INTRODUCTION
Homoscleromorpha represents a small group with two families (<100 described species) of exclusively marine sponges, generally located in shallow waters ranging from 8 to 60 m, but a few were also recorded from abyssal depths, up to 2460 m (Muricy & Diaz, Reference Muricy, Diaz, Hooper and van Soest2002). All species are dwellers of hard substrate communities often in semi-dark or dark conditions. Some species may exclusively grow on coralligenous substrate. In some places, Homoscleromorpha can be predominant and seem to be strong competitors for space, overgrowing massive sponges, sea fans and erect bryozoans (Diaz & van Soest, 1994; Muricy & Diaz, Reference Muricy, Diaz, Hooper and van Soest2002; Ereskovsky et al., 2009a; Pérez et al., 2011).
Homoscleromorpha have a great variability of shapes but their general organization and the shared features of their cytology and embryology argue for the monophyly of this group. This sponge clade is characterized by an aquiferous system of either sylleibid-like or leuconoid organization with eurypylous, diplodal or aphodal choanocyte chambers. Skeletal structures, when present, harbour a peculiar type of tetractinal spicules (calthrops), distinguishable from calthrops of Demospongiae and their derivatives by their small size, ramification of one to all four actines (lophose calthrops) or reduction (diods and triods) (Muricy & Diaz, Reference Muricy, Diaz, Hooper and van Soest2002). These spicules do not form a well-organized skeleton. Homoscleromorpha possess flagellated exopinacocytes and endopinacocytes, a cinctoblastula larva, cross-striated ciliar rootlets in larval cells, a basement membrane underlying both choanoderm and pinacoderm, and zonula adherens cell junctions in adults and larval epithelia (for review see Muricy & Diaz, Reference Muricy, Diaz, Hooper and van Soest2002; Ereskovsky et al., Reference Ereskovsky, Borchiellini, Gazave, Ivanišević, Lapebie, Pérez, Renard and Vacelet2009a; Gazave et al., 2010).
Until recently, Homoscleromorpha was classified as a subclass of the class Demospongiae, containing one order (Homosclerophorida Dendy, Reference Dendy1905), one family (Plakinidae Schulze, Reference Schulze1880) and seven genera (Boury-Esnault et al., Reference Boury-Esnault, Muricy, Gallissian and Vacelet1995; Muricy & Diaz, Reference Muricy, Diaz, Hooper and van Soest2002). Molecular phylogenies challenged this traditional classification schema: several phylogenetic/phylogenomic studies using several nuclear markers have corroborated the hypothesis suggesting that Homoscleromorpha forms a clade on its own, clearly separated from Demospongiae (Borchiellini et al., Reference Borchiellini, Chombard, Manuel, Alivon, Vacelet and Boury-Esnault2004). Recently, molecular phylogenetic studies of Homoscleromorpha, including six of the seven genera presently known, brought a new interpretation of the relationships within this group (Gazave et al., Reference Gazave, Lapébie, Renard, Vacelet, Rocher, Ereskovsky, Lavrov and Borchiellini2010, Reference Gazave, Lapébie, Ereskovsky, Vacelet, Renard, Càrdenas and Borchiellini2012; Ivanišević et al., Reference Ivanišević, Thomas, Lejeusne, Chevaldonné and Pérez2010). These studies restored the Oscarellidae Lendenfeld, Reference von Lendenfeld1887 and Plakinidae (Schulze, Reference Schulze1880) families, a suprageneric classification of Homoscleromorpha abandoned in 1995 (Boury-Esnault et al., Reference Boury-Esnault, Muricy, Gallissian and Vacelet1995). Therefore, Homoscleromorpha are considered to represent a separate class of sponges, with one order (Homosclerophorida Dendy, Reference Dendy1905), two families (Plakinidae Schulze, Reference Schulze1880 and Oscarellidae Lendenfeld, Reference von Lendenfeld1887) and seven genera (van Soest et al., Reference Soest, Boury-Esnault, Hooper, Rützler, de Voogd, Alvarez de Glasby, Hajdu, Pisera, Manconi, Schoenberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann and Díaz2013). Five genera belong to Plakinidae (Corticium Schmidt, Reference Schmidt1862; Plakina Schulze, Reference Schulze1880; Plakinastrella Schulze, Reference Schulze1880; Placinolopha Topsent, Reference Topsent1897; and Plakortis Schulze, Reference Schulze1880) and two belong to Oscarellidae (Oscarella Vosmaer, Reference Vosmaer1887; and Pseudocorticium Boury-Esnault et al., Reference Boury-Esnault, Muricy, Gallissian and Vacelet1995) (van Soest et al., Reference Soest, Boury-Esnault, Hooper, Rützler, de Voogd, Alvarez de Glasby, Hajdu, Pisera, Manconi, Schoenberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann and Díaz2013). The two latter genera are characterized by the lack of spicules and by an aquiferous system consisting of spherical, eurypylous choanocyte chambers regularly organized around large and even exhalant canals. To countervail this lack of morphological characters, attempts were made to recognize secondary metabolites, but only Oscarella lobularis from Mediterranean brought to light cytotoxic epoxy-sterols (Aiello et al., Reference Aiello, Fattorusso, Magno, Mayol and Menna1990).
Homoscleromorpha, with the exception of the genus Pseudocorticium, have a more or less worldwide distribution. Nevertheless, until now no Homoscleromorpha from the genera Placinolopha, Oscarella and Pseudocorticium have been registered in the Caribbean region.
The genus Oscarella has a worldwide distribution and consists of 16 valid species (van Soest et al., Reference Soest, Boury-Esnault, Hooper, Rützler, de Voogd, Alvarez de Glasby, Hajdu, Pisera, Manconi, Schoenberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann and Díaz2013), but with rare incursions into cooler temperate waters (Muricy & Pearse, Reference Muricy and Pearse2004; Ereskovsky, Reference Ereskovsky2006; Ereskovsky et al., Reference Ereskovsky, Sanamyan and Vishnyakov2009b; Pérez et al., Reference Pérez, Ivanišević, Dubois, Pedel, Thomas, Tokina and Ereskovsky2011). Until now the genus was reported but indeterminate in the Caribbean, from the Virgin Islands (Díaz & van Soest, Reference Díaz, van Soest, van Soest, van Kempen and Braekman1994), Belize (Rützler et al., Reference Rützler, Diaz, van Soest, Zea, Smith, Alvarez and Wulff2000; Díaz et al., Reference Díaz, Smith and Rützler2004; Díaz & Rützler, Reference Díaz and Rützler2009), Panama (Díaz, Reference Díaz2005; Díaz & Rützler, Reference Díaz and Rützler2009) and the Bahamas (Zea et al., Reference Zea, Henkel and Pawlik2009). Only one Oscarella sp. is registered from the south-east Brazilian coasts (Muricy & Hajdu, Reference Muricy and Hajdu2006) and two other ones from north-east (NE) Brazil (Muricy & Moraes, Reference Muricy and Moraes1998), all three yet to be described.
The genus Plakortis is cosmopolitan with 19 species known from different oceans around the world (Muricy, Reference Muricy2011; van Soest et al., Reference Soest, Boury-Esnault, Hooper, Rützler, de Voogd, Alvarez de Glasby, Hajdu, Pisera, Manconi, Schoenberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann and Díaz2013) and with six species described from the Caribbean Sea, north-eastern Brazil and north-western Atlantic: Plakortis simplex Schulze, Reference Schulze1880, P. angulospiculatus (Carter, Reference Carter1879), P. halichondrioides Wilson, Reference Wilson1902, P. zyggompha de Laubenfels, Reference Laubenfels1934, P. insularis Moraes & Muricy, Reference Moraes and Muricy2003 and P. microrhabdifera Moraes & Muricy, Reference Moraes and Muricy2003 (Carter, Reference Carter1879; de Laubenfels, Reference Laubenfels1934; Hechtel, Reference Hechtel1965; Boury-Esnault, Reference Boury-Esnault1973; Wiedenmayer, Reference Wiedenmayer1977; Pulitzer-Finali, Reference Pulitzer-Finali1986; Zea, Reference Zea1987; Mothes & Bastian, Reference Mothes and Bastian1993; Diaz & van Soest, 1994; Lehnert & van Soest, Reference Lehnert and van Soest1998; Moraes & Muricy, Reference Moraes and Muricy2003).
Plakortis species are mainly characterized by a skeleton formed by small diods with triods of varying abundance, even though some species may present diactine-derived microrhabds and deformed calthrops (Diaz & van Soest, 1994; Muricy & Diaz, Reference Muricy, Diaz, Hooper and van Soest2002; Muricy, Reference Muricy2011). Due to similarity of most species and simplicity of their spiculation (composed mainly of irregular diods of a single size-class and of rare triods), the species diagnosis is quite difficult without a detailed observation of external and anatomical features (e.g. architecture of the aquiferous system) and skeletal characters (Diaz & van Soest, 1994; Muricy & Diaz, Reference Muricy, Diaz, Hooper and van Soest2002; Moraes & Muricy, Reference Moraes and Muricy2003; Muricy, Reference Muricy2011).
Species from the genus Plakortis were proven to be a prolific source of secondary metabolites with a large structural diversity. They produce biologically active oxygenated polyketides, cyclic peroxides, peroxylactones, and other secondary metabolites (Faulkner, Reference Faulkner2002), many of which are biologically active (Gochfeld & Hamann, Reference Gochfeld and Hamann2001; Muricy & Diaz, Reference Muricy, Diaz, Hooper and van Soest2002; Rudi et al., Reference Rudi, Afanii, Gravalos, Aknin, Gaydou, Vacelet and Kashman2003; Berrué et al., Reference Berrué, Thomas, Bon, Reyes and Amade2005; Holzwarth et al., Reference Holzwarth, Trendel, Albrecht, Maier and Michaelis2005).
