Introduction
Insects collected from fields are reared in the laboratory on artificial diets for conducting various types of studies. However, contamination of insect artificial diets with microbial growth is a common problem. For successful rearing of insects in the laboratory, antibacterial and antifungal compounds are added to artificial diets to prevent microbial contamination. The tolerance of insects to antimicrobial agents varies with insect species as well as kinds and dietary levels of these compounds (Buyukguzel & Yazgan, Reference Buyukguzel and Yazgan2002). Many of these compounds can be toxic to insects, even at low concentrations and exert detrimental effects on the growth and development of insects (Singh & House, Reference Singh and House1970; Cohen, Reference Cohen2003) which ultimately affect bioassay results. However, insect diet lacking antimicrobial agents may become contaminated with bacteria/fungi which may cause biochemical changes in the diet leading to alteration in the nutritional value of the diet by producing toxins (Childress & Williams, Reference Childress and Williams1973; Bell et al., Reference Bell, King and Hamalle1981). Therefore these compounds should be used in right proportion to control microbial growth, while at the same time limiting their negative effects on the insects.
Most studies related to the effect of antibiotics on insects deal with the growth, development and biochemical changes taking place, however, their effect on gut microbial flora has not been investigated much. The use of antibiotics may lead to change the gut microflora of insects and ultimately influence the fitness of insect (Rosengaus et al., Reference Rosengaus, Zecher, Schultheis, Brucker and Bordenstein2011) as gut microbes are known to play significant role in host's nutrition and digestion (Brune, Reference Brune, Resh and Cardé2003; Moran et al., Reference Moran, Russell, Koga and Fukatsu2005). In light of this we investigated the effect of streptomycin sulphate on gut microbial diversity of a lepidopteran pest. Spodoptera litura (L.) (Fabricius) was used as a model insect because it is one of the most destructive pest of various crops like cruciferous vegetables, groundnut, cotton, tobacco etc. (Rao et al., Reference Rao, Rajasekhar, Venkataiah and Rao1994; Qin et al., Reference Qin, Ye, Huang, Ding and Luo2004). Pesticides have been extensively used for the management of this pest due to which it has developed resistance against a variety of insecticides belonging to almost all the insecticide groups used against it (Kranthi et al., Reference Kranthi, Jadhav, Kranthi, Wanjari, Ali and Russell2002; Sudhakaran, Reference Sudhakaran2002). Being an important economic pest it has been extensively used for various studies under laboratory conditions. As, its mass rearing is mainly carried out on artificial diet so the present study aims at to evaluate the effect of antibiotic on gut microbial diversity as well as survival and fitness of S. litura.
Materials and methods
Insect culture
The larvae of S. litura were collected from cauliflower fields around Guru Nanak Dev University, Amritsar (Punjab) India. The larvae brought to laboratory were reared on artificial diet as recommended by Gupta et al. (Reference Gupta, Rani, Birah and Raghuraman2005) with slight modifications for subsequent generations under controlled temperature and humidity conditions of 25 ± 2 °C and 65 ± 5%, respectively. The main ingredients of artificial diet were wheat germ, kidney bean flour, yeast powder, ascorbic acid, multivitamins, sorbic acid, streptomycin sulphate, methyl-p-hydroxybenzoate etc. The rearing was carried out in battery jars (15 × 10 cm2) with daily change of diet. The pupae were transferred to pupation jars containing 2–3 cm layer of moist sterilized sand covered with filter paper. The freshly emerged adults were shifted to oviposition jars similar to pupation jars except for a cotton swab soaked with honey solution (1 part honey:4 parts water) as food, hanging from the muslin cloth covering the jar. The oviposition jars were lined with filter paper to facilitate egg laying. The larvae from third generation of laboratory culture were used for experimental purpose.
Bioassay studies
Artificial diet of S. litura was supplemented with three concentrations of streptomycin sulphate (S+) i.e., 0.03, 0.07 and 0.15% (w/v). Diet without streptomycin sulphate (S−) served as control. Experiments were performed with 25 second instar larvae (five larvae per replicate) with five replications for each treatment group. The experiments were carried at 27 ± 2 °C temperature and 65 ± 5% relative humidity along with photoperiod of 16:8 L:D. Observations were made daily on various biological parameters of S. litura viz. larval mortality, larval and pupal period, total development period, per cent pupation and adult emergence.
