan, Wong and Fung144 propose that in patients without identified tumors, and particularly in those whose initial episode was not treated with immunomodulatory therapy, the period of ongoing tumor surveillance and antibody titer monitoring should be extended by as much as ten years.
Uchida et al. Reference Uchida, Kato, Yamashita, Ozaki and Matsukawa145 report a case that illustrates the need to keep looking for tumors as the means to questioning why some patients are refractory to extensive treatment. In their case, they suggest that failure to improve after ovarian resection could be a marker of recurrent ovarian teratoma in this disorder: a woman with anti-NMDAr encephalitis with bilateral ovarian teratomas who was refractory to tumor resection and early institution of immunotherapy, with persistently high titers of anti-NMDAr antibodies over time, had a recurrent ovarian teratoma detected by pelvic CT, and after total enucleation of bilateral cystic teratomas, her titers decreased and she clinically improved.
Life after the acute event and subsequent rehabilitation is, however, not an easy process. After recovering consciousness, the psychiatric manifestations can reemerge—in fact, there is no evidence that effective treatment of the disorder prevents recurrence of catatonic or isolated psychiatric symptomsReference Yoshimura, Yada, Horigome and Kishi123—and impulsivity, behavioral disinhibition, problems of attention, planning, social interaction, and memory deficits are usually difficult to manage, either at home or in rehabilitation centers. The latter usually have limited experience with the disorder, which tends to culminate in back-and-forth transfers with the hospital. A multidisciplinary approach involving nursing, psychiatrists, cognitive rehabilitators, and physiatrists, among others, is a necessary route to take in the future as it is highly rewarding for patients and families.Reference Armangue, Petit-Pedrol and Dalmau91, Reference Kayser, Kohler and Dalmau146–Reference Houtrow, Bhandal, Pratini, Davidson and Neufeld148 Curiously, van de Riet et al. Reference van de Riet, Esseveld, Cuypers and Schieveld105 published a report describing how the admission of one teenager presenting with a severe first onset psychosis, with later deterioration to catatonia and autonomic instability, and with a subsequent diagnosis of anti-NMDAr encephalitis, was enough for the nursing team to recognize the symptomatic pattern of the disease and suspect its presence on a following admitted teenager with similar symptoms, which allowed for an earlier diagnosis.
Anti-NMDAr encephalitis in pregnancy may have a good outcome for both mother and baby, Reference McCarthy, Dineen and McKenna109 but deaths of both mother and fetus have been described, like the case published by Keskin et al., Reference Keskin, Tanburoglu, Idiman and Ozturk149 where a 27-year-old pregnant woman who developed catatonia and autonomic instability died of septic shock after the demise of the fetus.
Refratory catatonia secondary to anti-NMDAr encephalitis
Psychiatric symptoms often show improvement that is gradual yet continuous, leading to full recovery without targeted intervention.Reference Chapman and Vause58 As discussed previously, it is rather frequent that psychiatric symptoms are indirectly managed through the etiologic management of the disease. An example that portrays this occurrence regarding catatonic symptoms is the case described by Ponte et al., Reference Ponte, Gama Marques, Carvalhão Gil, Nobrega, Pinheiro and Brito150 who described the case of a 33-year-old man diagnosed with paranoid schizophrenia in 2009 and who presented to the psychiatric emergency department, 6 years after having abandoned follow-up, with persistent headaches, abnormal behavior and loss of motor skill, having been admitted to the psychiatric ward under the diagnosis of Catatonic Schizophrenia. He later developed fluctuating catatonia, which did not respond to neuroleptics and benzodiazepines, and deteriorated to dysautonomic symptoms and seizures. He tested positive for anti-NMDAr antibodies but had no occult tumor. Etiologic treatment was initiated with high-dose steroids and IVIg, followed by cyclophosphamide, and the patient improved clinically. Similarly, a case described by Palakkuzhiyil et al. Reference Palakkuzhiyil, Uvais, Moideen and Shihabudheen151 reported on a middle-aged man whose catatonia did not respond to benzodiazepines, but improved with immunotherapy.
