Diabetic autonomic neuropathy, while affecting considerably the quality of life of diabetic patients, is one of the most difficult to determine among diabetic complications.Reference Maser, Lenhard and DeCherney 1 The long nerves are affected early in autonomic neuropathy; its first signs may therefore originate in the gastrointestinal, genitourinary, and cardiovascular systems.Reference Vinik, Maser, Mitchell and Freeman 2 Diabetic autonomic neuropathy is observed in one of about six diabetic patients.Reference Vinik, Maser, Mitchell and Freeman 2 , Reference Acharya, Joseph, Kannathal, Lim and Suri 3 Autonomic dysfunction can be hidden by compensating mechanisms. This increases the importance of determining the presence of autonomic dysfunction before the onset of clinical disorders.Reference Kardelen, Akçurin, Ertuğ, Akçurin and Bircan 4 Of the serious complications of diabetes mellitus, autonomic neuropathy is both the least evident and the most significant. Owing to injury to nerve endings in the heart and vessels, changes in heart rate control and vasoactive mechanisms occur.Reference Vinik, Maser, Mitchell and Freeman 2 They result from changes in heart rate control and vasoactive mechanisms, owing to injury to nerve endings in the heart and vessels.Reference Vinik, Maser, Mitchell and Freeman 2 Signs of cardiac autonomic neuropathy are subclinically present in 20–36% of patients with type 1 diabetes mellitus.Reference Stella, Ellis and Maser 5 Clinical tests that require the participation and collaboration of the patient, such as the Valsalva manoeuvre or the tilt table test, are evidently not easy to perform in children. These tests are difficult to standardise because of the differences among the various investigators and observers; their potential side effects are unacceptable for children.Reference Kardelen, Akçurin, Ertuğ, Akçurin and Bircan 4 , Reference Vinik and Ziegler 6 Heart rate variability is a reproducible, non-invasive electrocardiographic method to measure the effect on the sinus node of the sympathetic and parasympathetic components of the autonomous nervous system. 7 , Reference Javorka, Trunkvalterova, Tonhajzerova, Javorka, Javorka and Baumert 8 Heart rate variability is believed to be an index of the heart's capability to adapt to sudden and unexpected stimuli. The objective of this study was to evaluate heart rate variability by Holter monitoring in type 1 diabetic children compared with a healthy control group, and to determine the factors modifying heart rate variability
Materials and methods
Study population and study design
This study was conducted in 28 patients followed up between August, 2010 and January, 2011 at the Pediatric Endocrinology Department of the Dr. Sami Ulus Teaching and Research Hospital for Obstetrics, Gynecology and Pediatrics. The control group consisted of 27 healthy patients who had normal two-dimensional and colour-Doppler echocardiography and 24-hour Holter monitoring results after having consulted the Pediatric Cardiology Department for various complaints such as murmur or chest pain. Data were collected from patients at only one visit. Patients were not followed up except routine endocrinological controls.
Patients
All patients were being treated by subcutaneous insulin injections. Their age, weight, body surface area, diabetes duration, haemoglobin A1c levels, and daily insulin doses were recorded. The average duration of diabetes in the study patient group was 3.78 ± 3.5 years. The exclusion criteria were as follows: patients who received drugs that may affect the autonomous nervous system and those with conditions that may cause neuropathy; patients with diabetic peripheral neuropathy, retinopathy, or nephropathy signs and symptoms; family history of hypertension, hyperlipidaemia, or early-onset cardiovascular disease; and patients with cardiac lesions of haemodynamic and clinical significance.