Effect of different concentrations of streptomycin sulphate on nutritional physiology of S. litura
The effect of different concentrations of streptomycin sulphate i.e., 0.03, 0.07 and 0.15% (w/v) on food utilization of second instar larvae of S. litura, was studied following the procedure of Farrar et al. (Reference Farrar, Barbour and Kennedy1989). The artificial diet was prepared and amended with above mentioned concentrations of streptomycin sulphate. The diet without streptomycin sulphate served as control. The larvae starved for 3–4 h were weighed individually and placed in plastic containers (4 × 6 cm2) containing known amount of control or treated diets. The temperature and humidity conditions were maintained at 25 ± 2 °C and 65 ± 5%, respectively. The experiment was carried out using 25 larvae for each treatment and the observations were made after 72 h on larval weight, residual diet and faecal matter. The overall change in each variable was compared with the last recorded value. At the end of each experiment after 72 h dry weight of the larvae, diet and faecal matter were determined by incubating at 60 ± 2 °C to assess the loss of water under experimental conditions. Nutritional indices were calculated as per Wheeler & Isman (Reference Wheeler and Isman2001) by using following formulae:
where RGR = relative growth rate, RCR = relative consumption rate, ECI = efficiency of conversion of ingested food, ECD = efficiency of conversion of digested food, AD = approximate digestibility.
Biochemical studies
Biochemical studies were carried out on third instar larvae of S. litura feeding on S− and S+ (0.15%) diet. All experiments were replicated thrice with ten larvae per replication. For the analysis of digestive and detoxifying enzymes, third instar larvae were randomly selected from those fed on streptomycin mediated diet (0.15%) as well as from control diet.
Digestive enzymes
Activity of various digestive enzymes viz α-amylase, α, β-glucosidases and galactosidases, lipase and protease was analyzed by using standard protocols. α-amylase assay was performed by the dinitrosalicylic acid (DNS) procedure (Bernfeld, Reference Bernfeld1955), α, β-glucosidases, α, β- galactosidases activities were assayed as per the protocol of Ferreira & Terra (Reference Ferreira and Terra1983). Lipase activity was determined as per Tsujita et al. (Reference Tsujita, Ninomiya and Okuda1989). General proteases assay was performed by using haemoglobin (20 mg ml−1) as substrate according to Cohen (Reference Cohen1993).
Detoxifying enzymes
The activity of glutathione-s-transferases was measured according to the method given by Chien & Dauterman (Reference Chien and Dauterman1991). The methodology of Katzenellenbogen & Kafatos (Reference Katzenellenbogen and Kafatos1971) was used to extract and estimate esterases. The activities of acid and alkaline phosphatases were determined by methodology of Mac Intrye (Reference Mac Intyre1971).
Isolation of the culturable bacteria from larvae
S. litura larvae were reared on artificial diet supplemented with different concentrations of streptomycin sulphate i.e., 0.03, 0.07 and 0.15% (w/v) as well as without streptomycin. To isolate the gut bacteria, ten third instar larvae were randomly selected from all the treatments. All the larvae were starved for 24 h. The starved larvae were surface disinfected with 70% (v/v) ethanol followed by 5% (v/v) sodium hypochlorite solution (NaOCl). This was followed by thorough rinsing of larvae with sterilized distilled water to remove the disinfectant. The larvae were dissected with the help of sterilized microscissor and whole gut was removed and suspended in 1 ml 0.1 M phosphate buffer (pH 7.0). Ten larval guts per treatment were pooled and homogenized in a homogenizer (Eltek, India). All operations were carried out under the laminar flow cabinet (ESCO, USA). The homogenized suspensions were diluted upto 105 times and plated on Luria Bertani (LB) agar plates. The plates were incubated at 30 °C and observed after every 24 h for appearance of morphologically distinct colonies up to 72 h. The colonies were differentiated based on their size, colour and morphology, and a single representative isolate of each morphotype was transferred to a fresh plate. After five–six repeated streaking, the purity and morphology of the cells were ascertained by Gram staining. The purified isolates were maintained in 50% (v/v) glycerol at −80 °C.
Identification of bacterial isolates
Bacterial isolates were identified by Gram staining and various biochemical tests. The results obtained were evaluated according to Bergey's Manual of Systematic Bacteriology, Vols. 1 and 2 (Krieg & Holt, Reference Krieg, Holt and Palleroni1986; Sneath et al., Reference Sneath, Mair, Sharpe, Holt, Kandler and Weiss1986).