However, despite the expanding knowledge available, and as noted by Wilson et al., Reference Wilson, Shuster and Fuchs111 information on the management of psychiatric symptoms in these complex, often critically ill patients, is scarce, with medical literature mainly focusing on immunotherapy as the treatment for anti-NMDAr encephalitis. Given that anti-NMDAr encephalitis routinely first presents with psychiatric manifestations, including catatonia, that may persist and evolve throughout the illness course, it’s necessary to address the importance of targeting persistent signs of catatonia related to the physiologic mechanisms of anti-NMDAr antibodies on the brain.Reference Dalmau, Lancaster and Martinez-Hernandez 75, Reference Florance-Ryan and Dalmau78, Reference Wilson, Shuster and Fuchs111 Of the little information available on specific management measures and responses regarding catatonia in a setting of anti-NMDAr encephalitis, it is often mentioned in passing as part of the case description or, if at all given some attention, is under a context of unexpected or negative response to a given treatment. Whereas the best treatment approach for anti-NMDAr encephalitis encompasses a combination of tumor resection, immunotherapy, intensive care, and rehabilitation, including physical therapy, discussion regarding the optimal approach to behavioral management is lacking.Reference Chapman and Vause58, Reference Sansing, Tüzün, Ko, Baccon, Lynch and Dalmau98
Some articles have reported on the use of ECT as a means to target catatonia in patients with autoimmune encephalitis, Reference Kaestner, Mostert and Behnken4, Reference Lee, Glick, Dinwiddie, Rosa and Marcolin12, Reference Novillo-López, Rossi, Dalmau and Masjuan100 but reports on the use of ECT in patients with catatonia specifically secondary to anti-NMDAr encephalitis are still equally scarce and urgently needed.Reference Vitaliani, Mason, Ances, Zwerdling, Jiang and Dalmau72, Reference Dalmau, Lancaster and Martinez-Hernandez 75, Reference Florance-Ryan and Dalmau78, Reference Braakman, Moers-Hornikx , Arts, Hupperts and Nicolai152, Reference Dhossche, Fink, Shorter and Wachtel153
So how are physicians addressing the catatonic syndrome in patients with anti-NMDAr encephalitis in the clinical practice? By following the general guidelines for the management of catatonia associated with psychiatric illness, which include the liberal use of benzodiazepines (lorazepam at regular intervals (eg, 2 mg lorazepam every 6 hoursReference Mann, Grebenciucova and Lukas154)/doses of up to 20–30 mg of lorazepam per 24-hour period)Reference Barry, Byrne, Barrett, Murphy and Cotter125 and ECT, as previously discussed.Reference Kruse, Jeffrey, Davis, Dearlove, IsHak and Brooks55 These are measures with known effective treatment for catatonia in psychotic and mood disorders, but little is known about their therapeutic usefulness in catatonia associated with general medical and neurological illnesses.Reference Chapman and Vause58 Despite this, ECT is generally considered to be safe, even in medically compromised patients, and can be lifesaving in the setting of malignant catatonia, Reference Wilson, Shuster and Fuchs111 regardless of etiology. Although adverse side effects from ECT are widely feared, even despite the lack of published data on this topic, with subsequent barriers to its routine implementation in adolescents, medical literature reports it as being effective in treating 80% of cases of catatonia in young people.Reference Rey and Walter155
In the case of catatonia associated with anti-NMDAr encephalitis, some reportsReference Lee, Glick, Dinwiddie, Rosa and Marcolin12, Reference Braakman, Moers-Hornikx , Arts, Hupperts and Nicolai152, Reference Coffey and Cooper156 have shown that 7 to 8 bilateral ECT treatments over 2–4 weeks have led to symptom remission.Reference Mann, Grebenciucova and Lukas154
In the case of a 14-year-old with anti-NMDAr encephalitis described by Wilson et al., Reference Wilson, Shuster and Fuchs111 management of the primary immune-mediated encephalitis was accomplished using immunotherapy (rituximab) and malignant catatonia was addressed with ECT and high-dose lorazepam, until a dermoid cyst tumor was found and removed. ECT was then continued for 9 more treatments (before the tumor was removed, the patient had had 5 ECT treatments) and IVIg was added to rituximab. The patient gradually improved, received rehabilitation, and was well at 1-year follow-up. The authors hypothesized that ECT might have reversed the effect of antibodies on the brain, therefore providing protection until immunotherapy and tumor removal successfully stopped antibody production. Following this train of thought, the underlying argument would be that ECT could be beneficial in stabilizing the dysautonomy, hypermetabolism and agitated and psychotic states associated with anti-NMDAr encephalitis while a primary diagnosis and treatment are initiated.