Diagnostic criteria for diabetes mellitus
Diagnosis of diabetes mellitus followed the diagnostic criteria of the American Diabetes Association, viz. (1) postprandial plasma glucose level 200 mg/dl (11 mmol/L) or higher in the presence of polyuria, polydipsia, or (2) fasting plasma glucose level 126 mg/dl (7 mmol/L) or higher, or (3) 2-hour plasma glucose level 200 mg/dl (11 mmol/L) or higher during an oral glucose tolerance test. The oral glucose tolerance test was performed with doses corresponding to anhydrous glucose, 75 g total dose for patients weighing 43 kg or more, and 1.75 g/kg of body weight for those under 43 kg. 9 Diabetic patients who had generally acute-onset disease in childhood presenting with ketoacidosis, with positive anti-pancreatic autoantibodies – anti-glutamic acid decarboxylase or islet cell antibodies – were diagnosed with type 1 diabetes mellitus. 9
Holter monitoring and echocardiography
All cases, both in the study patient and control groups, were evaluated by two-dimensional and colour-Doppler echocardiography for left ventricular function, left ventricular end-diastolic diameter, valvular insufficiencies, and additional abnormalities. The 24-hour electrocardiography recordings were performed in all cases of both study patient and control groups on three channels, DII, V1, and V5. All recordings were evaluated by computer using the Pathfinder 700 Series (Reynolds Medical Ltd., Hertford, United Kingdom) and the ElatecHolter system (Ela Medical, Montrouge, France) software. Heart rate variability analysis, based on the measurement of change in consecutive RR intervals, was performed dynamically on 24-hour recordings during the study. Heart rate variability evaluation was conducted by a time-domain and a frequency–domain method.
Time-domain variables
Time-domain measurements were calculated from the standard deviation of heart rate variation based on the RR distances between consecutive sinus rhythm beats during a determined interval and considered under two different headings: The first of these was calculated based on the RR distances within a determined time interval. Average RR and the standard deviation of RR variation were measured. The standard deviation of all the 5-minute NN (normal to normal) interval means and the mean of all the 5-minute standard deviations of NN were calculated. Variables in the second category were evaluated on the basis of the differences between two consecutive RR distances: root-mean-square successive difference calculates the square root of the mean of the squared differences between successive NN intervals for all intervals, whereas percentage of differences between adjacent RR intervals >50 ms indicates the percentage of differences between successive NN intervals that are >50 ms. Time-domain heart rate variability metrics were evaluated according to the standards defined by the Task Force of the European Society of Cardiology and American Society of Pacing and Electrophysiology. 7 Table 1 showed normal values of standard measures of heart rate variability. Electrocardiographic recordings were also analysed for rhythm and QTc intervals.
Table 1 Normal values of standard measures of HRV.

HF = high frequency; LF/HF = low frequency/high frequency; LF = low frequency; rMSSD = square root of the mean of the sum of squares of differences between adjacent NN intervals; SDANN = standard deviation of all the 5-minute NN intervals; SDNN = standard deviation of all NN intervals.
Frequency–domain variables
The frequency–domain metrics examined were total power, very low-frequency, low-frequency, and high-frequency band power. Fluctuations observed in the RR intervals were transformed into wave lengths showing sympathetic and parasympathetic oscillation. The 0.15–0.4 Hz range was accepted as high frequency, representing parasympathetic activation, 0.05–0.15 Hz as low frequency and 0.003–0.04 Hz as very low frequency. The low-frequency/high-frequency ratio was calculated as an index of the sympathetic–parasympathetic relationship. Frequency-domain heart rate variability metrics were evaluated according to the standards defined by the Task Force of the European Society of Cardiology and American Society of Pacing and Electrophysiology. 7 Table 1 showed normal values of standard measures of heart rate variability.
Authorisation for the study was obtained from the local Ethics Committee. Consent forms were signed by the families of all subjects in the study.
Statistical analysis
The SPSS 16 software package for Windows (SPSS Inc., Chicago, Illinois, United States of America) was used for statistical evaluation. Values were expressed as mean ±1 standard deviation. Normally distributed continuous variables were compared by Student's t-test. The Mann–Whitney U-test was used for between-groups comparison of variables that were not normally distributed. Bivariate correlation tests were used in studying the prognostic effect of different variables. Whereas simple correlation – Pearson's correlation coefficient – test was used for parametric data, χ2 test was used for non-parametric data. Correlation test for age, gender, and body surface area was performed on the entire study group, but for haemoglobin A1c and diabetes duration only on the diabetes group. Correlation test was not done separately for girls and boys. A p-value under 0.05 was accepted as statistically significant.