The genus Plakina includes 25 species (van Soest et al., Reference Soest, Boury-Esnault, Hooper, Rützler, de Voogd, Alvarez de Glasby, Hajdu, Pisera, Manconi, Schoenberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann and Díaz2013) with worldwide distribution. In the Atlantic, six species were described: Plakina brachylopha Topsent, Reference Topsent1927 from the Azores, P. versatilis (Schmidt, Reference Schmidt1880) from the Gulf of Mexico, P. elisa (de Laubenfels, Reference Laubenfels1936a), P. jamaicensis Lehnert & van Soest, Reference Lehnert and van Soest1998 and P. tetralopha (Hechtel, Reference Hechtel1965) from the Caribbean Sea and P. trilopha sensu Boury-Esnault (Reference Boury-Esnault1973), from NE Brazil.
Plakina species have a distinctive spiculation consisting of diods, triods, and calthrops of a single size-class, and homolophose calthrops with one to four lophate rays. Antimicrobial steroidal alkaloids were reported in Plakina sp. (Rosser & Faulkner, Reference Rosser and Faulkner1984).
Finally, the genus Corticium with only six species has also a wide-ranging distribution, from NW Mediterranean to Indian Ocean, Western Pacific. A single species was recorded in the Caribbean, Corticium quadripartitum (Topsent, Reference Topsent1923). Corticium species are defined as thin to thick plakinids, with a skeleton dominated by non-lophose calthrops and heterolophose calthrops (candelabra). Certain species also possess homolophose calthrops. Some news steroidal alcaloids have been described from different Corticium species (De Marino et al., Reference De Marino, Zollo, Iorizzi and Debitus1998, Reference De Marino, Iorizzi, Zollo, Roussakis and Debitus1999; Lee et al., Reference Lee, Seo, Rho, Shin and Paul2001; Borbone et al., Reference Borbone, De Marino, Iorizzi, Zollo, Debitus, Esposito and Iuvone2002; Ridley & Faulkner, Reference Ridley and Faulkner2003; Zampella et al., Reference Zampella, D'Orsi, Sepe, De Marino, Borbone, Valentin, Debitus, Zollo and D'Auria2005).
In this paper we describe a new Oscarella occurring in Martinique and Jamaica, three new Plakortis from Jamaica and a new Corticium from Martinique. In addition a revision of Plakina jamaicensis Lehnert & van Soest Reference Lehnert and van Soest1998 is based on newly collected material from Jamaica and the type specimen. A key to Caribbean species of Homoscleromorpha is provided.
MATERIALS AND METHODS
In Martinique, specimens were collected by SCUBA in early June 2003 from the vertical walls and roof of a coralligenous reef cave (‘Fer à Cheval’ Cave) off Le Diamant at depths of 22–26 m. In Jamaica, specimens were collected by SCUBA in March and April 2005 from vertical walls of coralligenous reef caves at Pear Tree Bottom, 5 km east of Discovery Bay at depths of 28 m and at Chalet Caribe, west of Montego Bay (Figure 1). Geographical coordinates are given in taxonomic descriptions. All underwater photographs were taken with a Nikon Coolpix 950 digital camera. Vouchers for microscopy were fixed in 4% glutaraldehyde in 0.2 M sodium cacodylate buffer, pH 7.4, supplemented with 0.35 M sucrose and 0.1 M NaC1 to obtain a final osmotic pressure of 1700 mOsM for 24 h at 4°C. Specimens were washed six times for 10 min in 0.2 M sodium cacodylate buffer, pH 7.4. Samples were postfixed for 3 h in 1% osmium tetroxide in 0.2 M sodium cacodylate and 0.3 M NaC1, dehydrated through a graded ethanol series, and embedded in ERL 4206 according to Spurr (Reference Spurr1969). Sections were obtained with a diamond knife on a Leica Ultracut UCT ultramicrotome. Prior to sectioning, the siliceous spicules at the section plane were dissolved with 15 to 20% hydrofluoric acid in distilled water for 5 min. Semithin sections were stained with toluidine blue, observed in light microscopy (LM) and photographed with a Nikon Coolpix 950 digital camera. For transmission electron microscopy (TEM), thin sections double-stained with uranyl acetate and lead citrate (Reynolds, Reference Reynolds1963) were examined with a Zeiss EM 900, a FEI Tecnai 10 and a LEO 906 E transmission electron microscopes at 60 and 80 kV. For scanning electron microscopy (SEM), specimens were fractured in liquid nitrogen, critical-point-dried, sputter-coated with gold-palladium, and observed under a XL30 ESEM Philips SEM. Whole samples destined to reference collection were fixed and preserved in ethanol 70°. For skeletal architecture observation, sections were cut from ERL embedded fragments with a low speed diamond saw (Bennet Labcut 1010) using a diamond wafering blade, mechanically wet ground on a series of diamond grinding disks (Buehler ultra-prepTM) using a semiautomatic grinder (Buehler Minimet 1000) to a thickness of 5–10 μm and observed under a Nikon Optiphot-2 microscope.

Fig. 1. Collection sites of Homoscleromorpha from Jamaica and Martinique. 1, Chalet Caribe; 2, Pear Tree Bottom; 3, Le Diamant.
Total DNA was prepared from a small piece of each specimen preserved in ethanol by proteinase K digestion in the CTAB (hexadecyltrimethylammonium bromide) buffer, followed by phenol-chloroform extraction and ethanol precipitation (Saghai-Maroof et al., Reference Saghai-Maroof, Soliman, Jorgensen and Allard1984). A region of cob was PCR amplified with Promega PCR kit using the diplo-cob primers (Lavrov et al., Reference Lavrov, Wang and Kelly2008) and the sequences were determined at the Eurogene company. Partial mitochondrial cob sequences from species described in this study and complete cob sequences from species used in our previous study were aligned with the MAFFT program (Katoh & Toh, Reference Katoh and Toh2008) and the ML phylogeny was estimated with the PhyML 3 program (Guindon et al., Reference Guindon, Dufayard, Lefort, Anisimova, Hordijk and Gascuel2010). Abbreviations used: DBML (Discovery Bay Marine Laboratory), MNHN (Muséum National d'Histoire Naturelle, Paris, France), RBINS (Royal Belgian Institute of Natural Sciences), SME (Station Marine d'Endoume, Marseille, France), RBINS (Royal Belgian Institute of Natural Sciences), WPD (World Porifera Database), ZIN RAS (Zoological Institute of the Russian Academy of Science). All type material described here is deposited at RBINS under the general iventory number IG 32243.
SYSTEMATICS
Phylum PORIFERA Grant, Reference Grant and Todd1836
Class HOMOSCLEROMORPHA Bergquist, Reference Bergquist1978
Order HOMOSCLEROPHORIDA Dendy, Reference Dendy1905
Family OSCARELLIDAE Lendenfeld, Reference von Lendenfeld1887
Genus Oscarella Vosmaer, Reference Vosmaer1887
SYNONYMY
[Oscaria] Vosmaer, Reference Vosmaer1881: 163 (preocc. by Oscaria Gray, 1873 – Reptilia). Oscarella Vosmaer, 1884: pl. 8 (explanation). Oscarella Vosmaer, Reference Vosmaer1887: 326; pl. 2 fig. 3, pl. 8 (nom. nov. for Oscaria Vosmaer). Octavella Tuzet and Paris, Reference Tuzet and Paris1963: 88 (no type specimens designated). Taxonomic decision after Vosmaer (Reference Vosmaer1887: 326); Boury-Esnault et al., (1984: 13, 1992b: 282); Diaz & van Soest (1994: 102).
TYPE SPECIES
Halisarca lobularis Schmidt, Reference Schmidt1862 (by monotypy).
DIAGNOSIS (MODIFIED FROM MURICY & DIAZ, 2002)
Homoscleromorpha without skeleton, with thinly encrusting to lobate shape. Thin ectosome (<100 μm), often limited to pinacoderm; true cortex absent. Mesohyl poorly developed, with a proportion of mesohyl to chambers varying from 0.5:1 to 1.2:1. The aquiferous system has a sylleibid organization, with spherical, eurypylous choanocyte chambers uniformly arranged around large, regular exhalant canals, and a large basal cavity.
Oscarella nathaliae sp. nov.