The case described by Matsumoto et al. Reference Matsumoto, Matsumoto, Kobayashi and Kato157 of an 18-year-old man with anti-NMDAr encephalitis who fully recovered from catatonia after 13 ECT treatments together with antipsychotics but no immunotherapy, and the case described by Mann et al.Reference Mann, Machado, Liu, Mazin, Silver and Afzal158 of a 14-year-old girl failed to improve with IVIg + risperidone + rituximab but whose progressively worsening catatonia rapidly and partially improved with ECT (allowing for the subsequent institution of immunotherapy which lead to a gradual recovery) were interpreted by Jones et al. Reference Jones, Schwartz, Hermida and Kahn74 as 2 examples of how improvement with ECT is independent from the response to the usual standard treatments (immunotherapy, tumor removal). The authors suggest that ECT may have a direct disease-modifying effect in anti-NMDAr encephalitis and, if that is indeed the case, that ECT might improve outcomes over standard therapy alone. As such, ECT as an adjuvant measure to surgical and immunological therapy in the acute care of this serious yet often reversible cause of catatonia warrants further study.
Ramanathan et al. Reference Ramanathan, Wong and Fung144 reported 2 cases where patients demonstrated complete recovery without immunomodulatory therapy or tumor identification, with Patient 2 being only the second report of complete recovery with ECT as the sole therapeutic intervention, the first being described recently by Matsumoto et al. Reference Matsumoto, Matsumoto, Kobayashi and Kato157
Braakman et al. Reference Braakman, Moers-Hornikx , Arts, Hupperts and Nicolai152 presented the case of a previously healthy 47-year-old male who presented with progressive psychiatric symptoms following an upper respiratory tract infection, whose extensive investigations did not reveal any abnormality, and whose CSF showed pleocytosis. These limited findings motivated initial therapy for a viral encephalitis and subsequently for encephalitis lethargica with intravenous lorazepam and 3 days of intravenous methylprednisolone. However, his psychiatric symptoms failed to resolve and so he underwent 7 sessions of bilateral ECT, which eventually induced remission. All symptoms, including mutism, hallucinations, oculogyric crises, and extrapyramidal symptoms resolved and he returned to work within 2 years. The definite diagnosis of anti-NMDAr encephalitis was only made later with retrospective analysis of his CSF, which revealed anti-NMDAr antibodies.
Gough et al. Reference Gough, Coebergh, Chandra and Nilforooshan159 described a case of a previously healthy 71-year-old female who presented to her general practitioner (GP) with malaise and bilateral shoulder pain. Because her erythrocyte sedimentation rate (ESR) was raised at 45 mm/h and C-reactive protein (CRP) levels were raised as well, she was prescribed prednisolone, with polymyalgia rheumatica as the suspected diagnosis. By the start of the medication she started to show signs of low mood, and obsessional and paranoid thoughts. Once completed her course of steroids, she presented again to her GP, which decided to commence her on fluoxetine, buspirone and diazepam as symptomatic relief. Despite this, her psychiatric symptoms continued to deteriorate, and she developed catatonia, posturing with psychotic symptoms, paranoid thoughts, nihilistic delusions, and auditory hallucinations, associated with significant loss of weight and inability to fulfil her activities of daily living. By this time, she was admitted to a psychiatric ward (2 months after her initial presentation to the GP): she appeared withdrawn, confused, and disorientated, and was responding to auditory hallucinations. Her general physical examination was normal, as was her neurological one. She maintained a raised ESR and was found to have a raised leukocyte count (with neutrophilia), raised urea and creatinine, alanine transaminase (ALT) and bilirubin. Lumbar puncture for CSF analysis was not performed, as the doctors did not feel there was indication for such, and a CT scan of her head did not show any abnormality. The patient was started on risperidone and sertraline but stopped shortly afterwards because she became dizzy and had an unresponsive episode, which motivated an admission to the emergency department for further investigation, with no physical cause being identified. Mirtazapine, venlafaxine, and olanzapine also failed to improve her symptoms. By then she remained severely depressed with persistent catatonia and psychotic features, and so an ECT regime was started. However, after the fourth cycle of ECT, the patient fell and fractured the neck of her femur, which required surgery and a 2-week admission to an orthopedic ward, and thus stopped her ECT regimen. Once she returned to the psychiatric unit her serum autoantibodies screen test results (sent on admission) became available and showed low positivity for anti-NMDAr encephalitis. No evidence of malignancy was detected on CT scan of chest, abdomen, and pelvis. The Neurology unit was contacted, and plasmapheresis was started associated with methylprednisolone, followed by prednisolone. However, her antibodies titers remained on the low-positive range and despite the plasmapheresis, corticosteroids and multiple psychotropics later administered (quetiapine, lithium, and further venlafaxine), the patient’s symptoms remained severe. A second course of ECT was then initiated, with 8 cycles being completed. This time, however, the patient presented a marked recovery, with her mood improving significantly and with no psychotic symptoms. Her antibody titers were repeated and undetectable and the patient was discharged home at her premorbid level.