Results
General results
The patients were aged (average ± 1 standard deviation) 9.9 ± 4.2 years (range 2.5–16) in the diabetic group, including 13 (46.5%) girls and 15 (53.5%) boys. The healthy controls comprised 20 (74%) girls and seven (26%) boys, with an average age 8.6 ± 3.7 (range 2–17) years. No significant difference was established between the study and control groups with regard to age and weight (p > 0.05), but there was a significant difference in the distribution of the two groups according to the gender (p < 0.05). The average duration of diabetes was 3.78 ± 3.5 years. The average haemoglobin A1c level was 9.9 ± 2.5 mg/dl (see Table 1). In the study patient group, left ventricular ejection fraction was 72 ± 7% (63–88%) and shortening fraction 39.1 ± 6.4 (32–46%). Minimal mitral regurgitation was observed in two (7.1%) patients; it was grade 1 in another patient (3.5%). There was one patient (3.5%) with patent foramen ovale. With regard to the control group, its average ejection fraction was 70 ± 6 (61–82%) and shortening fraction 35.9 ± 4.3 (31–50%). When evaluating rhythm on the patient group's Holter monitor recordings, two (7.1%) of them presented rare supraventricular extrasystoles, whereas another patient (3.5%) had a moderate frequency of ventricular extrasystoles with occasional trigeminy. The control group had no rhythm disturbance except for rare supraventricular extrasystoles in one (3.7%) patient. The QTc values were 408 ± 14 ms (385–439) in the study patient group and 412 ± 10 ms (400–458) in the controls, with no statistical difference (p = 0.456). Table 2 shows the calculated time-domain and frequency–domain variables of the groups.
Table 2 Characteristics of the patient and control groups.

BSA = body surface area; HbgA1c = haemoglobin A1c.
Heart rate variability findings
Both time-domain and frequency–domain heart rate variability parameters seemed to be reduced in the study patient group when compared with those of the healthy control group (Table 3). Minimum and maximum heart rate and the percentage of differences between adjacent RR intervals >50 ms value, calculated on the base of mean RR interval, were the only variables with statistically significant difference between the groups (p < 0.05). The search for factors modifying heart rate variability yielded the following correlations significant at a p level <0.05: for the time-dependent variables, negative between age and both average and maximal heart rate (r = −0.263 and −0.460, respectively). The average heart rate and percentage of differences between adjacent RR intervals >50 ms was significantly higher in the girls than the boys in all groups. For only diabetic patients, it was negative between haemoglobin A1c and percentage of differences between adjacent RR intervals >50 ms (r = −0.494) and positive between diabetes duration and square root of the mean of the sum of squares of differences between adjacent NN intervals (r = 0.460). With regard to the frequency-dependent factors affecting heart rate variability in only the diabetes group, correlations were found between haemoglobin A1c level and both total power and very low frequency (r = −0.751 and −0.644, respectively) and between very low frequency and diabetes duration (r = 0.69). All the mentioned correlations were significant, with a p-value <0.05. Findings are summarised in Table 4.
Table 3 Calculated time-domain and frequency-domain variables in the patient and control groups.

HF = high frequency; LF = low frequency; pNN50 = percentage of differences between adjacent RR intervals >50 ms; rMSSD = square root of the mean of the sum of squares of differences between adjacent NN intervals; SDANN = standard deviation of all the 5-minute NN intervals; SDNN = standard deviation of all NN intervals; SDNNi = mean of the standard deviations of all NN intervals for all 5-minute segments; VLF = very low frequency.
Table 4 Evaluation of factors influencing time-domain and frequency-domain parameters of heart rate variation.