16S rRNA gene sequencing
DNA extraction was done according to the standard protocol of Sambrook et al. (Reference Sambrook, Fritsch, Maniatis, Ford, Nolan and Ferguson1989) with slight modifications. DNA pellets were dissolved in 50 µl of TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0) and isolated DNAs were stored at –20 °C until use. Polymerase chain reaction (PCR) amplification of the gene coding for 16S rRNA was performed by using universal bacterial forward primer 27F (5′-AGAGTTTGATCATGGCTCAG-3′) and reverse primer 1492R (5′-TACGGCTACCTTGTTACGACTT-3′). PCR amplification was carried out with the following program: 94 °C for 5 min; 35 cycles at 94 °C for 1 min, 50 °C for 1 min and 72 °C for 2 min and a final extension at 72 °C for 20 min. Amplification products were analyzed by electrophoresis on a 1.5% (w/v) agarose gel and visualized under ultraviolet light after staining with ethidium bromide. The nucleotide sequences of isolates SL1, SL5, SL8, SL9, SL11 and SL13 were submitted to the GenBank database with accession numbers KP058531, KP058542, KP058543, KP058534, KP058541, KP058540, respectively. Sequencing of the 16S rRNA gene was outsourced from Chromous Biotech Pvt Ltd, Bangalore, India.
Phylogeny
The sequences were used to perform basic local alignment search tool (BLAST) searches using the national center for biotechnology information (NCBI) GenBank database to look for similar sequences. In addition, evolutionary relationships of the five bacterial isolates and their ten closely related species were evaluated. Sequences were assembled and edited with BioEdit and aligned (Hall, Reference Hall1999). Cluster analyses of the sequences were performed using BioEdit (version 7.09) with Clustal W followed by neighbour joining analysis on aligned sequences performed with MEGA 4.0 software (Tamura et al., Reference Tamura, Dudley, Nei and Kumar2007). Alignment gaps were treated as missing data. Reliability of dendrograms was tested by bootstrap analysis with 1000 replicates using MEGA 4.0.
Statistical analysis
To compare difference in means one way analysis of variance (ANOVA) with Tukey's test at P ≤ 0.05 was performed. To study the differences in enzyme activities between S− and S+ diets Student's ‘t’ test was performed. SPSS software for windows version 16.0 (SPSS Inc, Chicago) and Microsoft office Excel 2007 (Microsoft Corp., USA) were used to perform the statistical analysis.
Results
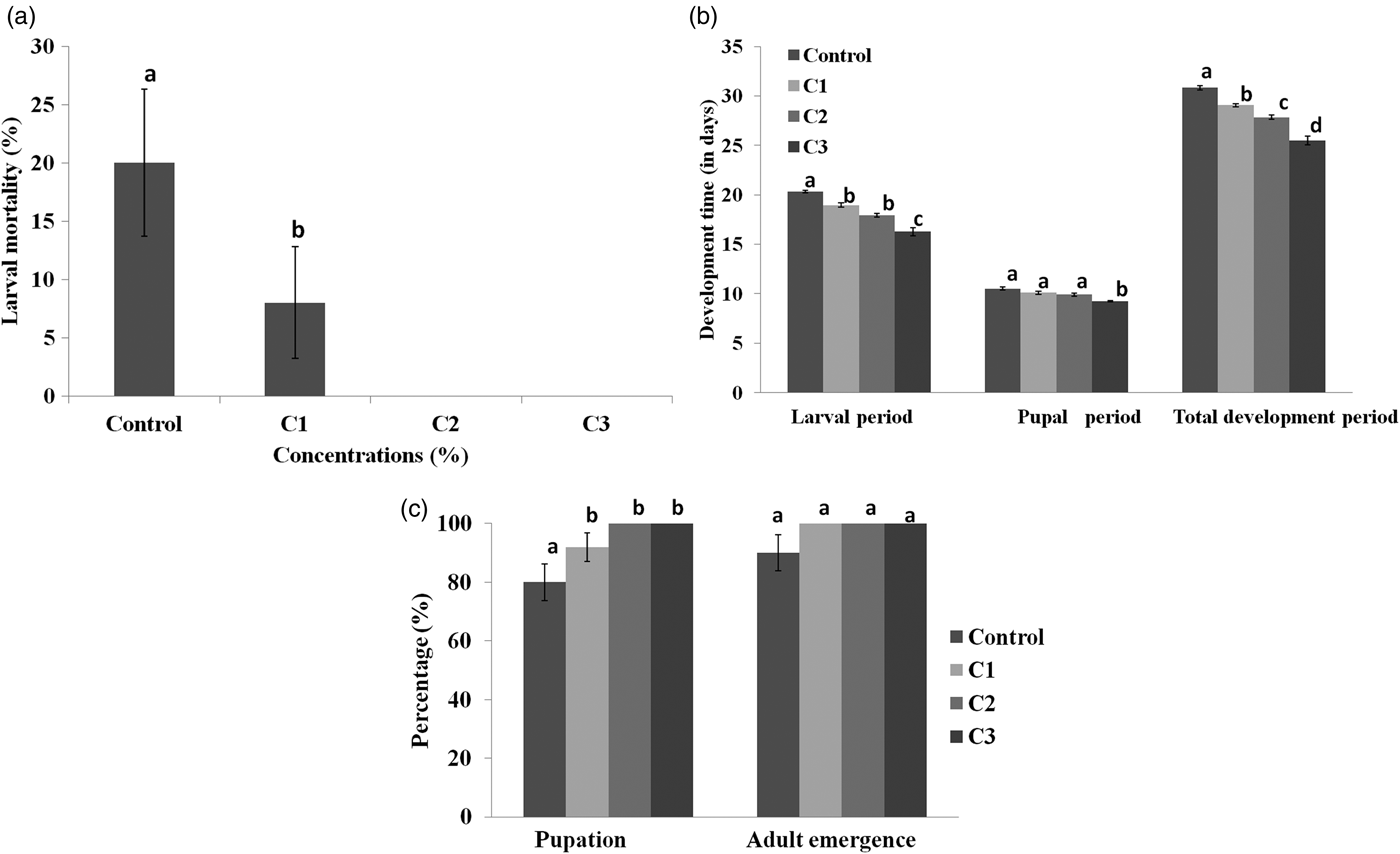