Sunwoo et al. Reference Sunwoo, Jung and Choi160 defend that ECT may be an option for life-threatening catatonia and medically refractory dyskinesia in patients with anti-NMDAr encephalitis; their 27-year-old female patient developed catatonia even after the disease had been detected early and an ovarian tumor removed followed by IVIg and administration of rituximab. Catatonia was refractory to benzodiazepines and the dyskinesia was so severe she had to have continuous infusions of a neuromuscular blocker, with escalating doses, suggesting that she was not improving, even with coadministration of rituximab. Finally, ECT was started and she responded to it.
Amorim et al. Reference Amorim and McDade161 describe an interesting case of a 73-year-old woman that was referred to the neurology clinic for evaluation of cognitive decline and catatonia. Despite treatment with benzodiazepines, antipsychotics and 12 ECT sessions (with no electrocardiographic seizures documentation despite escalating electrical doses), her catatonic symptoms persisted. She was later found to have a mature ovarian tumor, which was removed, but catatonia failed to improve, even with benzodiazepines. ECT was repeated, this time with achievement of electrographic seizures, and there was partial improvement. Anti-NMDAr antibodies were negative in the CSF, but the authors stress that these were obtained nearly 10 months after the surgery. In the meanwhile, due to ongoing sleep disturbance, she was prescribed zolpidem (a GABA-A receptor agonist, routinely used as a hypnotic in the short-term treatment of insomnia) at bedtime and shortly after its administration, the patient improved dramatically. However, the effect lasted only 6-10 hours until she would return to her baseline catatonic-state, with progressing shorter periods of response. A few case reportsReference Zaw and Bates162, Reference Thomas, Rascle, Mastain, Maron and Vaiva163 of catatonia responsive to zolpidem have been described in the literature, and it has been proposed that it could be used as a pharmacological test for the clinical diagnosis of catatonia.
Medina and CooperReference Medina and Cooper164 report on the case of a highly educated woman in her late 20s whose refractory catatonia secondary to anti-NMDAr encephalitis was successfully treated with ECT. What’s interesting in this case is that, contrary to other reports where the authors only described the improvement of the catatonic syndrome, this patient performed the clock-drawing test (CDT) pre-ECT, after the second ECT session, after the sixth and final ECT session and at 6-months follow-up, with the difference between the first and second drawing being remarkable, with a clear improvement following the first 2 sessions of ECT.
Recently, in February 2019, Moussa et al. Reference Moussa, Afzal, Cooper, Rosenberger, Gerstle and Wagner-Weiner 165 described the case of a previously healthy 16-year-old with anti-NMDAr encephalitis and catatonia, whose catatonic symptoms remained refractory to trials of lorazepam and zolpidem. Similarly, catatonia did not resolve with etiologic treatment; despite intensification of both first-line treatment with IVIg and second-line treatment with rituximab and a second course of 1g methylprednisolone daily for 3 days. In fact, she persisted with episodes of agitation, rigidity, sleep disturbance, mutism, facial twitching, drooling, and autonomic instability, leading to the diagnosis of malignant catatonia. Her BFCRS was 27. Because of this, she was started on ECT. Over the course of her 8 treatments given thrice weekly, her BFCRS dropped from 27 to 2 and her mental status evaluation (through the CDT) showed marked improvement in visuospatial, motoric, and cognitive functioning, with the patient’s mother noting that the patient displayed more of her typical personality and mannerisms. However, the patient remained mute post-ECT and was discharged home on melatonin, levetiracetam, tapering doses of prednisone and lorazepam, and additional IVIg treatments, as well as intensive outpatient rehabilitation including speech, physical and occupational therapy. At her 6-week post-ECT clinic appointment, her verbal abilities and communication skills appeared normal.
