About This Column
Aaron Kesselheim serves as the editor for Health Policy Portal. Dr. Kesselheim is the JLME editor-in-chief and director of the Program On Regulation, Therapeutics, And Law at Brigham and Women’s Hospital/Harvard Medical School. This column features timely analyses and perspectives on issues at the intersection of medicine, law, and health policy that are directly relevant to patient care. If you would like to submit to this section of JLME, please contact Dr. Kesselheim at akesselheim@wh.harvard.edu.
On May 1, 2020, the US Food and Drug Administration (FDA) issued an emergency use authorization (EUA) to allow use of remdesivir — an unapproved drug — for the treatment of hospitalized patients with severe coronavirus disease 2019 (Covid-19).Reference Food1 Remdesivir is a viral RNA polymerase inhibitor with antiviral activity against the coronaviruses that cause severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). It was identified early in January 2020 as a promising therapeutic candidate for Covid-19 due to its ability to inhibit the in vitro activity of SARS-CoV-2.Reference Wang, Cao, Zhang and Yang2 While at the time of the EUA decision there was limited evidence on the safety and efficacy of using remdesivir to treat Covid-19, the drug was later shown to shorten the time to recovery by 4 days in a recent clinical trial sponsored by the National Institute of Allergy and Infectious Diseases (NIAID).Reference Beigel, Tomashek and Dodd3 It was the first therapeutic shown in a wellcontrolled prospective trial to have any activity against Covid-19.
Although remdesivir has not been shown to improve survival or longterm health, optimism has fueled concern about its accessibility both in the US and around the world. After the EUA was issued, Gilead announced that it would donate 1.5 million doses of remdesivir worldwide, of which 607,000 vials were to be made available in the US, enough to treat an estimated 78,000 hospitalized patients.4 The federal government was later able to secure 500,000 treatment courses through September 2020. Despite these efforts, it is unclear whether adequate supplies will be available to treat all patients with Covid-19 who require hospitalization each month nationwide.
Concerns have also been raised that not all patients would be able to afford the treatment. Early price estimates have suggested that it could range from $10 per course of treatment as a minimum cost of productionReference Hill, Wang, Levi, Heath and Fortunak5 to $4,500 per treatment course as measured by cost-effectiveness using a $50,000/QALY threshold.6 This estimate was later lowered to a range of $2,520 to $2,800 per treatment, assuming dexamethasone as standard of care.Reference Provides7 On June 29, 2020, Gilead announced a price of $520 per vial, or $3,120 per treatment, for privately insured patients, along with a commitment to make the limited supply of the drug available to US patients first.8 As clinical trials continue, we review the discovery and ownership of remdesivir, focusing on contributions from the US federal government during the course of its development.
Funding the Discovery of Remdesivir
Remdesivir (GS-5734) was synthesized by Gilead Sciences, but its development was the culmination of several years of collaboration between Gilead, the U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID), and the U.S. Centers for Disease Control and Prevention (CDC). Prior to the 2014 Ebola outbreak in West Africa, the CDC and USAMRIID had formed a collaboration with Gilead to screen for possible antiviral candidates in Gilead’s library of approximately 1,000 nucleoside analogues.Reference Siegel, Hui, Doerffler and Clarke9 When the Ebola epidemic erupted, global efforts to identify and develop antiviral candidates intensified. The Gilead- USAMRIID-CDC collaboration identified the precursor to remdesivir, which researchers from USAMRIID and Gilead refined and further developed.10
Led by USAMRIID Science Director Sina Bavari, the USAMRIID research team determined the compound’s antiviral activity against several pathogens, including the Ebola virus, in cell cultures and animal models. In October 2015, USAMRIID announced promising results: treatment initiated on day 3 postinfection was followed by a 100% survival rate in rhesus monkeys infected with the Ebola virus.Reference Warren, Jordan and Lo11 Although the study’s initial aim focused on the drug’s antiviral activity against Ebola, interest soon turned to investigating remdesivir as a potential treatment for Coronaviridae viruses after the compound successfully inhibited in vitro replication of other pathogenic RNA viruses, including filoviruses (such as Ebola), arenaviruses and coronaviruses. This investigation was in part supported by the Joint Science and Technology Office for Chemical and Biological Defense (JSTO-CBD) of the Defense Threat Reduction Agency (DTRA), a part of the US Department of Defense.12
The results published by USAMRIID and Gilead led to more joint public-private research on the use of remdesivir to treat other singlestranded RNA viruses. For example, a collaboration among Gilead, the University of North Carolina Chapel Hill, Vanderbilt University, and the Antiviral Drug Discovery and Development Center (AD3C) at the University of Alabama investigated remdesivir (known as GS-5734) as a candidate for treating Middle East Respiratory Syndrome (MERS) and severe acute respiratory syndrome (SARS).Reference Koplon, researchers and News13 The study found that the drug could inhibit SARS-CoV and MERS-CoV replication in invitro systems.Reference Sheahan, Sims, Graham, Agostini, Andres and Sims14 Specifically, it was shown to inhibit MERS-CoV replication in primary human airway epithelial cell cultures (IC50 = 0.025 μM). The collaboration further revealed that remdesivir was more effective than other antiviral drugs, such as lopinavir and ritonavir, against MERS-CoV in mice and human cell cultures.Reference Sheahan, Sims and Leist15 It suppressed MERS-CoV replication with a half maximal effective concentration (EC50) of 0.09 µM, while the EC50 levels generated for lopinavir and ritonavir were much higher, at 11.6 and 24.9 µ M, respectively.