A significant effect of different concentrations of streptomycin sulphate mediated (S+) diet was observed on survival and development of S. litura. Significantly higher larval mortality i.e., 20% was recorded on diet without streptomycin sulphate as compared to diet amended with streptomycin sulphate (F = 3.73, P ≤ 0.05) (fig. 1a). No larval mortality was recorded on diet amended with 0.07 and 0.15% concentrations of streptomycin sulphate whereas the lowest concentration resulted in 8% larval mortality. At the highest concentration larvae turned into pupal stage 4.06 days earlier than those fed on S− diet (F = 43.02, P ≤ 0.05) (fig. 1b). Similarly, pupae completed their development earlier on streptomycin sulphate mediated diet but as compared to control significant differences were recorded only at the highest concentration (F = 12.83, P ≤ 0.05) (fig. 1b). All the concentrations of streptomycin sulphate significantly influenced the development period of S. litura from the larva to adult emergence. As compared to 30.84 days on S− diet, S. litura completed its development in 25.52 days when the highest concentration of streptomycin sulphate was added to diet (F = 62.22, P ≤ 0.05) (fig. 1b). Relative to control, per cent pupation was significantly higher when the larvae were fed on S+ diet (F = 5.58, P ≤ 0.05) (fig. 1c). However, adult emergence did not differ significantly among streptomycin amended and control diets (fig. 1c).

Fig. 1. (a) Larval mortality (b) larval period, pupal period and total development period (c) percent pupation and adult emergence of Spodoptera litura when second larvae were fed on diet containing different concentrations of streptomycin sulphate (S+) (C1 = 0.03%, C2 = 0.07% and C3 = 0.15% ) as well as control diet (S−). Means and SE are given. Means within a column followed by the same letter are not significantly different (P ≤ 0.05) based on Tukey’s test.
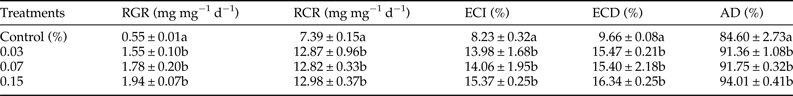
The addition of streptomycin sulphate to diet positively influenced the nutritional physiology of S. litura larvae. The RGR of S. litura larvae feeding on S+ diet significantly increased by 2.81–3.52-fold over control (F = 26.71, P ≤ 0.05)) (table 1). Similarly, relative to control a significant rise of 1.73–1.75-fold in RCR was recorded on S+ diet (F = 24.42, P ≤ 0.05). Both ECI and ECD values also showed significant increase of 1.69–1.86 and 1.59–1.69-fold, respectively on S+ diet (ECI: F = 5.91, P ≤ 0.05; ECD: F = 4.10, P ≤ 0.05) (table 1). AD also significantly increased when streptomycin was added to the diet (6.98, P ≤ 0.05) (table 1).
Table 1. Influence of different concentrations of streptomycin sulphate on growth, feeding and food utilization of S. litura larvae after 3 clays of feeding.

Means (±SE) followed by different letters within a column are significantly different at P ≤ 0.05 according to Tukey's test. RGR, relative growth rate; RCR, relative consumption rate; ECI, efficiency of conversion of ingested food; ECD, efficiency of conversion of digested food; AD, approximate digestibility.