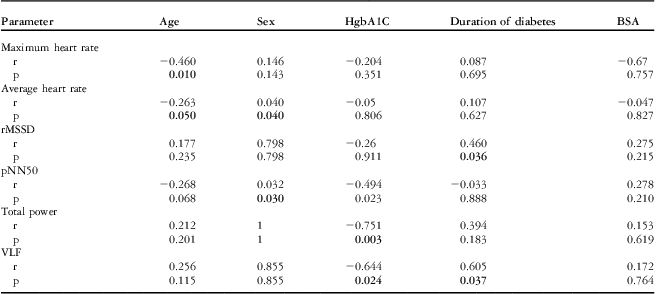
p = statistical significance level; pNN50 = percentage of differences between adjacent RR intervals >50 ms; r = correlation coefficient; rMSSD = square root of the mean of the sum of squares of differences between adjacent NN intervals; VLF = very low frequency. Bold values indicate statistically significant.
Discussion
Cardiac autonomic neuropathy, one of the long-term complications of diabetes, may develop over the years without clinical signs. Different tests have therefore been used in order to determine the presence of a pathologic condition in its subclinical period. Although examinations such as the 30–15 intermittent fitness test, the Valsalva ratio, the tilt-table test, and blood pressure response are readily performed in adults, they are not popular for paediatric use because of cooperation problems in working with children. Another disadvantage of these tests is their subjective nature, which causes differences among different observers’ evaluations.Reference Kardelen, Akçurin, Ertuğ, Akçurin and Bircan 4 , Reference Vinik and Ziegler 6 Heart rate variability is an easy, reliable, and reproducible method based on 24-hour (long-range) and 5-minute (short-term) Holter recordings. 7 , Reference Javorka, Trunkvalterova, Tonhajzerova, Javorka, Javorka and Baumert 8 Differences were observed in only a limited number of variables; heart rate variability parameters were particularly reduced in type 1 diabetes patients who had a long disease duration and poor metabolic control.
Some published reports have shown that the heart rate variability is significantly lower in diabetic patients when compared with age-adjusted controls.Reference Kardelen, Akçurin, Ertuğ, Akçurin and Bircan 4 , Reference Chessa, Butera and Lanza 10 , Reference Faulkner, Hathaway, Milstead and Burghen 11 Different publications have discussed which of the time-domain and frequency–domain variables are more sensitive than the others. Kardelen et al observed a statistically significant reduction in all time-domain metrics except standard deviation of all the 5-minute NN intervals in a group of 47 diabetic children, compared with 46 healthy controls.Reference Kardelen, Akçurin, Ertuğ, Akçurin and Bircan 4 Some authors have indicated that parameters calculated based on the RR interval variation average, such as percentage of differences between adjacent RR intervals >50 ms and square root of the mean of the sum of squares of differences between adjacent NN intervals, are less influenced by the cardiac circadian rhythm. These same variables are thought to be the most sensitive of all heart rate variability metrics to the parasympathetic condition in the body. It has been reported that because autonomic dysfunction in diabetic patients starts with the parasympathetic system to then extend to the sympathetic, reductions in percentage of differences between adjacent RR intervals >50 ms and square root of the mean of the sum of squares of differences between adjacent NN intervals may precede those in standard deviation of all NN intervals, the mean of standard deviation of all RR for all 5-minute segments, standard deviation of all the 5 minute NN intervals, and similar indexes.Reference Faulkner, Hathaway, Milstead and Burghen 11 We similarly observed, in this study, a statistically significant difference in minimum and maximum heart rate and a significant reduction in percentage of differences between adjacent RR intervals >50 ms in the patients, compared with the control group.
With regard to frequency–domain parameters, whereas high-frequency spectral analysis mainly represents the parasympathetic nervous system, the low-frequency spectral analysis informs us about both the sympathetic and the parasympathetic. Very low-frequency and ultra-low-frequency thermoregulation is influenced by peripheral vasomotricity and the renin–angiotensin–aldosterone system. The low-frequency/high-frequency ratio expresses in part the balance between the parasympathetic and sympathetic nervous systems. Total power, on the other hand, is a reflection of the global autonomous nervous system.Reference Acharya, Joseph, Kannathal, Lim and Suri 3 , 7 A study by Chessa et al. compared 50 children with type 1 diabetes mellitus with 30 healthy controls. Although a significant difference was found between the groups for high-frequency and the low-frequency/high-frequency ratio, there was none with respect to total frequency and low frequency.Reference Chessa, Butera and Lanza 10 No statistically significant difference was found in our study for any of the frequency–domain variables.