The NIH Research Portfolio Online Reporting Tools (RePORTER) and Knowledge Ecology International16 indicate that the research of the AD3C collaboration involving remdesivir were funded by a few key NIH grants (Table 1).
Table 1 NIH funding for research in antiviral activity of remdesivir (GS-5734) against coronaviruses
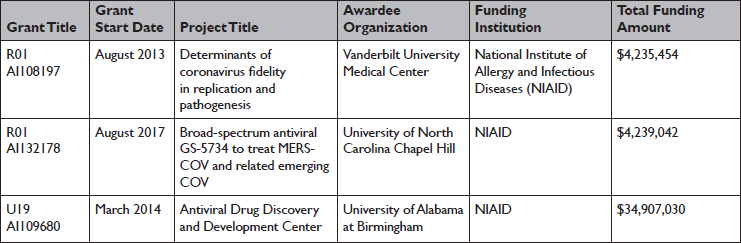
* Common federal funding associated with key coronavirus-related publications from the AD3C collaboration were found using NIH Research Portfolio Online Reporting Tools (RePORTER)34 and a report from Knowledge Ecology International.35 General grants for supporting centers and training programs were excluded.
In response to the Ebola virus outbreak, the NIAID sponsored two randomized controlled clinical trials of remdesivir. No results from the first trial (NCT02818582), completed October 2019, were available as of May 2020.17 The second trial, which ran from November 2018 to August 2019 in hospitals across the Democratic Republic of Congo, enrolled 681 patients with Ebola infection and randomized them to four different investigational treatments: mAb114, REGN-EB3, ZMapp, and remdesivir (NCT03719586).Reference Mulangu, Dodd, Davey and Mbaya18 The preliminary analysis found that both mAb114 and REGN-EB3 provided a survival advantage greater than that seen with ZMapp or remdesivir. At 28 days, death had occurred in 61 of 174 patients (35%) in the MAb114 group, as compared with 84 of 169 (50%) in the ZMapp group (p=0.007). In the case of REGN-EB3, 52 of 155 (34%) deaths occurred, as compared with 79 of 154 (51%) in the ZMapp subgroup (p=0.002). Despite the promising preclinical results, remdesivir performed similarly to ZMapp and was shown to be less efficacious than MAb114 and REGN-EB3. This study was co-sponsored by the government of the Democratic Republic of Congo (DRC) and the African Coalition for Epidemic Research, Response, and Training.