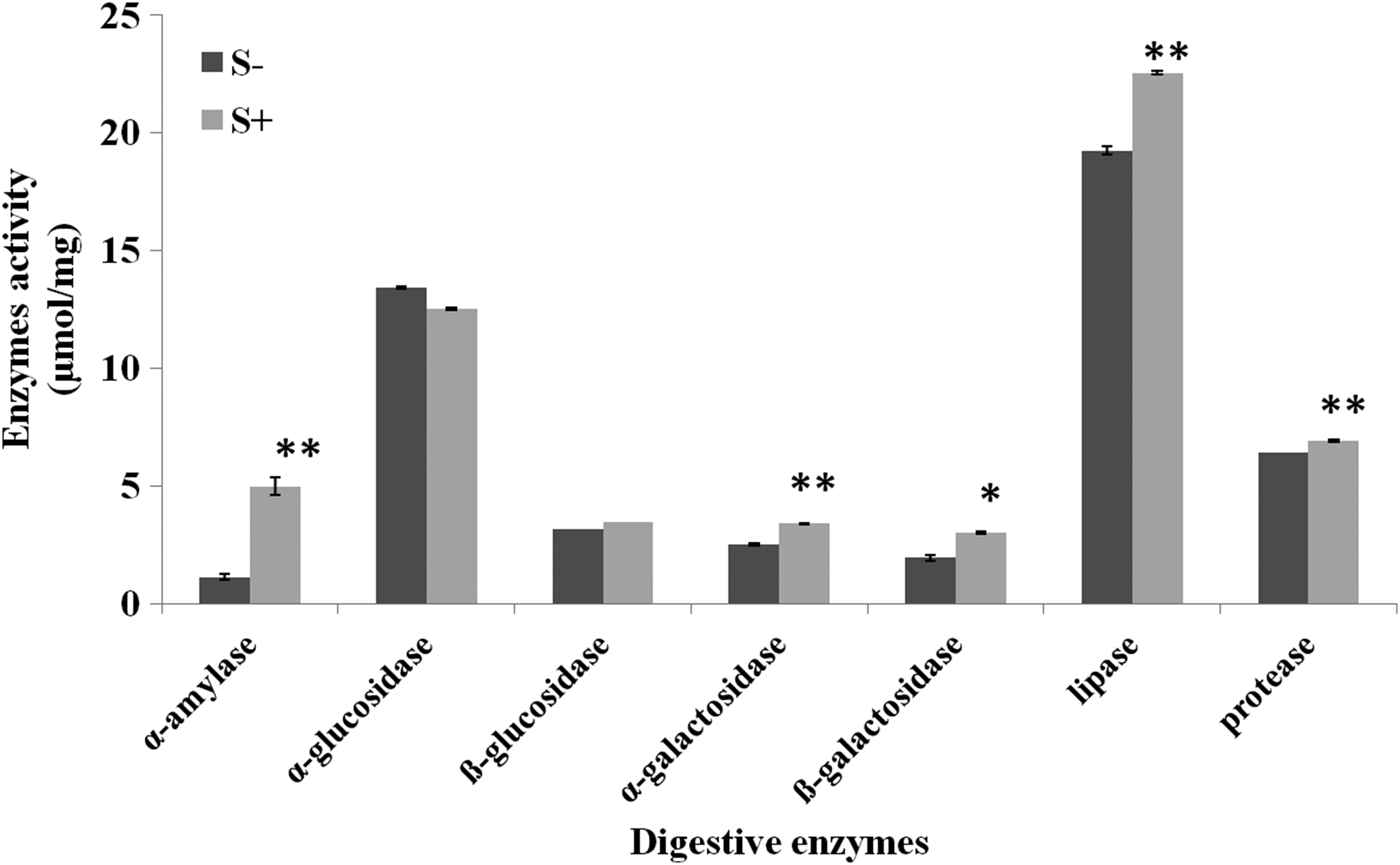
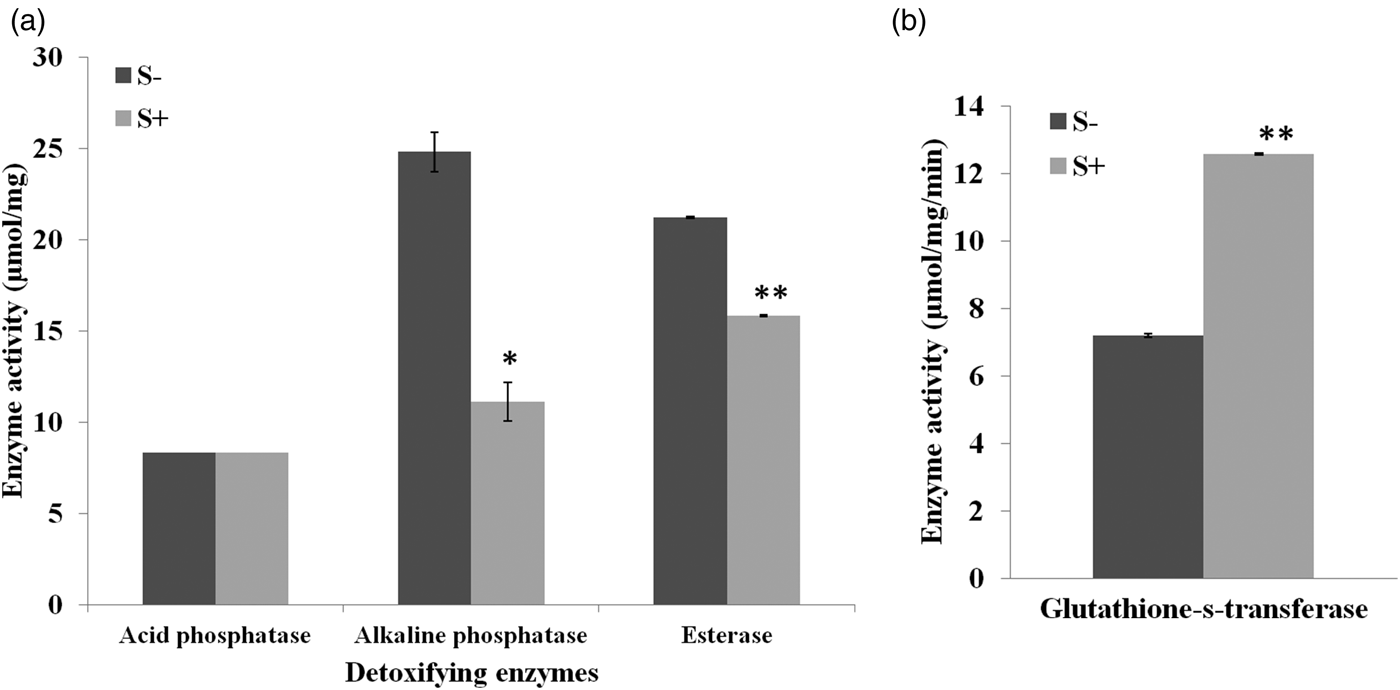
A significant influence of antibiotic was recorded on all the tested digestive enzymes. The level of α- amylase and lipase increased by 4.40 and 1.17-fold relative to control (α- amylase, t = 9.00, lipases, t = 13.97, P ≤ 0.05). No significant impact of streptomycin sulphate was observed on α, β-glucosidases however, a significant rise of 1.34 and 1.55-fold in α, β-galactosidases activity was recorded, respectively, over control (α-galactosidases, t = 19.00, P ≤ 0.05; β-galactosidases, t = 5.48, P ≤ 0.05). Similarly S+ diet induced the level of proteases in larvae (t = 7.80, P ≤ 0.05) (fig. 2). Addition of streptomycin sulphate to larval diet significantly influenced the detoxifying enzymes. The activity of alkaline phosphatases and esterases suppressed by 0.44 and 0.74-fold, respectively, over control (Akp, t = 6.38, P ≤ 0.05; Est, t = 215.00, P ≤ 0.05) while no significant effect was recorded on acid phosphatases (fig. 3a). The larvae feeding on antibiotic mediated diet showed an increase of 1.74-fold in the activity of glutathione-S-transferase with respect to control (t = 138.83, P ≤ 0.05) (fig. 3b).

Fig. 2. Effect of different concentrations of streptomycin sulphate (S+) (C1 = 0.03%, C2 = 0.07% and C3 = 0.15%) on digestive enzymes of third instar larvae of S. litura. Means and SE are given where * indicate significant difference at 5% level and ** at 1% based on student t-test.