Heart rate variability parameter values are known to decrease with age. In utero and in the early postnatal phase, heart rate variability increases along with the parasympathetic system maturation. The high heart rate variability values in early infancy, owing to the dominant sympathetic activation, fall rapidly at the age of 5–10.Reference Acharya, Joseph, Kannathal, Lim and Suri 3 Similarly, within the diabetic patient group in our study, age stands in an inverse correlation with maximum age and average heart rate. The question whether this negative correlation is a consequence of an increase in age or of the increased duration of diabetes has been discussed in certain reports of studies in diabetic patients. The consensus seems to be that the contribution of age-related reduction is relatively minor.Reference Rollins, Jenkins, Carson, McClure, Mitchell and Imam 12 Another point is that age directly affects and reduces high frequency, which represents the parasympathetic system, whereas the duration of diabetes affects principally low frequency, which reflects the sympathetic system, followed by total power.Reference Wawryk, Bates and Couper 13
An influence of gender on heart rate variability parameters is also known. Heart rate variability variables are generally higher in women. It is noted that the high-frequency band is significantly higher in women well into the fifth decade of life. A cardioprotective effect of this influence in women is readily apparent. Before the fifth decade, this difference between the sexes tends to disappear.Reference Acharya, Joseph, Kannathal, Lim and Suri 3 , 7 There was a significant difference in the distribution of the two groups according to the gender. However, as correlation test was applied in the whole study group, gender differences became meaningless. The correlation test showed that there is no effect of gender on heart rate variability parameters in our study, except for average heart rate and percentage of differences between adjacent RR intervals >50 ms. This may be attributed to the fact that the average age for both our study and control subjects was around 9 years, whereas the gender-related differences begin at ∼12 years of age.Reference Galaev, Igisheva and Kazin 14
Several published studies have shown that autonomic dysfunction and the resulting change in heart rate variability are closely correlated to metabolic control of the disease.Reference Traon, Fontaine, Tap, Guidolin, Senard and Hanaire 15 The best index, roughly, of metabolic control is the haemoglobin A1c level. Neuropathic and microangiopathic changes become inevitable with deteriorating metabolic control along with high haemoglobin A1c levels.Reference Traon, Fontaine, Tap, Guidolin, Senard and Hanaire 15 , Reference Burger, Weinrauch, D'Elia and Aronson 16 Some published reports have documented a slow recovery of heart rate variability after intensive insulin treatment in diabetic patients who had developed autonomic neuropathy.Reference Burger, Weinrauch, D'Elia and Aronson 16 , 17 Such an improvement, however, is possible only in the stage of reversible nerve dysfunction, in the absence of definitive morphologic change.Reference Acharya, Joseph, Kannathal, Lim and Suri 3 , Reference Kardelen, Akçurin, Ertuğ, Akçurin and Bircan 4 Recognising this stage with only the measurement of haemoglobin A1c level may not always be possible. In a study by Burger et al., diabetic patients were classified in two groups according to their scores at autonomic function tests such as the 30–15 intermittent fitness test and the Valsalva ratio; the response of these groups to intensive insulin treatment was compared. Duration of diabetes was similar in both groups; whereas patients with early autonomic dysfunction experienced a significant fall in heart rate variability parameter values, those in advanced stage failed to do so. The average haemoglobin A1c levels were similar in both groups. The average haemoglobin A1c level in our study group 9.9 ± 2.5 mg/dl. Although we worked with a relatively unfavourable patient population, we could not determine a difference from the control group in either time-domain or frequency–domain parameters, to the exception of percentage of differences between adjacent RR intervals >50 ms. A possible interpretation of this may be that although haemoglobin A1c is helpful and important in estimating the prognosis of autonomic neuropathy, it is not sufficient by itself. In addition, correlation testing in our study shows an inverse correlation of haemoglobin A1c with the time-domain parameter percentage of differences between adjacent RR intervals >50 ms and the frequency–domain parameters total power and very low frequency. The decrease in very low frequency could be a result of the relative increase in the low frequency band, which reflects sympathetic activation, in the presence of a fall of all frequency–domain parameters.