Repurposing of Remdesivir for Covid-19
Although remdesivir did not perform as well as early evidence suggested for the treatment of Ebola infection, its broad antiviral activity against RNA viruses demonstrated in the Ebola research led it to be considered as a response to the Covid-19 outbreak.Reference Joseph19 After Chinese scientists published the 2019 novel coronavirus (nCoV) genome in January 2020,Reference Cohen20 several reports suggested that there was an estimated 96-98% sequence alignment between RNA-dependent- RNA-polymerases (RdRp) of SARSCoV and SARS-CoV-2.Reference Morse, Lalonde, Xu, Liu, Zhang, Stephen, Theriault, Wang and Lin21 Given remdesivir’s ability to inhibit viral RNA polymerases, this suggested that the drug might be an effective therapy against Covid-19. Consistent with this hypothesis, remdesivir and chloroquine were shown to have some invitro antiviral activity against 2019- nCoV.22 Other studies published during the outbreak reported potential activity of remdesivir against the MERS virus and proposed its use for related coronaviruses, such as 2019- nCoV.Reference Feldmann, Cronin, Gordon, Tchesnokov, Feng, Porter and Gotte23 One study was supported by the Intramural Research Program of NIAID and federal funds from the Biomedical Advanced Research and Development Authority.24
In the absence of proven effective therapy, numerous clinical trials were launched across the globe to evaluate the safety and efficacy of remdesivir in hospitalized patients with severe Covid-19. As of May 2020, three clinical trials had reported results on remdesivir’s effects on Covid-19. A clinical trial of 237 patients in China sponsored by Capital Medical University compared remdesivir with placebo and found no benefit from the drug. However, the trial did not enroll enough patients to have adequate statistical power (NCT04257656).Reference Wang, Zhang and Du25 On April 10, 2020, Gilead published results from its expanded access program, in which 36 of 53 patients (68%) receiving remdesivir were reported to have had reduced need for oxygen support, but the absence of a control group prevented conclusions as to efficacy or safety compared to placebo (NCT04292899).Reference Grein, Ohmagari and Shin26
On February 21, 2020, the National Institute of Allergies and Infectious Diseases (NIAID) initiated the first double-blind randomized trial in the US to evaluate the safety and efficacy of remdesivir compared with placebo (NCT04280705).27 The primary outcome suggested a modest reduction in time to recovery, from a median of 15 days among placebo recipients to 11 days among those receiving remdesivir (p<0.001). A point estimate of approximately 4.8% lower mortality was observed among patients who received remdesivir (7.1%) compared to those receiving placebo (11.9%), but the difference was not considered statistically significant (hazard ratio for death, 0.70; 95% CI, 0.47 to 1.04). Serious adverse events were reported for 21% (114 of the 541) of patients in the remdesivir group compared to 27% (141 of the 522) in the placebo group.
With the announcement of remdesivir’s pricing, questions have arisen over its cost and availability. Notwithstanding federal contributions to remdesivir’s research and development, Gilead’s sponsorship of the drug through FDA approval would mean that a single company would control manufacturing, pricing, and distribution until a generic product became available, which is unlikely to occur for many years.
The results of the NIAID trial demonstrated that hospitalized patients recovered 4 days faster with the use of remdesivir. However, there remains a need for better evidence regarding remdesivir’s effects on mortality and long term health, and for more effective therapies.
Intellectual Property and Remdesivir
With the announcement of remdesivir’s pricing, questions have arisen over its cost and availability. Notwithstanding federal contributions to remdesivir’s research and development, Gilead’s sponsorship of the drug through FDA approval would mean that a single company would control manufacturing, pricing, and distribution until a generic product became available, which is unlikely to occur for many years. In the US, protection from generic competition occurs via two systems: (1) patents and (2) non-patent exclusivities. The most common sources of non-patent exclusivities are the Hatch-Waxman Act (at least 4 years of exclusivity for all new drugs), the Orphan Drug Act (7 years of exclusivity for new drugs treating rare diseases), the Biologics Price Competition and Innovation Act (12 years of exclusivity for new biologics) and the Best Pharmaceuticals for Children Act (6 months of add-on exclusivity for testing new drugs in children). Patent terms run concurrently with any nonpatent, exclusivity except pediatric exclusivity.
Gilead currently owns at least 12 patents on remdesivir, the last of which expires in 2039. Such patents can sometimes be successfully challenged or designed around, potentially allowing generic drugs to enter the market many years before expected patent expiration. In our review of the United States Patent and Trademark Office (USPTO) database and data from Knowledge Ecology International,28 all patents that have been identified as being linked to remdesivir are owned by Gilead. These include 6 active ingredient patents, 1 for the method to treat Paramyxoviridae infections, 2 for the method to treat Coronaviridae and Arenaviridae infections, and 3 for the method to treat Filoviridae infections (Table 2).