Tanguturi et al. Reference Tanguturi, Cundiff and Fuchs166 aimed to thoroughly review the use of ECT in the treatment of catatonia secondary to anti-NMDAr encephalitis, concluding that its use is indicated in the treatment of catatonia, particularly in cases of resistant catatonia, failure of first-line immunotherapy and when plasma exchange is not available. They also report that the use of synergistic ECT with benzodiazepines could help with faster recovery and shorter time spent in the hospital, and recommend the creation of a treatment algorithm for anti-NMDAr encephalitis that includes both benzodiazepines and/or ECT to manage catatonic symptoms in addition to immunotherapy to target the antibody development, with further research needed to identify the treatment specifications such as length of time of treatment and parameters for response to treatment.
In a 2019 systematic review of the use of ECT in anti-NMDAr encephalitis, Warren et al. Reference Warren, Grote, O’Gorman and Siskind167 looked to ascertain whether ECT in the treatment of anti-NMDAr encephalitis was safe and whether it could improve psychiatric outcomes. To do so, they analyzed 30 cases published between 2007 and June 2018. Cases selected had to have anti-NMDAr encephalitis formally diagnosed by positive serum or CSF IgG antibodies and catatonia had to be clearly documented under established criteria. Treatment response and outcome was based on the information provided in the case reports and categorized as resolved, improved, no improvement or deterioration. Results regarding catatonia were the following:
• 70% of patients (n = 21/30) presented initially with behavior abnormalities, 30% of which (n = 9/30) had catatonia.
• All patients (n = 30/30) had behavior abnormalities during the disease, with 86.7% having catatonia (n = 26/303) and 3 additional cases, despite not having a documented catatonia, had a BFCSI greater than 2, which indicates catatonic symptoms. Of these 26 catatonic patients, 11 had fluctuating catatonia.
• The most common catatonic symptoms were, in descending order: excitement, immobility/stupor, mutism, withdrawal, posturing, rigidity, stereotypy, and perseveration.
• Of the 24 cases documented to have started ECT due to catatonia, 20.8% (n = 5/24) had resolved catatonia, 33.3% (n = 8/24) had improved catatonia and 16.7% (n = 4/24) did not show any signs of improvement. The remaining cases—16.7% (n = 4/24)—had no statements on ECT outcomes.
• Both excited and stuporous catatonic symptoms were effectively treated in these cases at a comparable efficacy.
Of the 23 cases which provided information on effectiveness of ECT in anti-NMDAr encephalitis, 15 (65.2%) noted improvement of psychiatric symptoms, and in 9 of these cases the improvement was seen before initiation of immunotherapy. Data pertaining to the effectiveness of ECT on overall psychiatric symptoms were also reported: of the 23 cases on information regarding ECT effectiveness, 4 had complete resolution of psychiatric symptoms without any immunotherapy and after treatment resistance had been noted with psychotropics, and 15 (65.2%) noted improvement of psychiatric symptoms, with 9 of those having improvement prior to immunotherapy; in the 6 cases which improved with a combination of ECT and immunotherapy, ECT was started due to insufficient response to immunotherapy. Based on the analysis of symptomatic outcomes and data pertaining to anesthetic and follow-up complications, the authors concluded what other authors have suggested throughout time based on their personal clinical experience: ECT appears to be a safe, effective adjuvant treatment, especially for psychiatric symptoms, particularly for catatonia. Table 2 summarizes the case reports of catatonia secondary to anti-NMDA receptor encephalitis.
Table 1. Diagnostic criteria for anti-NMDA receptor encephalitis, as per Graus et al.