(Figures 2–6)

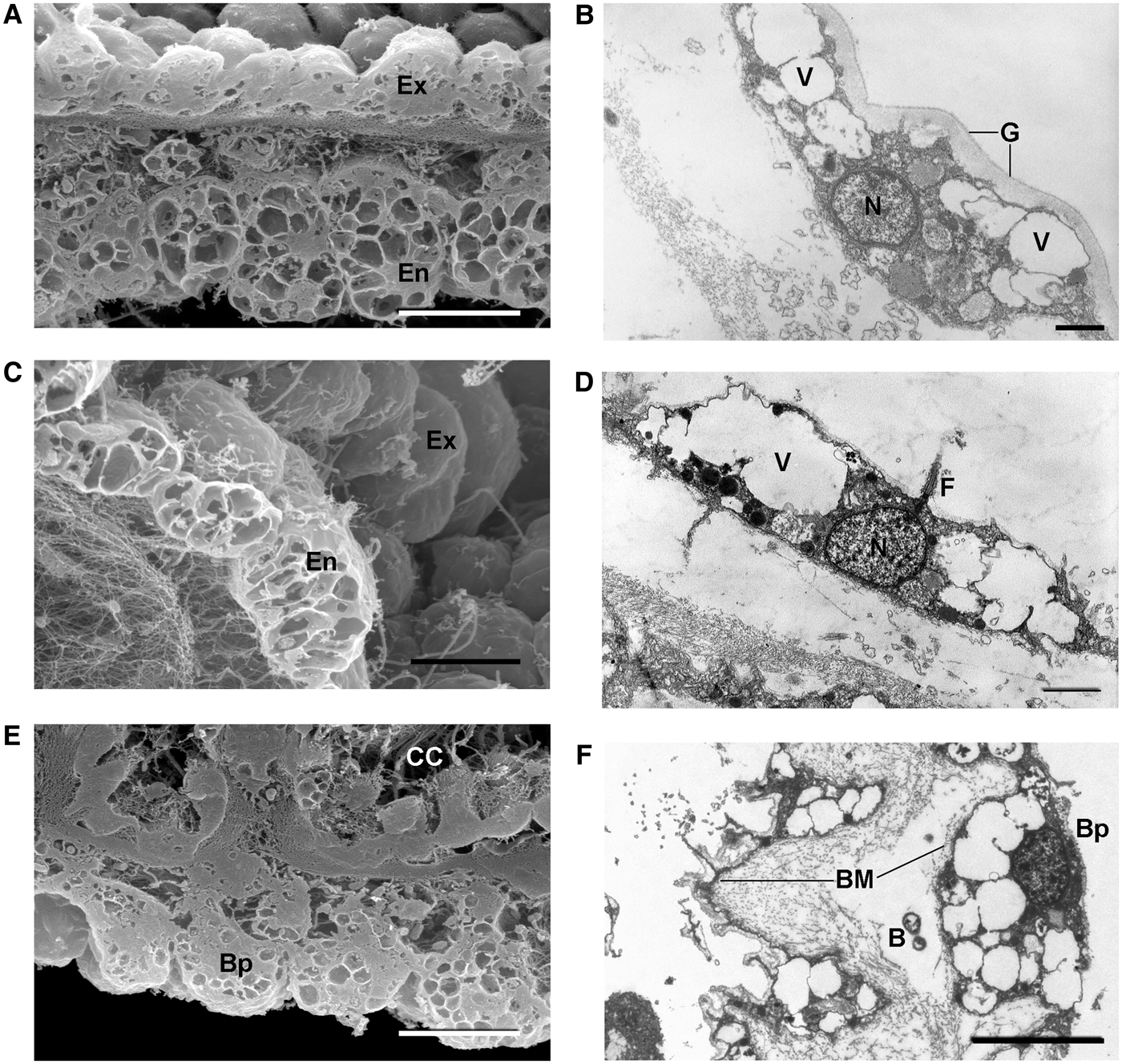
Fig. 2. Oscarella nathaliae sp. nov.: (A) from Martinique; (B) from Jamaica (in situ); (C) transverse cryofracture through ectosome and choanosome (SEM); (D) transverse section through ectosome and choanosome (LM); (E) cryofracture of a choanocyte chamber and its apopyle (SEM); (F) apopylar cell (TEM). Ap, apopyle; CC, choanocyte chamber; EC, exhalant canal; En, endopinacocyte; Ex, exopinacocytes; F, flagellum; IC, inhalant canals; N, nucleus; V, vacuole. Arrows, basement membrane. Scale bars: A, 3 cm; B, 2 cm; C, 200 µm; D, 50 µm; E, 20 µm; F, 1 µm.

Fig. 3. Oscarella nathaliae sp. nov.: Pinacocytes (SEM and TEM). (A, B) Exopinacocytes; (C, D) endopinacocytes; (E, F) basopinacocytes. B, endobiotic bacteria; BM, basement membrane; Bp, basopinacocytes; CC, choanocyte chamber; En, endopinacocytes; Ex, exopinacocytes; F, flagellum; G, glycocalyx; N, nucleus; V, vacuole. Scale bars: A, 10 µm; B, 1 µm; C, E, 10 µm; D, 2 µm; F, 5 µm.

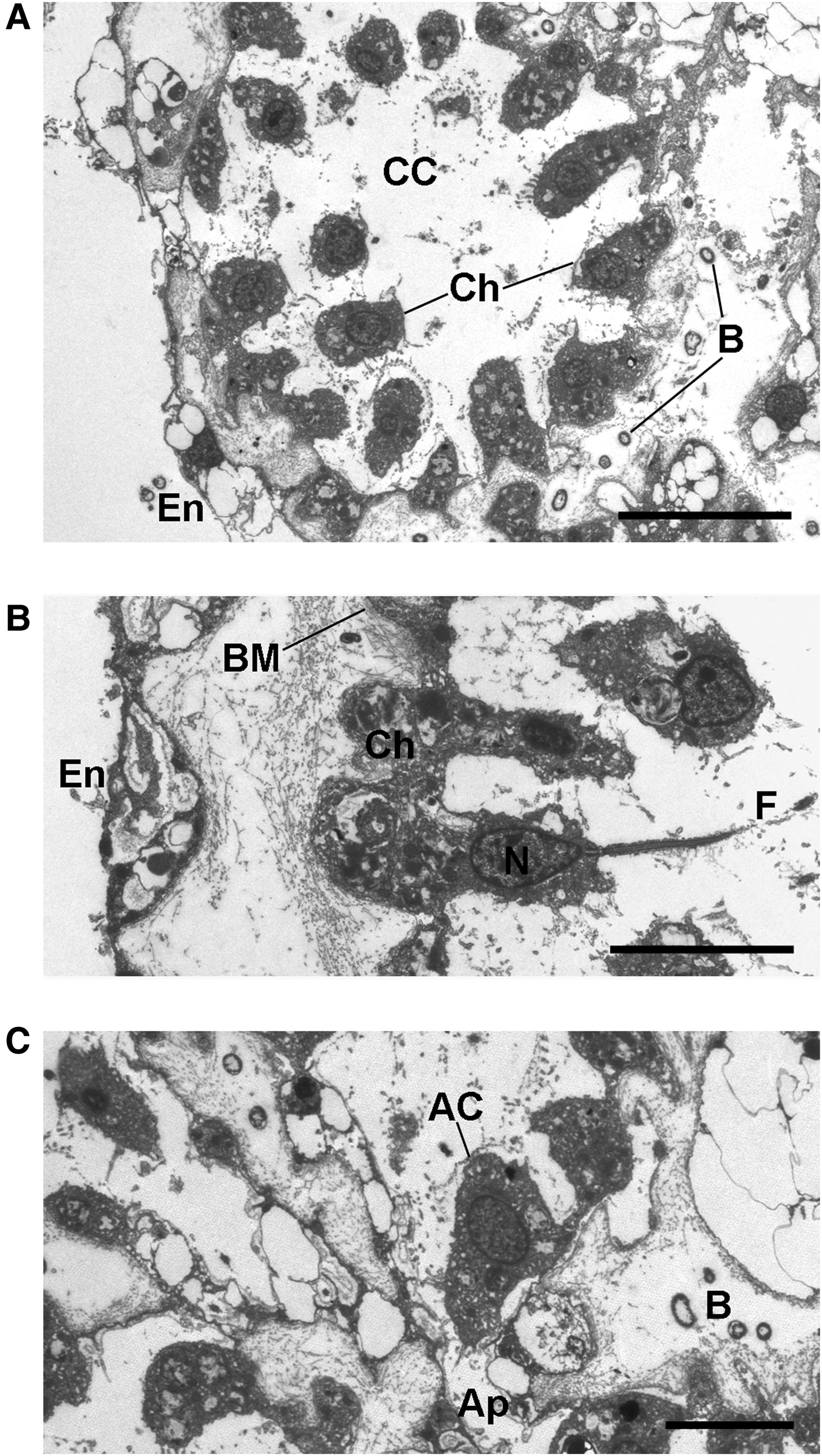
Fig. 4. Oscarella nathaliae sp. nov.: Choanocytes and apopylar cells (TEM). (A) Choanocyte chamber; (B) detail of a choanocyte; (C) apopylar cell. AC, apopylar cell; Ap, apopyle; B, endobiotic bacteria; BM, basement membrane; CC, choanocyte chamber; Ch, choanocytes; En, endopinacocytes; F, flagellum; N, nucleus. Scale bars: A, 10 µm; B, C, 5 µm.

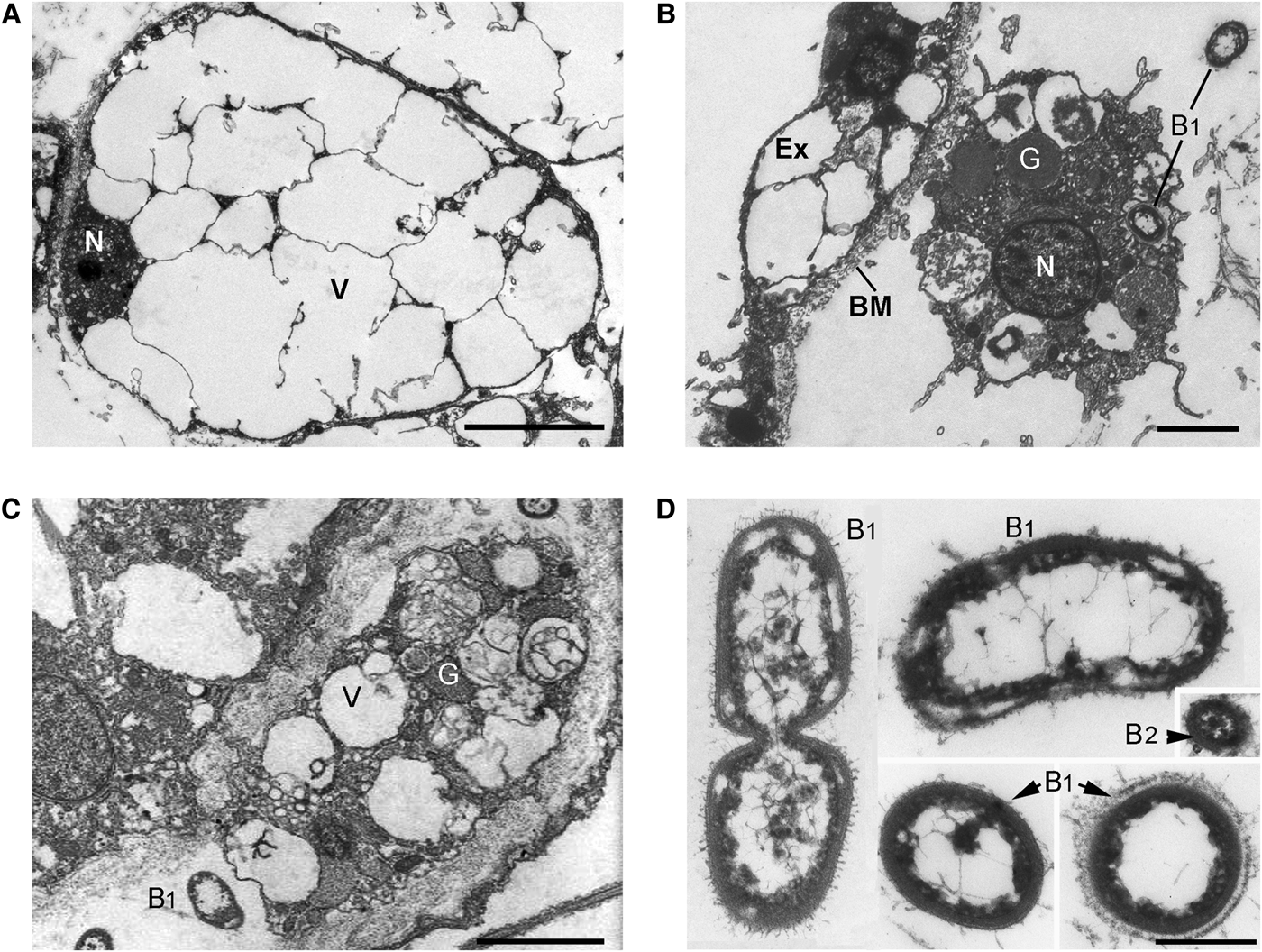
Fig. 5. Oscarella nathaliae sp. nov.: Mesohylar cells (TEM). (A) Vacuolar cell; (B, C) granular cells; (D, E) endobiotic bacteria. B, endobiotic bacteria; B1, endobiotic bacteria of morphotype 1; B2, endobiotic bacteria of morphotype 2; BM, basement membrane; Ex, exopinacocyte; G, granules; N, nucleus; V, vacuole. Scale bars: A, 5 µm; B, C, 3 µm; D, 0.5 µm.