Fig. 3. Effect of different concentrations of streptomycin sulphate (S+) (C1 = 0.03%, C2 = 0.07% and C3 = 0.15%) on detoxifying enzymes of third instar larvae of S. litura (a) Acid phosphatases, alkaline phosphatases and esterase (b) Glutathione-s-transferases. Means and SE are given where * indicate significant difference at 5% level and ** at 1% based on student t-test.
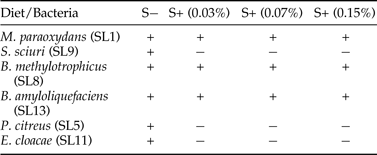
Six different morphotypes were isolated from the gut of third instar larvae of S. litura fed on S− diet (control diet). On the basis of identification tests and sequencing analysis these were identified as Microbacterium paraoxydans (SL1), Planococcus citreus (SL5), Bacillus methylotrophicus (SL8), Staphylococcus sciuri (SL9), Enterobacter cloacae (SL11) and Bacillus amyloliquefaciens (SL13). Addition of streptomycin sulphate to artificial diet resulted in change of larval gut microbial community as well as its abundance. Only three bacterial cultures i.e., M. paraoxydans (SL1), B. methylotrophicus (SL8) and B. amyloliquefaciens (SL13) were isolated from the gut of S. litura larvae feeding on S+ diet (table 2). However, the gut microbial diversity of larvae fed on different concentrations of streptomycin sulphate did not differ (table 2). The colony growth of M. paraoxydans B. methylotrophicus and B. amyloliquefaciens was more on S+ diet in comparison with S− diet where E. cloacae, P. citreus and S. sciuri were the dominant colonies.