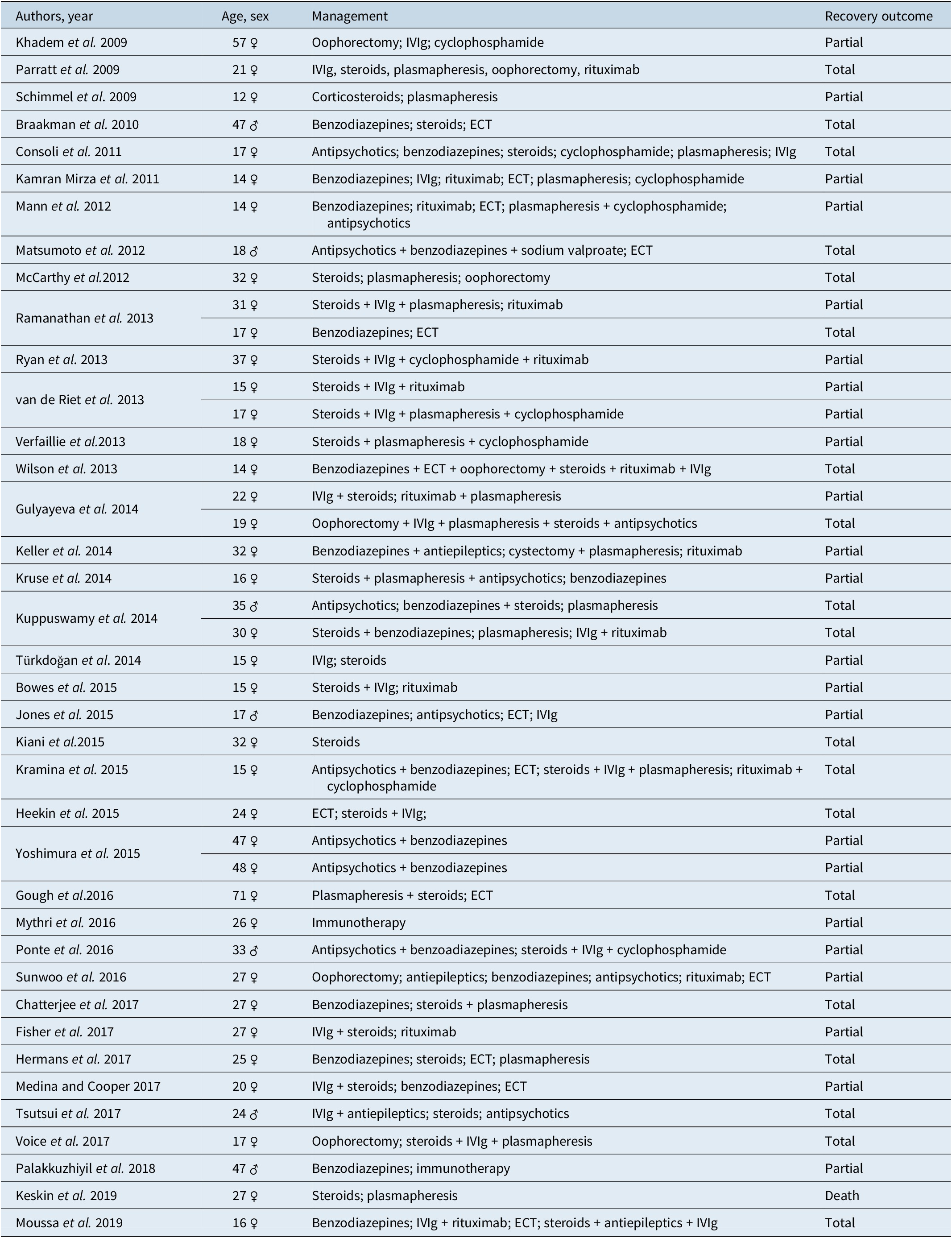
* Patients with a history of herpes simplex virus encephalitis in the previous weeks might have relapsing immune-mediated neurological symptoms (post-herpes simplex virus encephalitis).
† Antibody testing should include testing of CSF. If only serum is available, confirmatory tests should be included (eg, live neurons or tissue immunohistochemistry, in addition to cell-based assay).
Conclusion
Two main groups of conclusions can be drawn from this article: (1) a group based on the results of the literature review and (2) a group based on the compilation of the clinical cases of catatonia secondary to anti-NMDAr encephalitis.
(1) Catatonia is a psychiatric syndrome that may be associated with primary psychiatric disease or medical conditions, such as anti-NMDAr encephalitis. As such:
a. This disease must be considered in the differential diagnosis of a catatonic patient;
b. When dealing with a patient with a diagnosis of, or suspected of having, anti-NMDAr encephalitis, physicians must be aware that catatonia may develop, and that malignant catatonia must be acutely managed as it may be fatal. In clinical practice, psychiatric symptoms, including catatonia, are often indirectly managed through the etiologic treatment of the underlying encephalitis, with immunotherapy and tumor removal, if any;
c. Management of catatonia secondary to this type of encephalitis is based on etiologic treatment, but symptomatic remission is variable. Benzodiazepines and ECT can be used, are safe, and improve catatonic outcomes, even more so if used as a combination and as adjunct therapy to immunotherapy and tumor removal.