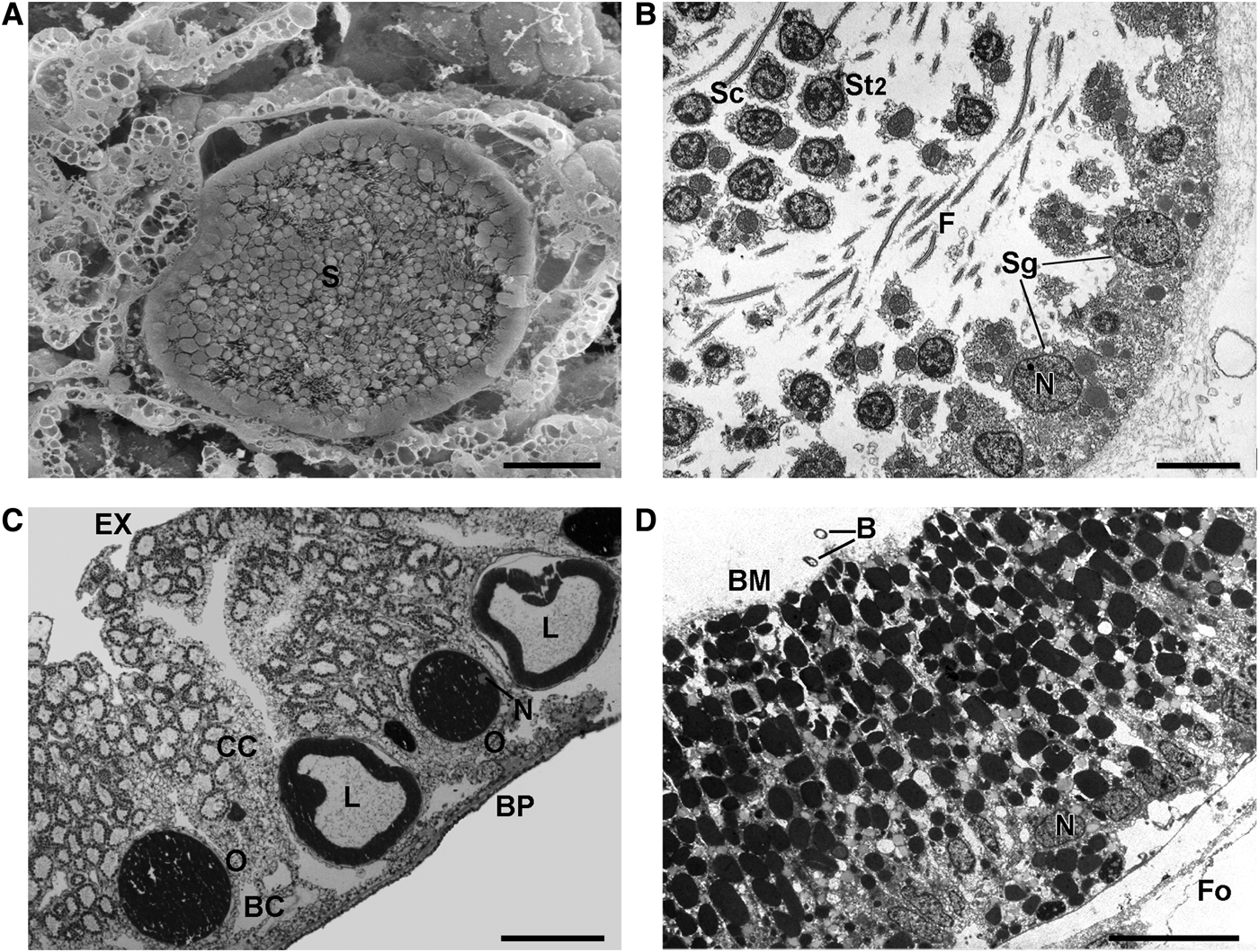
Fig. 6. Oscarella nathaliae sp. nov.: Reproduction (A) Spermatocyste in the choanosome (SEM); (B) detail of a spertmatocyste (TEM); (C) oocytes and cinctoblastulae larvae in the choanoderm (LM); (D) cell wall of a larva (TEM). B, endobiotic bacteria inside a larva; BC, basal cavity; BP, basopinacoderm; BM, basement membrane; CC, choanocyte chamber; EX, exopinacoderm; F, flagella; Fo, follicle; L, larva; N, nucleus; O, oocyte; S, spermatic cyste; Sg, spermatogonia; St, spermatide; St2 spermatocyte 2. Scale bars: A, 20 µm; B, 5 µm; C, 100 µm; D, 50 µm.
TYPE MATERIAL
Holotype: RBINS POR 90, Martinique, Le Diamant, ‘Fer à Cheval’ Cave, 14°28′04.72″N 61°00′59.37″W, 22 m depth, Coll. Ph. Willenz and B. Rosenheim, 7 June 2003.
Paratypes: RBINS POR 91; RBINS POR 92, same locality, fixed for EM, coll. Ph. Willenz and B. Rosenheim, 9 June 2003; RBINS POR 67; RBINS POR 78, Jamaica, Pear Tree Bottom Cave, 5 km east of Discovery Bay, 18°27′57.31″N 77°21′18.49″W, 28 m depth, coll. A. Ereskovsky and Ph. Willenz, 1 April 2005. RBINS POR 94 Guadeloupe, Cathedral Cave, Pointe de la Fontaine (Anse-Bertand) 16°27′40.48″N 61°31′50.67″W; depth 15 m, coll. A. Ereskovsky, 18 May 2012.
COMPARATIVE MATERIAL EXAMINED
Oscarella malakhovi Ereskovsky, Reference Ereskovsky2006 (ZIN RAS 10697, ZIN RAS 10698: Japan Sea). Oscarella kamchatkiensis Ereskovsky, Sanamyan & Vishnyakov, 2009 (ZIN RAS 11058, ZIN RAS 11059 and ZIN RAS 11060: North Pacific, Avacha Gulf). Oscarella lobularis (Schmidt, Reference Schmidt1862) (SME AE 008) and Oscarella tuberculata (Schmidt, Reference Schmidt1868) (SME AE 003). NE Mediterranean Sea (Marseille region), underwater cave of Maire Island. Oscarella microlobata Muricy, Boury-Esnault, Bézac, Vacelet, 1996 (SME AE 010) and Oscarella viridis Muricy, Boury-Esnault, Bézac, Vacelet, 1996 (SME AE 009). NE Mediterranean Sea (Marseille region), underwater cave of Jarre Island. Oscarella balibaloi, Pérez, Ivanišević, Dubois, Pedel, Thomas, Tokina, Ereskovsky, 2011 (MNHN-DJV 129). NE Mediterranean Sea (Marseille region), underwater cave of Maire Island.
DIAGNOSIS
Cave dwelling thin and translucent Oscarella, yellowish-pale green or milky-beige in colour, forming hanging lobes with perforated surface curled up at the periphery, soft, slimy consistency and two mesohylar types of cells with inclusions: large, abundant vacuolar cells and small rare granular ones.
DESCRIPTION (FIGURE 2)
Sponge leaf-like, thinly encrusting on vertical surfaces, flat, irregular with fragile lobes hanging from the surface. Size up to 3 × 8 cm wide by 1–4 mm thick. Colour in vivo yellowish-pale green or milky-beige. Preserved fragments are light brown to ivory white. Sponge loosely attached to the substrate at intervals only. Surface is finely lobate and is perforated by abundant pores 9–12 µm in diameter. Bulge of tissue without perforation along periphery of individuals corresponding to exhalant canals. Oscula rare and located at the edge of the sponge, oscular tubes about 1–2 mm high. Consistency soft, slimy, very fragile and easy to tear.
SOFT TISSUE ORGANIZATION (FIGURE 2C, D)
Spicule and (or) fibre skeleton absent. Ectosome from 6 to 15 µm thick. Inhalant canals (15–40 µm in diameter) run perpendicular to the surface (Figure 2C). Choanocyte chambers ovoid to spherical, eurypilous, 27.1–49.6–61.2 µm in diameter (Figure 2C, D, E). Exhalant canals run towards a well developed system of basal cavities with diameter 7.2–37.6–78.4 µm, leading to the oscula situated on the external border of specimens. Ostia 8–23 µm in diameter.
CYTOLOGY (FIGURES 2F; 3A–F; 4A–C; 5A–C)
Exopinacocytes flagellated with oval to flat (in the ostia regions) shape, 7.6 µm wide by 5.5 µm high (N = 15; Figure 3A, B). Flagellae very poorly developed. Cytoplasm filled with electron translucent vacuoles 4.5–7.5 µm in diameter. Nucleus about 2.2 µm in diameter with basal or central position in flat cells. External surface of exopinacocytes covered by dense layer of glycocalyx 0.14–0.30 µm thick (Figure 3B).