Table 2. Gut microbial diversity of S. litura larvae feeding on streptomycin mediated (S+) and control diets (S−).

Discussion
The present findings demonstrated higher mortality in larvae fed on S− diet while addition of antibiotic reduced the mortality rate. No larval mortality was observed at higher concentrations of streptomycin mediated diet. Similarly streptomycin (100 mg litre−1) significantly reduced larval mortality in Plutella xylostella (L.) which were susceptible to B. thuringiensis (Liu et al., Reference Liu, Tabashnik, Moar and Smith1998). Artificial diet used for rearing of insects lacking antimicrobial agents may have contamination of bacteria or fungi which reduce the nutritive value of diet by producing toxins which can lead to death of insect (Childress & Williams, Reference Childress and Williams1973; Bell et al., Reference Bell, King and Hamalle1981). Streptomycin sulphate has been reported to be safe as compared to other antibiotics i.e., rifampicin, ampicillin, tetracycline and chloramphenicol (Lin et al., Reference Lin, Kang, Pan and Liu2015). Our results demonstrated increased values of nutritional indices i.e., RGR, RCR, ECI, ECD and AD on S+ diet which depict faster growth of S. litura larvae. A significant reduction in average time of larvae to reach the pupal stage of Pimpla turionellae (L.) was earlier reported by Buyukguzel & Yazgan (Reference Buyukguzel and Yazgan2002) at a concentration of 30 mg of penicillin. However, addition of rifampicin at 5 and 10 mg concentrations to diet resulted in a significant decrease in the postlarval survival of P. turionellae. Survival of Galleria mellonella (L.) larvae increased with increasing doses of antibiotics daptomycin or vancomycin when larvae were infected with Staphylococcus aureus (Desbois & Coote, Reference Desbois and Coote2011). Previously Buyukguzel & Kalender (Reference Buyukguzel and Yazgan2008) also documented no adverse effects of streptomycin sulphate on survivorship of G. mellonella when used at lower concentrations while higher concentration prolonged the development period, reduced pupation and adult emergence. However, in the present study streptomycin sulphate did not show negative effects on pupation, adult emergence as well as morphology of S. litura.
The activity of all the tested digestive enzymes viz. α- amylase, α- β-galactosidases, lipases and proteases significantly increased on streptomycin sulphate mediated diet. The increased values of digestive enzymes might have improved the efficiency of conversion of ingested food to insect biomass and further resulted in faster development. Overall activity of detoxifying enzymes significantly decreased on antibiotic mediated diet except for glutathione-s-transferase which showed a significant increase in activity. This increase in enzyme activity on S− diet might be attributed to their capacity to adapt according to diet.
The present study revealed that addition of streptomycin sulphate in artificial diet of S. litura caused alteration in gut microbial flora as well as their abundance. M. paraoxydans, S. sciuri, B. methylotrophicus, B. amyloliquefaciens, P. citreus, E. cloacae were isolated from control larvae. In comparison to this only M. paraoxydans, B. methylotrophicus and B. amyloliquefaciens were present in the gut of S. litura larvae feeding on S+ diet. Some of the gut bacteria isolated from S. litura have previously been reported to be associated with lepidopteran guts as normal symbionts e.g., E. cloacae in laboratory reared Peridroma saucia (Huber) (Armstrong et al., Reference Armstrong, Porteous and Wood1989), S. sciuri in laboratory reared Mythimna separata (Walker) (He et al., Reference He, Nan, Zhang and Menglou2013) and M. paraoxydans in wild larvae of Ostrinia nubilalis (Shil et al., Reference Shil, Mojumder, Sadida, Uddin and Sikdar2014). Similar to our studies Rosengaus et al. (Reference Rosengaus, Zecher, Schultheis, Brucker and Bordenstein2011) documented variation in diversity of gut microflora of two termite species, Zootermopsis angusticollis (Hagen) and Reticulitermes flavipes (Kollar) due to rifampin. The diet may influence the physical and chemical milieu of the gut (Flint et al., Reference Flint, Bayer, Rincon, Lamed and White2008; Ley et al., Reference Ley, Lozupone, Hamady, Knight and Gordon2008; Clissold et al., Reference Clissold, Tedder, Conigrave and Simpson2010; Sorensen et al., Reference Sorensen, Mayntz, Simpson and Raubenheimer2010) and thus will constrain the type of bacterial strains that can survive in the gut ecosystem.