Although cardiac autonomic neuropathy can appear as early as 2 years after the start of diabetes, the risk of autonomic neuropathy increases with disease duration.Reference Valensi, Ngoc and Lormeau 18 The incidence of autonomic neuropathy among patients without signs of marked nephropathy or retinopathy, who had been followed up for 2 years or shorter, was 13.6% as reported by Valensi et al.Reference Valensi, Ngoc and Lormeau 18 The average duration of diabetes in our group was 3.78 years and disease duration was correlated, positively, to the square root of the mean of the sum of squares of differences between adjacent NN intervals and very low frequency only. Although the increase in very low-frequency values with longer diabetes duration may be explained by a fall in other frequency bands, the square root of the mean of the sum of squares of differences between adjacent NN intervals increase contradicts published studies15. General opinion is that the duration of disease alone does not exert a negative influence globally on heart rate variability metrics, whereas the incidence of autonomic dysfunction increases with increased disease duration only in individual patients who have some precipitating factors.Reference Piha and Halonen 19 It is interesting to note, with Piha et al. who compared two groups followed up for 3 years and 16 years, that there was, however, no significant difference in heart rate variability metrics, and that all five patients who exhibited marked cardiac autonomic dysfunction were in the group with the longer follow-up.Reference Piha and Halonen 19 If there is a good metabolic control and adherence to treatment, heart rate variability parameters are not affected for a long time. Probably, to see the change in heart rate variability parameters, there must be a greater number of patients. In addition, duration of diabetes in childhood may not be as long as adulthood disease.
An evaluation according to body surface area did not disclose any correlation, in either direction, with heart rate variability parameters. Essentially, weight along with body surface area appears as a risk factor in type 2 diabetes patients rather than type 1. Although obesity itself can cause a reduction in heart rate variability parameter values, no influence of body surface area on autonomic dysfunction could be found in children followed up for type 1 diabetes.Reference Valensi, Ngoc and Lormeau 18 This, however, should not necessarily lead to conclude that regular exercise or weight control are unnecessary. It has been known for a long time that regular physical activity improves heart rate variability parameters and protects the body from the injury caused by an uncontrolled sympathetic system.Reference Acharya, Joseph, Kannathal, Lim and Suri 3 , Reference Ewing, Campbell and Clarke 20
Study limitations
There were a limited number of cases. Gender differences exist between the two groups. These gender differences may contribute to an increase in some of the heart rate variability parameters in the control group. Patients who were chosen did not have diabetic nephropathy and retinopathy; however, heart rate variability could be correlated with them. Spot measurement was performed. Longitudinal follow-up could be done.
Conclusion
We observed that among the time-domain variables percentage of differences between adjacent RR intervals >50 ms is influenced by the haemoglobin A1c level, square root of the mean of the sum of squares of differences between adjacent NN intervals by the duration of diabetes, minimum and maximum heart rate by, respectively, age and gender; with regard to the frequency–domain variables, total power and very low frequency correlate with haemoglobin A1c level and only very low frequency is affected by the duration of diabetes. In conclusion, an impairment of heart rate variability parameters was observed in type 1 diabetes mellitus patients who had a long disease duration and with poor metabolic control, as compared with healthy controls. We believe that more studies on this topic will allow a better evaluation of the effect of diabetes on autonomic neuropathy.
Acknowledgement
Dr Filiz Senocak is the guarantor of this work, has full access to all the data, and takes full responsibility for the integrity of data and the accuracy of data analysis.
Financial Support
The authors received no financial support for the research and/or authorship of this article.
Conflicts of Interest
None.
Author Contributions
F.S., S.Ö., Ö.C., and U.A.Ö. contributed to idea, concept, design, and writing. S.K., Ö.C., S.O., Z.A., and F.S. contributed to supervision and critical review. S.Ö., Ö.C., V.D., O.Y., and M.K. contributed to the literature search, material and data collection, analysis and interpretation.