Endopinacocytes flagellated, about 12.1 µm wide by 4.8 µm high (N = 15; Figure 3C, D). Cell free surface smooth or wavy (Figure 3C). Nucleus ovoid (2.2 µm in diameter), without nucleolus. Cytoplasm filled with electron translucent vacuoles 0.8–3.8 µm in diameter and rare phagosomes 0.8–1.1 µm in diameter.
Basopinacocytes oval, rare, flat, 10.5 µm wide by 6.5 µm high and similar to exopinacocytes (N = 8; Figure 3E, F). Flagellae absent. Cytoplasm filled with electron translucent vacuoles 0.6–3.2 µm in diameter. Nucleus centrally located about 2.5 µm in diameter.
Choanocytes ovoid to pyramidal, irregular, basal part about 4.2 µm wide by 5.4 µm high (N = 20; Figure 4A, B). Nucleus apical or centrally positioned, about 2.2 µm in diameter with rare small nucleolus. Cytoplasm includes from one to eight phagosomes 0.7–2.2 µm in diameter. Collar 4 µm in width, with about 38 microvilli. Choanocytes contact each other at their middle or basal parts (Figure 4A, B). Basal parts often show large outgrowth, anchoring the cell to the underlining collagen layer.
Apopylar cells roughly triangular in section, 6.9 µm wide by 4.3 µm high (N = 8; Figures 2F, 4C). Nucleus spherical, up to 2.5 µm in diameter. Cytoplasm contains mitochondria, digestive vacuoles, and small osmiophilic inclusions.
Surface of endopinacocytes, choanocytes and apopylar cells covered by thin irregular layer of glycocalyx. Choanoderm and pinacoderm underlined by continuous basement membrane-like dense collagen layer 0.9–1.7 µm thick (Figures 3B, D, F, 4B).
Two types of cells with inclusions occur within the mesohyl.
Vacuolar cells ovoid to triangular, 18.3 µm × 11.3 µm (N = 10; Figure 5A). Nucleus ovoid, about 2.7 µm in diameter, with nucleolus. Cytoplasm thick with large electron translucent vacuoles 1.6–14.5 µm in diameter, often connected to each other and with very rare small electron-dense homogeneous granules 0.5 µm in diameter. Abundant cells often forming compact aggregates.
Granular cells ovoid to elongate, approximately 6.5 × 10.4 µm, sometimes amoeboid-like or irregular (N = 8; Figure 5B, C). Cytoplasm filled with small (approximately 0.2 µm in diameter) and larger (0.9–2.4 µm in diameter) electron translucent vacuoles, with vacuoles 1.6–3.4 µm in diameter, containing heterogeneous material, and irregular (0.5–1.2 µm) vacuoles with opaque homogeneous contents. Nucleus irregular, about 1.9 µm in diameter.
Archaeocytes absent in all samples examined.
ENDOBIOTIC BACTERIA (FIGURE 5D, E)
Two morphological types of extracellular endobiotic bacteria occur in the mesohyl: B1, B2 (Figure 5E). Type B1 are the most abundant, rod-like, slightly curved shape 0.9–1.56–2.0 µm in length and 0.7–0.73–0.87 µm in diameter (N = 22; Figure 5E). Cell wall consists of two membranes, and a tight periplasmic space. Cytoplasm forming a dark irregular layer under the cell wall. Small cavities often formed between cell wall and dark cytoplasm layer. Nucleoid filamentous network is irregular, with thick elements in the centre and thin filaments closer to the periphery. Surface covered with thin filamentous outgrowths, but in some bacteria, this layer forms a well developed capsule. Type B1 appears sometimes in the vacuoles of granular cells. Type B2 are very rare and small, spherical to oval about 0.3 µm in diameter (N = 3; Figure 5E). Cell wall consists of two membranes with a developed intermediate space. Cytoplasm forms a dark irregular layer under the cell wall. Nucleoid region with dense filamentous network.
REPRODUCTION (FIGURE 6A, B)
In sponges, collected in Martinique spermatogenesis observed in early June. Spermatic cysts surrounded by a thick layer of collagen located in the choanosome (Figure 6A). Spermatocysts resulting from choanocyte chambers differentiation ovoid, about 65 µm in diameter. Spermatogenesis asynchronous. Cysts generally with several generations of male germ cells forming clusters (Figure 6A, B). Mature spermatozoa with long terminal flagella and slightly elongated head containing acrosome and a large mitochondria (Figure 6B). Oogenesis asynchronous; oocytes occur simultaneously at different stages in the same individual, as well as embryos and larvae (Figure 6C). Embryos at different stages of development and mature larvae in the lower part of the choanosome of specimens collected at the end of March and early April in Jamaica and in May in Guadeloupe. Cinctoblastula larvae typical for Homoscleromorpha with oval shape about 153 × 255 µm. Posterior pole pink, surrounded by a belt of cells with intranucleolar paracrystalline inclusion. Basal side of ciliated larval cells lined by basement membrane (Figures 6D). Larval cavity filled with extracellular matrix (Figure 6C) including endobiotic bacteria of both morphotypes (Figure 6D).
HABITAT
Exclusively sciaphilous, occurring on vertical walls or ceilings of reef caves between 15 and 28 m.
DISTRIBUTION
Caribbean: north of Jamaica, Martinique, Guadeloupe (present study).
ETYMOLOGY
The specific name is given in honour of Nathalie Deneumoustier for her support while describing this sponge.
TAXONOMIC REMARKS
The external morphology and colour of Oscarella nathaliae sp. nov. are unique. The surface is unusually perforated by abundant pores. Oscarella nathaliae sp. nov. has small lobes hanging from the surface and a milky-grey or yellowish-pale green colour in vivo. These colours are different from any known Oscarella species. Unlike other members of this genus, the new species shows a leaf-like flat body with a bulgy edging lacking perforation. The loose, irregular and punctual attachment to the substrate is another external exclusive character of this new species. Cytological characters, such as cell types with different inclusions or pinacocytes features are important clues to identify Oscarella at the species level in modern taxonomy. Spherulous cells, vacuolar cells and granular cells are abundant in Oscarella and possess different varieties of structures in each species (Boury-Esnault et al., Reference Boury-Esnault, Solé-Cava and Thorpe1992; Muricy et al., Reference Muricy, Boury-Esnault, Bézac and Vacelet1996; Muricy & Pearse, Reference Muricy and Pearse2004; Ereskovsky, Reference Ereskovsky2006; Ereskovsky et al., Reference Ereskovsky, Borchiellini, Gazave, Ivanišević, Lapebie, Pérez, Renard and Vacelet2009a, Reference Ereskovsky, Sanamyan and Vishnyakovb; Pérez et al., Reference Pérez, Ivanišević, Dubois, Pedel, Thomas, Tokina and Ereskovsky2011). The cell contents of O. nathaliae sp. nov. like in all other Oscarella species are simple, with only two kinds of cells with inclusions: vacuolar and granular cells. Vacuolar cells are the most characteristic cells of O. nathaliae sp. nov. As in O. tuberculata, they are abundant and large, forming compact masses. However, they are much larger in O. nathaliae sp. nov. (up to 18.3 × 11.3 µm) than in O. tuberculata (10.0 × 7.0 µm) (Boury-Esnault et al., Reference Boury-Esnault, Solé-Cava and Thorpe1992). As for many other Oscarella species, the mesohyl of O. nathaliae sp. nov. lacks archaeocytes.
In contrast with the other Oscarella species, the pinacoderm cells (exo- and endopinacocytes) of O. nathaliae sp. nov. have large electron translucent vacuoles. An additional character useful to differentiate Homoscleromorpha is the presence of different types of endosymbiotic bacteria. Their ultrastructure and the number of types are specific to each species studied so far (Boury-Esnault et al., Reference Boury-Esnault, Solé-Cava and Thorpe1992; Muricy et al., Reference Muricy, Boury-Esnault, Bézac and Vacelet1996, Reference Muricy, Bézac, Gallissian and Boury-Esnault1999; Ereskovsky et al., Reference Ereskovsky, Sanamyan and Vishnyakov2009b; Vishnyakov & Ereskovsky, Reference Vishnyakov and Ereskovsky2009; Gloeckner et al., Reference Gloeckner, Hentschel, Ereskovsky and Schmitt2012). Oscarella nathaliae sp. nov. has two types of endosymbiotic extracellular bacteria, like O. tuberculata, O. viridis, O. carmela, O. malakhovi and O. balibaloi. While O. lobularis, O. imperialis and O. kamchatkensis have three bacterial types, and O. microlobata has six distinct morphotypes (Vishnyakov & Ereskovsky, Reference Vishnyakov and Ereskovsky2009).
Reproduction features of the new species are typical to all Oscarella (Ereskovsky, Reference Ereskovsky2010).
Family PLAKINIDAE Schulze, Reference Schulze1880
Genus Plakortis Schulze, Reference Schulze1880
SYNONYMY
Plakortis Schulze, Reference Schulze1880: 449; Placortis Topsent, Reference Topsent1895:557; Roosa de Laubenfels, Reference Laubenfels1934: 2 (after Topsent, Reference Topsent1937:7).
TYPE SPECIES
Plakortis simplex Schulze, Reference Schulze1880.
DIAGNOSIS (FROM MURICY & DIAZ, 2002)
Thinly to massively encrusting plakinids with a skeleton mostly formed by small (50–200 µm) diods with triods in varying abundance. Deformed calthrops can be found in some specimens. Some species have microrhabds (5–20 µm) distributed regularly in the sponge body. Aquiferous system intermediate between sylleibid-like and leuconoid, with eurypylous choanocyte chambers regularly distributed around exhalant canals. Both ectosomal inhalant cavities and basal exhalant cavities are usually present. Skeleton confused, dense, without ectosomal specialization or differential location of spicules.
Plakortis myrae sp. nov.