Table 2 U.S. patents issued for remdesivir (GS-5734)
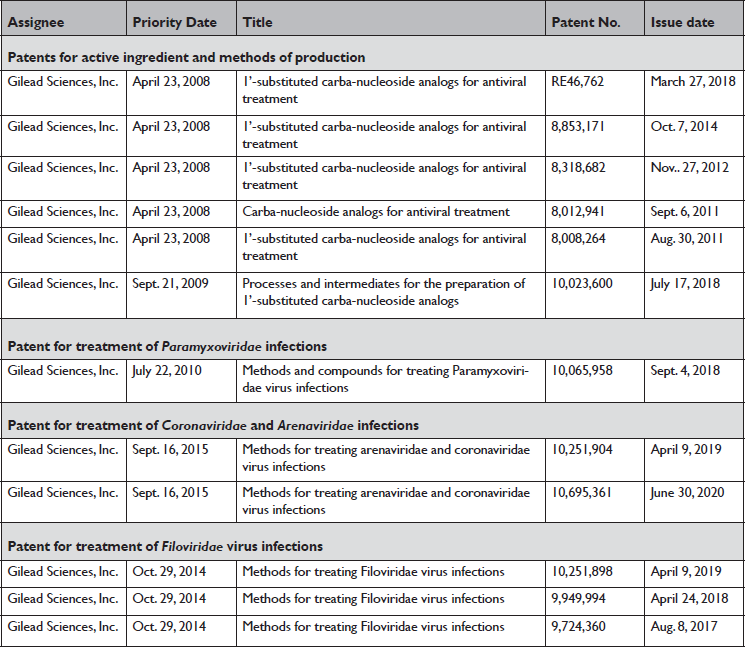
* United States Patent and Trademark Office (USPTO) database search on “GS-5734,” provisional patent application numbers found on published patents related to “GS-5734,” and a report by Knowledge Ecology International36 were used to identify U.S. patents issued for remdesivir.
Statutory exclusivity provides greater certainty of a competition-free period. Remdesivir was granted Orphan Drug Act status in 2015 for the treatment of the Ebola virus infection.Reference Food29 In 2020, Gilead requested Orphan Drug status for its use for the treatment of Covid-19 infection on the grounds that at that time the disease affected fewer than 200,000 people in the US — the statutory ceiling for Orphan Drug Act designation.Reference Fang and Lerner30 This status was granted by the FDA on March 23, 2020, despite the emerging evidence that the condition’s prevalence was increasing exponentially and would quickly pass the benchmark.31 Under the Orphan Drug Act, a generic version of Gilead’s remdesivir would have been blocked until at least 2027; the orphan designation would also have allowed Gilead to claim a tax credit equal to 25% of all its qualified clinical trial expenses — a benefit potentially totaling $40 million.Reference Hemel and Ouellette32
However, under public scrutiny for seeking Orphan Drug Act protections, Gilead voluntarily withdrew its designation.33
Following the Hatch-Waxman Act, it has become common for drugs to be patented solely by private pharmaceutical companies even when their development relied heavily on publicly funded research. Efforts to account for public funding have not been successful, including a requirement that the drug be sold at a “reasonable price,” profit-sharing between the government funder and the drug manufacturer, and eligibility of the government funder to claim the rights to a drug if availability or affordability are at risk (such as via “march-in rights” under the Bayh-Dole Act).
If remdesivir is approved by the FDA for Covid-19 treatment as a new chemical entity, Gilead will likely receive at least 4 years of exclusivity under the Hatch-Waxman Act. This statutory exclusivity would bar the FDA from accepting any abbreviated new drug application (ANDA) or 505(b)(2) application for a generic drug containing the same active ingredient as remdesivir. The Hatch-Waxman Act also provides that if a patent holder initiates litigation against a generic manufacturer within 1 year after the 4-year period ends, the exclusivity period will be extended to 7.5 years.
Conclusion
Academic researchers and public funding have made critical contributions to the discovery and clinical development of remdesivir since the 2014 Ebola virus outbreak. However, those contributions are not reflected in Gilead’s sole ownership of the patents on remdesivir, and there are no limits on pricing new drugs in the US. If remdesivir is approved for the treatment of Covid-19, policymakers should consider the extent to which pricing and availability should be considered in light of the substantial contributions by governments and researchers across the world, evaluating the needs of current Covid-19 patients and the ongoing need to encourage the development of therapies to treat future patients.