The difference in growth and development of S. litura on S+ and S− diet may also be attributed to change in gut microbial diversity. Indigenous gut bacterial community contributes to the nutrition of the host insect in various forms such as helping in survival on suboptimal diets, improved digestion efficiency, acquisition of digestive enzymes, provision of vitamins and also protecting from toxic compounds and pathogens (Berenbaum, Reference Berenbaum, Barbosa and Letourneau1988; Douglas, Reference Douglas1992; Teixeira et al., Reference Teixeira, Ferreira and Ashburner2008; Osborne et al., Reference Osborne, Leong, O′Neill and Johnson2009; Koch & Schmid, Reference Koch and Schmid2011; Jones et al., Reference Jones, Sanchez and Fierer2013) as they possess some metabolic properties which are absent in insects, thus acting as ‘microbial brokers’. Certain microbiota may be responsible for the production of digestive enzymes in some insects (Terra et al., Reference Terra, Ferreira, Jordao, Dillon, Lehane and Billingsley1996). M. paraoxydans, B. methylotrophicus and B. amyloliquefaciens isolated from S. litura larvae have already been known to produce digestive enzymes (Madhaiyan et al., Reference Madhaiyan, Poonguzhali, Kwon and Sa2010; Ojha et al., Reference Ojha, Mishra, Kapoor and Chand2013; Saha et al., Reference Saha, Maity, Roy, Pahan, Pathak, Majumdar and Gupta2014). Higher abundance of these bacteria on S+ diet in comparison with S− diet may have resulted in increased level of digestive enzymes in larvae feeding on S+ diet. Thus it is assumed that these microbes might have helped the insect to utilize the nutrients of diet and further increased its survival and fitness.
However, some gut microbes have also been reported as opportunistic pathogens e.g., Enterobacter genus contains insect pathogenic strains (Grimont & Grimont, Reference Grimont and Grimont1978) which are usually considered opportunistic or facultative pathogen as these are often avirulent to insects when present in digestive tract but are lethal upon entering insect haemocoel following injury or stress (Bucher, Reference Bucher and Steinhaus1963). Its insecticidal potential has been demonstrated against Bemisia argentifolii (Bellows & Perring), Chrysoperla rufilabris (Burmeister), Oberea linearis (L.), Phyllocnistis citrella (Stainton) etc. (Davidson et al., Reference Davidson, Rosell and Hend2000; Sandra & Douglas, Reference Sandra and Douglas2004; Bahar & Demirbag, Reference Bahar and Demirbag2007; Campos et al., Reference Campos, Sepúlveda and Tume2007). Increased activity of detoxifying enzymes in control diet may be due to the presence of pathogenic bacteria e.g., E. cloacae and P. citreus on control diet. In comparison with other isolates, the population of E. cloacae and P. citreus was found to be higher in larvae feeding on S− diet which may have resulted in reduced survival of larvae. P. citreus which was also present in larvae feeding on S− diet has also been reported to be an opportunistic pathogen. Planococcus sp. has earlier been reported to be pathogenic to Hylesia metabus at doses of 3–4 × 107 cfu ml−1 showing 10% mortality (Osborn et al., Reference Osborn, Berlioz, Vitelli-Flores, Monsalve, Dorta and Lemoine2002). Both these bacteria were found to be absent in larvae feeding on S+ diet. Previously Broderick et al. (Reference Broderick, Raffa and Handelsman2006) documented reduction in Lymantria dispar (L.) mortality and associated it with reduced population of Enterobacter species. Bt formulation at LC90 level resulted in 10.00% larval mortality in Helicoverpa armigera (Hubner) reared on diets with 250 and 500 µg ml−1 of four antibiotics (gentamicin, penicillin, rifampicin and streptomycin) as compared to 83.33% mortality in larvae reared on diets without antibiotics, suggesting that elimination of the gut microflora by antibiotics decreased the toxicity of Bt towards the larvae of H. armigera (Paramasiva et al., Reference Paramasiva, Sharma and Krishnayya2014).
In conclusion, present study demonstrated alteration in gut microbial diversity of insect in response to streptomycin sulphate however, no adverse effects of antibiotic were observed on survival and fitness of S. litura.
Acknowledgements
Financial assistance from Department of Biotechnology (DBT), Ministry of Science and Technology, Government of India, New Delhi, is duly acknowledged.