(Figure 7)

Fig. 7. Plakortis myrae sp. nov. (A) In situ close-up; (B) transverse section through the ectosomal skeleton (LM); (C) tangential section through the ectosome (LM); (D) transverse section through the choanosomal skeleton (LM); (E) diods; (F) triods; (G) microrhabds. Scale bars: A, 2 cm; B–D, 200 µm; E, 50 µm; F, 20 µm; G, 2 µm.
TYPE MATERIAL
Holotype: RBINS POR 68, Jamaica, Pear Tree Bottom Cave, 5 km east of Discovery Bay, 18°27′57.31″N 77°21′18.49″W, 28 m depth, coll. A. Ereskovsky and Ph. Willenz, 1 April 2005.
Paratype: none.
COMPARATIVE MATERIAL EXAMINED
Plakortis dariae (RBINS POR 65 and RBINS POR 76): Jamaica. Plakortis edwardsi sp. nov. (RBINS POR 69): Jamaica. Plakortis simplex Schulze, Reference Schulze1880 (SME AE 005): NE Mediterranean Sea (Marseille region), underwater cave 3PP.
DIAGNOSIS
Plakortis light brown. Diods of one size-class, 66.6–119.0 µm, with lightly to strongly marked centre, some sinuous-bent. Triods Y- or T-shaped with actines 17.8–53.0 µm long. Microrhabds abundant 5.0–12.4 µm long.
DESCRIPTION (FIGURE 7A)
Sponge thickly encrusting to cushion shaped, irregular. Size up to 4 × 10 cm wide by 1–2 cm thick. Colour in vivo is homogeneous light brown. Preserved specimens are dark brown. Sponge firmly attached to the substrate. Surface even and smooth. Oscules slightly elevated with translucent inner rim around the oscula 2–5 mm in diameter, contracted in alcohol. Consistency soft, compressible.
SKELETON (FIGURE 7B, C)
Ectosome is distinct in transversal sections, 0.5–0.6 mm thick with multispicular tracts 20–40 µm in diameter, oriented perpendicular to the surface; forming irregularly elliptical meshes, spicules rarely cross the surface. Ectosome with a tangential reticulation of spicule tracts forming elliptical meshes (Figure 7B, C). Choanosome formed by a dense and relatively confused alveolar arrangement of diods and microrhabds with rounded meshes 60–80 µm in diameter (Figure 7D).
SPICULES (FIGURE 7E–G)
Diods abundant, thin, irregular, slightly curved, lightly to sometimes strongly marked protuberant, sometimes sinuous S-bent centre and sharp endings with high length variations: 66.6–100.2–119.0/2–4 µm (N = 20; Figure 7E). Triods abundant, regular to irregular Y- or T-shaped, with sharp endings; actines 17.8–37.1–53.5/1.8–3.3 µm (N = 8; Figure 7F). Microrhabds abundant, irregularly twisted, sinuous: 5.0–7.5–12.4/0.44–0.97 µm (N = 5; Figure 7G). Microdiods or small diods absent.
HABITAT
Exclusively sciaphilous, occurring on vertical walls of a coralligenous reef cave between 26 and 28 m depth.
DISTRIBUTION
Caribbean: north of Jamaica (present study).
ETYMOLOGY
The specific name is given in honour of Myriam Doriaux to celebrate 40 years of patient assistance to the last author.
TAXONOMIC REMARKS
Nineteen species of the genus Plakortis are known throughout the world, four of which occur in the Caribbean Sea and more precisely in the Jamaican region: P. angulospiculatus, P. zyggompha, P. halichondrioides and P. simplex (Carter, Reference Carter1879; Schulze, Reference Schulze1880; de Laubenfels, Reference Laubenfels1934; Hechtel, Reference Hechtel1965; Boury-Esnault, Reference Boury-Esnault1973; Wiedenmayer, Reference Wiedenmayer1977; Pulitzer-Finali, Reference Pulitzer-Finali1986; Zea, Reference Zea1987; Mothes & Bastian, Reference Mothes and Bastian1993; Diaz & van Soest, 1994; Lehnert & van Soest, Reference Lehnert and van Soest1998; Moraes & Muricy, Reference Moraes and Muricy2003). Two other species are known from the Western Atlantic Ocean: Plakortis insularis and P. microrhabdifera (Moraes & Muricy, Reference Moraes and Muricy2003).
Plakortis myrae sp. nov. is part of the P. lita species group which is characterized by the presence of microrhabds. The group includes three species (P. hooperi Muricy, Reference Muricy2011 from Papua New Guinea, P. lita de Laubenfels, Reference Laubenfels1954 from Indonesia and Papua New Guinea, and P. microrhabdifera Moraes & Muricy, Reference Moraes and Muricy2003 from NE Brazil). Plakortis myrae sp. nov. differs from P. microrhabdifera in that it presents triods which are absent in P. microrhabdifera; it differs from P. hooperi in its reticulated ectosome, whereas the latest is characterized by a confused ectosomal skeleton; and it differs from P. lita in its light brown colour (in P. lita the colour is reddish-brown externally and lighter internally). Plakortis myrae sp. nov. differs from all other Plakortis species in that it has irregular microrhabds.
Plakortis edwardsi sp. nov.
(Figure 8)

Fig. 8. Plakortis edwardsi sp. nov. (A) In situ close-up; (B) transverse section through the ectosome (LM); (C) tangential section through the ectosome (LM); (D) transverse section through the choanosome (LM); (E) diods; (F) small diods; (G) triods. Scale bars: A, 2 cm; B–D 200 µm; E, 25 µm; F, 5 µm; G, 20 µm.
TYPE MATERIAL
Holotype: RBINS POR 69 Jamaica, Pear Tree Bottom Cave, 5 km east of Discovery Bay, 18°27′57.31″N 77°21′18.49″W, 25 m depth, coll. A. Ereskovsky and Ph. Willenz, 28 March 2005.
Paratype: none.
COMPARATIVE MATERIAL EXAMINED
Plakortis dariae sp. nov. (RBINS POR 65 and RBINS POR 76): Jamaica. Plakortis myrae sp. nov. (RBINS POR 68): Jamaica. Plakortis simplex Schulze, Reference Schulze1880 (SME AE 005): NE Mediterranean Sea (Marseille region), underwater cave 3PP.
DIAGNOSIS
Plakortis light to dark brown with different colour patches on the same specimens. Consistency soft, compressible, ectosome without alveolar arrangement of skeleton. Diods abundant of one size-class (110–128 µm) with thick, sinuous, S-bent centres; small diods rare, slightly twisted, sinuous (22–31 µm), triods not abundant Y- or T-shaped, some sinuous close to the centre, actines 28–59 µm. Microrhabds absent.
DESCRIPTION (FIGURE 8A)
Sponge thickly encrusting to massive, lobate, irregular. Size up to 2 × 8 cm by 1.0 – 2.5 cm thick. Colour in vivo light to dark brown with different colour patches on the same specimens. Choanosome is green-brown. Preserved specimens are dark brown. Sponge firmly attached to the substrate. Surface smooth, regular. Oscules flush with the surface, 2–4 mm in diameter, contracted in alcohol. Consistency soft, compressible, fragile.
SKELETON (FIGURE 8B–D)
Ectosome in transversal sections 60–80 µm thick, with a loose confused arrangement of diods in low density, without signs of alveolar arrangement (Figure 8B). Spicules never cross the surface. Ectosome with a tangential reticulation of spicule tracts forming rounded meshes (Figure 8C). Choanosome formed by a confused, dense mass of diods without a clear alveolar arrangement (Figure 8D).
SPICULES (FIGURE 8E–G)
Diods of two categories: large and small. Large diods irregular to almost straight, slightly curved, centre thick, sinuous, S-bent centre, sometimes with small protuberance; endings acerate: 110.0–118.0–128.0/2.6–3.0 µm (N = 20; Figure 8E). Small diods rare, slightly irregularly twisted, sinuous with rugged surface and stub edges: 22.4–27.9–31.1/0.56–0.77–1.09 µm (N = 5; Figure 8F). Triods not abundant, regular to irregular, Y- or T-shaped, generally sinuous close to the centre, with sharp endings; actines sometimes slightly curved 28.1–42.9–59.4/2–2.6 µm (N = 7; Figure 8G). Microrhabds absent.
HABITAT
Occurs on vertical shaded sides of massive reef boulders among coralline algae between 23 and 26 m depth.
DISTRIBUTION
Caribbean: north of Jamaica (present study).
ETYMOLOGY
The specific name is given to honour Tracey Edwards, who first collected this sponge.
TAXONOMIC REMARKS
Plakortis edwardsi sp. nov. is the only species of this genus with very small diods (< 30 µm). See the same section after Plakortis dariae sp. nov. description.
Plakortis dariae sp. nov.
(Figure 9)

Fig. 9. Plakortis dariae sp. nov. (A) In situ close-up; (B) transverse section through the ectosome (LM); (C) tangential section through the ectosome (LM); (D) transverse section through the choanosome (LM); (E) diods; (F) small diods; (G) triods. Scale bars: A, 2 cm; B–D, 200 µm; E, 50 µm; F, G, 20 µm.
TYPE MATERIAL
Holotype: RBINS POR 65, Jamaica, Pear Tree Bottom Cave, 5 km east of Discovery Bay, 18°27′57.31″N 77°21′18.49″W, 28 m depth, coll. A. Ereskovsky and Ph. Willenz, 27 March 2005.
Paratype: RBINS POR 76, the same site, coll. A. Ereskovsky and Ph. Willenz, 28 March 2005.
COMPARATIVE MATERIAL EXAMINED
Plakortis myrae sp. nov. (RBINS POR 68): Jamaica. Plakortis edwardsi sp. nov. (RBINS POR 69): Jamaica. Plakortis simplex Schulze, Reference Schulze1880 (SME AE 005): NE Mediterranean Sea (Marseille region), underwater cave 3PP.
DIAGNOSIS
Plakortis light green with thin slightly brownish patches. Diods abundant of two size-classes; large: 67.3–112.2 µm, with lightly to strongly marked centre, some sinuous-bent; small diods rare, irregular, slightly curved, with protuberant centre, often deformed with one end blunt: 30–59.5 µm. Triods rare, regular; actines 20–43.5 µm long. Microrhabds and microdiods absent.