(2)
a. We found that women of child-bearing age were the most commonly affected, which is congruent with data on anti-NMDAr encephalitis found elsewhere;
b. A significant proportion of patients only have partial remission;
c. There does not seem to be a consensus on the sequence of what different treatment modalities to apply, which attests to the necessity of creating guidelines for this type encephalitis including catatonia management.
Table 2. List of case reports of catatonia secondary to anti-NMDAr encephalitis.
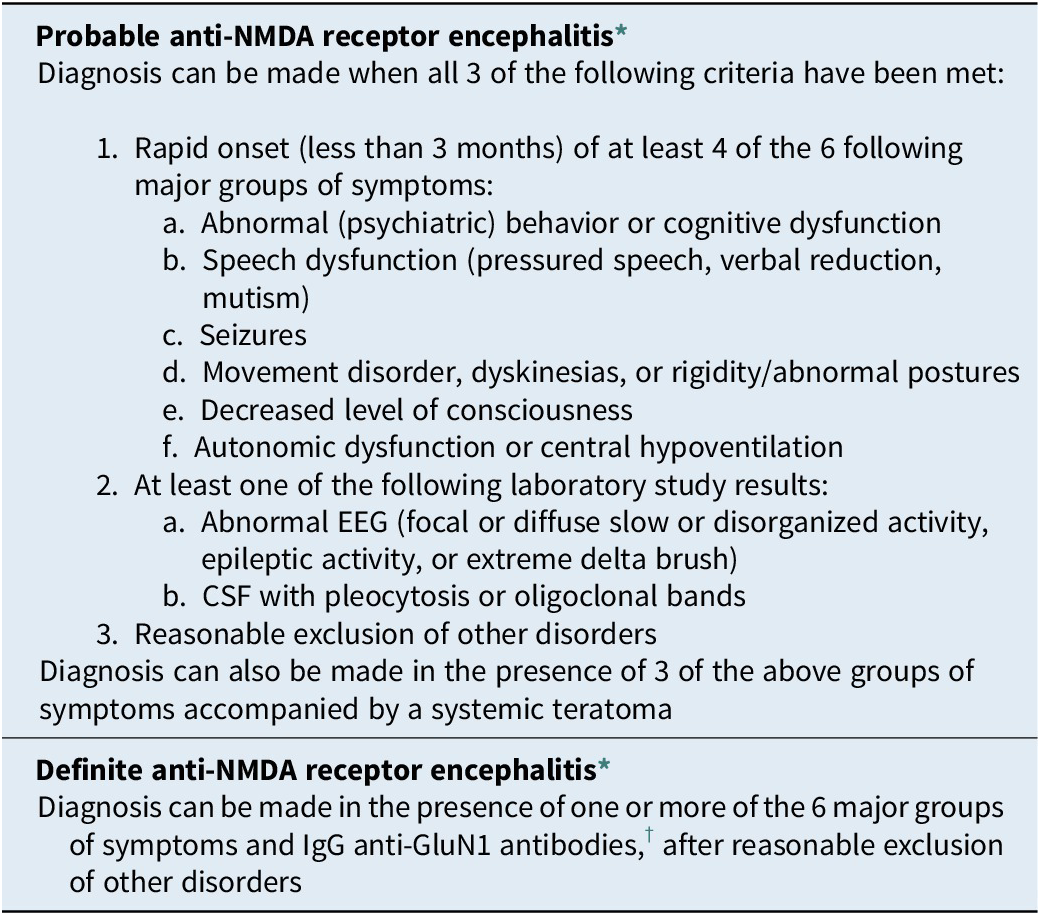
The main aim of this article is, however, to alert the medical and scientific community of the close relation between these 2 entities—anti-NMDA receptor encephalitis and catatonia—and that they may not be as rare as once thought and, as so, cannot go unnoticed by any psychiatrist or neurologist. Further attention and research on this relation should be encouraged (eg, Figure 2).
Disclosure.
The authors have nothing to disclose.