DESCRIPTION (FIGURE 9A)
Sponge thickly encrusting to cushion shaped, irregular. Size up to 2 × 8 cm wide by 1–3 cm thick. Colour in vivo is light green with thin slightly brownish patches. Sponge firmly attached to the substrate. Surface even and smooth. Oscules flush with the surface, 3–8 mm in diameter, contracted in alcohol. Consistency soft, compressible.
SKELETON (FIGURE 9B–D)
Ectosomal skeleton is distinctly reticulate, with multispicular tracts oriented perpendicular to the surface, forming circular or irregular meshes in tangential sections (Figure 9C). Spicules cross the surface (Figure 9B). The ectosome (120–170 µm thick) is poorly differentiated, slightly denser than the choanosome, without subectosomal lacunae. Choanosomal skeleton confused to vaguely reticulate (Figure 9D).
SPICULES (FIGURE 9E–G)
Diods of two categories: large and small. The large diods are abundant, thin, irregular, slightly curved, lightly to sometimes strongly marked protuberant centre, sometimes sinuous S-bent centre and sharp endings: 67.3–89.7–112.2/1.6–2.8 µm (N = 20; Figure 9E). The small diods are rare, irregular, slightly curved, marked protuberant, often deformed with one end blunt: 30–46.1–59.5/0.8–2.1 µm (N = 9; Figure 9F). Triods rare, regular to irregular form, with sharp endings; actines 20–35.8–43.5/2.2–2.7 µm (N = 6; Figure 9G). Microrhabds and microdiods are absent.
HABITAT
Exclusively sciaphilous, occurring on vertical walls of coralligenous reef caves between 26 and 28 m depth.
DISTRIBUTION
Caribbean: north of Jamaica (present study).
ETYMOLOGY
The specific name is given in honour of Daria Tokina, without whom this research would have been impossible.
TAXONOMIC REMARKS
Based on the spicule size and composition, Plakortis edwardsi sp. nov. and P. dariae sp. nov. should be placed in the P. simplex species group, which is characterized by the absence of any distinctive characters such as microrhabds, quasiamphiasters, or large spicules (Muricy, Reference Muricy2011). This group includes 12 species (P. albicans Cruz-Barraza & Carballo, Reference Cruz-Barraza and Carballo2005, P. communis, P. copiosa Pulitzer-Finali, Reference Pulitzer-Finali1993, P. edwardsi sp. nov., P. erythraena Lévi, Reference Lévi1958, P. galapagensis, P. insularis, P. japonica (Hoshino, Reference Hoshino1977), P. nigra Lévi, Reference Lévi1953, P. dariae sp. nov., P. simplex and P. zyggompha). However, such placement is not supported by molecular data for which P. edwardsi sp. nov. is grouped with P. halichondroides/P. angulospiculatus while P. dariae sp. nov. is grouped with P. simplex (Figure 13).
Plakortis edwardsi sp. nov. and P. dariae sp. nov. both differ from other members of this group and from other Plakortis species by the presence of diods of two size-classes separated by a size gap. Although both species display diods of similar sizes (P. edwardsi sp. nov.: smaller 22–31 µm and larger 110–128 µm; P. dariae sp. nov.: smaller 30–59.5 µm and larger: 67.3–112 µm) their skeletons are notably different. The ectosomal skeleton of P. edwardsi sp. nov. has a loose confused arrangement of diods in low density, without signs of alveolar arrangement and the spicules do not cross the surface. The ectosomal skeleton of P. dariae sp. nov. is distinctly reticulate, with multispicular tracts oriented perpendicular to the surface, forming circular or irregular meshes. Spicules cross the surface. Two other Plakortis species with diods of two size-categories were previously reported: P. bergquistae Muricy, Reference Muricy2011 and P. galapagensis Desqueyroux-Faúndez and van Soest, Reference Desqueyroux-Faúndez and van Soest1997.
Plakortis bergquistae differs from the new species described here in diods sizes and shape: smaller 91–163/2–6 µm, larger 202–356/5–11 µm; both are straight or slightly curved, smooth, regular, with slightly thickened centre. On the contrary, diods of P. edwardsi sp. nov. are almost straight, slightly curved, but irregular, with a thick sinuous S-bent centre, sometimes with small protuberance. Diods of P. dariae sp. nov. are slightly curved, irregular, with a lightly to sometimes strongly marked protuberant, sometimes sinuous S-bent centre. Plakortis galapagensis differs also from both new species in that it has larger diods: smaller 27–92/1.5–4 µm and larger 126–165/4–8 µm.
Genus Plakina Schulze, Reference Schulze1880
SYNONYMY
[Achinoe] Gray, Reference Gray1867a: 546 (unavailable name). Plakina Schulze, Reference Schulze1880: 448. Placina Topsent, Reference Topsent1890d: 231. Plakoosa de Laubenfels, Reference Laubenfels1936b: 462 (after Topsent, Reference Topsent1937: 7).
TYPE SPECIES
Plakina monolopha Schulze, Reference Schulze1880.
DIAGNOSIS (MURICY & DIAZ, 2002)
Thinly to massively encrusting Plakinidae with a spiculation of diods, triods, and calthrops, and with homogeneously ramified lophocalthrops with one, two, three, or four lophate rays. Candelabra (heterolophose calthrops) absent. Lophocalthrops usually concentrated at the sponge surface and along bordering canals. Development of the ectosome is variable, and subectosomal cavities may be present. A large basal cavity is present in most species. Proportion of mesohyl to chambers varies from 0.7 to 1.8:1. Choanocyte chambers are eurypylous or aphodal, usually with a radial arrangement around incurrent and excurrent canals (called sylleibid-like arrangement).
Plakina jamaicensis Lehnert and van Soest, Reference Lehnert and van Soest1998
(Figures 10, 11)

Fig. 10. Plakina jamaicensis RBINS POR 70. (A) In situ close-up; (B) transverse section through the tissue, showing the general organization of the skeleton and aquiferous system (LM); (C) transverse section through the ectosome (SEM); (D) transverse section through the choanosome (LM); (E) transverse section through the choanosome (SEM). CC, choanocyte chambers; EC, exhalant canals; Ex, exopinacoderme; SC, subdermal inhalant cavities. Scale bars: A, 2 cm; B, 500 µm; C, 200 µm; D, 50 µm ; E, 100 µm.

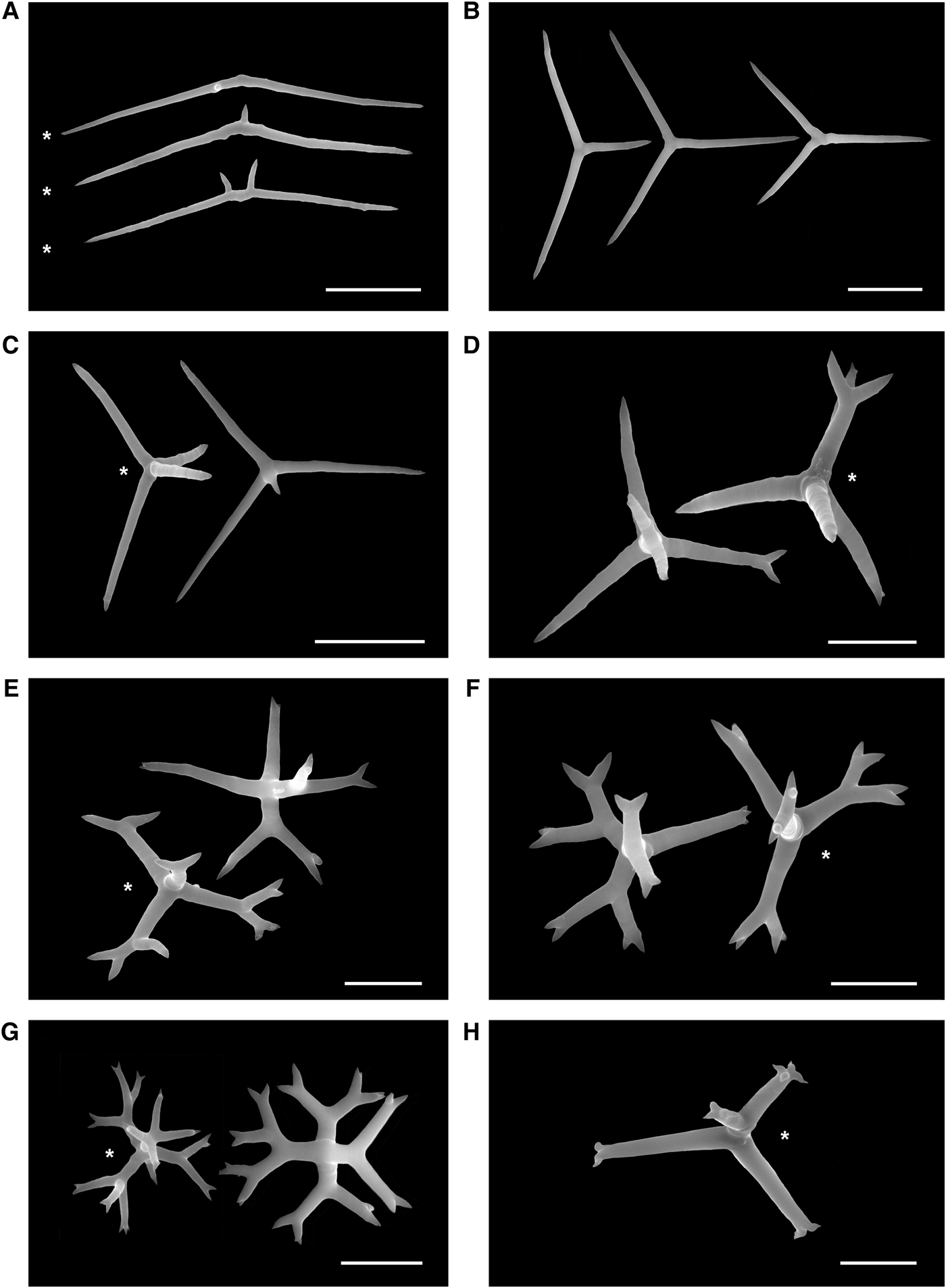
Fig. 11. Spicules of Plakina jamaicensis. Holotype ZMA POR 12736; paratype RBINS POR 70 (*) (SEM). (A) Diods; (B) triods; (C) calthrops; (D) monolophous calthrops; (E) dilophous calthrops; (F) trilophous calthrops; (G) tetralophous calthrops; (H) distally tetralophose calthrop. Scale bars: A–C, 20 µm; D–H, 10 µm.
MATERIAL EXAMINED
Holotype: ZMA POR. 12736, Jamaica, Discovery Bay, # J319, 17.7.1993, fore reef, underneath Montastrea annularis, 35 m depth.
Paratype: RBINS POR 70 and RBINS POR 79, Jamaica, Chalet Caribe Cave, west of Montego Bay, 18°27′14.78″ N 77°58′17.29 W, 23 m depth, coll. A. Ereskovsky and Ph. Willenz, 3 April 2005.
COMPARATIVE MATERIAL EXAMINED
Plakina trilopha Schulze, Reference Schulze1880 (SME AE 006), Plakina jani Muricy, Boury-Esnault, Bézac, Vacelet, Reference Muricy, Boury-Esnault, Bézac and Vacelet1998 (SME AE 007), NE Mediterranean Sea (Marseille region, La Ciotat), underwater cave 3PP.
DIAGNOSIS
Plakina slightly tough, compressible, creamy orange to yellowish or fawn, encrusting, up to 1 cm thick, surface brain-like convoluted. Spicule types irregular and variable in shape. Diods common with central swelling smooth or with one to two spines. Triods abundant, never lophose. Monolophose calthrops rare (1m, ts or only 1m). Dilophose calthrops rare (1m, 2d, ts). Trilophose calthrops very rare (1m, 2d, ts). Tetralophose calthrops common (1m, 2d, ts).
DESCRIPTION (FIGURE 10A)
Sponge firm but compressible, firmly attached to the substrate, thinly encrusting with convoluted brain-like aspect due to swelled sub-ectosomal exhalant canals converging to rare and short oscules. Size from 9 × 9 to 30 × 40 cm wide by about 1 cm thick. Colour in vivo is yellowish or fawn. Preserved fragments are light brown. Surface smooth, irregular borders. Oscules, about 5 mm in diameter, surrounded by a thin transparent oscular rim. Found on vertical shaded areas of coral reefs.
GENERAL ORGANIZATION (FIGURE 10B–E)
Ectosome is 17–43 µm thick, separated from the choanosome by well developed system of irregular inhalant/exhalant canals 30–140 µm wide (Figure 10B, C). Aquiferous system (Figure 10C–E) is leuconoid, choanocyte chambers spherical or ovoid, diplodal, 35–50 µm in diameter. Spicules of all kinds are haphazardly dispersed throughout the mesohyl. The spiculation is denser in the ectosome, The choanosome is somewhat less densely packed with spicules.
SPICULES (FIGURE 11A–H)
All spicule types are irregular and variable in shape.
Diods abundant, irregular, sinuous, with actines gradually pointing to sharp endings. Central swelling typically S-bent, with one or two spines or almost smooth: 69.0–78.2–91.1/2.3–2.9 µm (N = 20; Figure 11A). Triods abundant, central swelling, actine size, and angle between actines variable. Never lophose or with bifurcated rays. Actines: 22.1–27.5–32.1/1.3–2.0 µm (N = 9; Figure 11B). Calthrops common, actines unequal, never lophose. Actines: 5.5–9.6–11.6/0.8–2.9 µm (N = 9; Figure 11C). Monolophose calthrops rare, irregular, with some bifurcated or trifurcated actines (ramification pattern 1m, ts or only lm). Total length: 22.4–27.1–31.7/1.1–1.6 µm (N = 4; Figure 11D). Dilophose calthrops rare, irregular, some lophose bifurcated actines with one or two distal spines (ramification pattern 1m, 2d, ts). Total length: 24.9–28.3–35.7/1.3–1.9 µm (N = 6; Figure 11E). Trilophose calthrops rare, actines bifurcated or trifurcated, lophose actines with two distal spines (ramification pattern 1m, 2d, ts). Total length: 25.7–28.4–29.2/1.4–1.54 µm (N = 3; Figure 11F). Tetralophose calthrops common, actines bifurcated, rarely trifurcated or quadrifurcated, with one or two distal spines (ramification pattern 1m, 2d, ts). Total length: 17.6–19.8–23.3/1.1–1.8 µm (N = 12; Figure 11G). Distally tetralophose calthrops very rare with two to four distal spines (ramification pattern 1d, ts). Total length 26–30–31.8 µm (N = 2; Figure 11H).
HABITAT
On vertical shaded areas of coral reef, 20–25 m depth.
DISTRIBUTION
Caribbean: Bahamas, Panama (Diaz and Rützler, 2009), North Jamaica (Lehnert & van Soest, Reference Lehnert and van Soest1998; present study).
TAXONOMIC REMARKS
Our redescription of Plakina jamaicensis Lehnert and van Soest, Reference Lehnert and van Soest1998, based on the revision of the holotype as well as on a newly collected specimen, reveals essential differences with the original description of spicule composition and size. In addition to diods, triods, rare calthrops and tetralophose calthrops originally reported, our investigations reveal abundant simple calthrops and additional monolophose, dilophose and trilophose calthrops.
Plakina jamaicensis differs markedly from the two other known Caribbean Plakina by its convoluted brain-like surface, spicule assortment and dimensions, as well as from the six Atlantic species. However, among Mediterranean Plakina species, P. jani and P. trilopha display a similar external shape. Plakina brachylopha has only calthrops and monolophose calthrops, both larger than in P. jamaicensis (Topsent, Reference Topsent1927). Plakina elisa possesses diods, triods and monolophose calthrops which are smaller than in P. jamaicensis and in addition it has a typical blue colour in vivo (de Laubenfels, Reference Laubenfels1936a). Spicules of P. tetralopha include triods, calthrops, trilophose and tetralophose calthrops that all have different dimensions than in P. jamaicensis (Hechtel, Reference Hechtel1965). Plakina versatilis (Schmidt, Reference Schmidt1880) differs from P. jamaicensis in the presence of monolophose and trilophose calthrops only, according to the original description. The type specimen, Corticium versatile TYPE = MCZ8140 (Orig n° 342) Coll A. Agassiz, was accidentally mixed up with a Halichondria and lost in the Harvard University Museum collection as previously mentioned by B. Austin (personal communication to A. Johnson, Museum of Comparative Zoology, Harvard University, 1986). Our analysis of the spicules of this specimen confirmed the only presence of oxea.
Interestingly, part of the Mediterranean Plakina species shows the same spicules composition as P. jamaicensis (P. trilopha, P. jani, P. endoumensis Muricy, Boury-Esnault, Bézac, Vacelet Reference Muricy, Boury-Esnault, Bézac and Vacelet1998, and P. weinbergi Muricy, Boury-Esnault, Bézac, Vacelet, Reference Muricy, Boury-Esnault, Bézac and Vacelet1998), whereas the other Mediterranean species have a different spicule combination (P. monolopha Schulze, Reference Schulze1880, P. dilopha Schulze, Reference Schulze1880 and P. crypta Muricy, Boury-Esnault, Bézac, Vacelet, Reference Muricy, Boury-Esnault, Bézac and Vacelet1998). Here we provide the comparative analysis of P. jamaicensis with P. trilopha, P. jani, P. endoumensis, and P. weinbergi (Table 1).
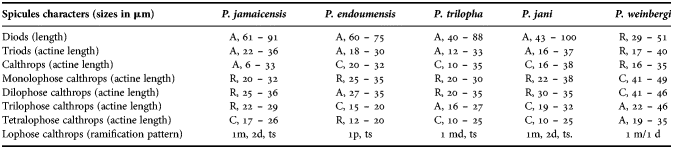
Table 1. Comparison of spicule abundance and characteristics in Plakina jamaicensis and Mediterranean Plakina species. A, abundant; C, common; R, rare.

Diods of P. jamaicensis are different from other species by their size; triods are abundant with size similar only with P. jani and P. weinbergi but in the later these spicules are rare. Calthrops of P. jamaicensis are smaller than all compared species. Rare monolophose calthrops have similar sizes in P. jamaicensis, P. endoumensis, P. trilopha, P. jani, as well as dilophose calthrops, in addition, in P. endoumensis these spicules are abundant. Rare trilophose calthrops of P. jamaicensis are close in size with the same but abundant spicule type of P. trilopha and P. jani. The dimensions of tetralophose calthrops of P. jamaicensis are different from all other Plakina species. Finally the ramification patterns of the lophose calthrops actines of P. endoumensis and P. weinbergi are very different compared to P. jamaicensis, P. jani and P. trilopha (Table 1). The ramification pattern of P. jamaicensis and P. jani is the same (1m, 2d, ts) except that monolophose calthrops of P. jamaicensis have 1m, ts or only lm.
Genus Corticium Schmidt, Reference Schmidt1862
SYNONYMY
Corticium Schmidt, Reference Schmidt1862, p. 42.
TYPE SPECIES
Corticium candelabrum Schmidt, Reference Schmidt1862.
DIAGNOSIS (AMENDED AFTER MURICY & DIAZ 2002)
Thinly encrusting to cushion-shaped Plakinidae with a spiculation consisting almost exclusively of non-lophose calthrops in one size-class and heterolophose calthrops (‘candelabra’). Homolophose calthrops may also be present, and nonlophose calthrops are absent in some species. Aquiferous system leuconoid, with aphodal choanocyte chambers. The species of this genus present variable but usually thick cortex (100–300 µm), and a proportion of mesohyl to chambers of about 1:1.
Corticium diamantense sp. nov.
(Figure 12)

Fig. 12. Corticium diamantense sp. nov. (A) In situ close-up; (B) transverse section through ectosome and choanosome (LM); (C) transverse section through the ectosomal skeleton (LM); (D) transverse section through the choanosomal skeleton (LM); (E) calthrops; (F) tetralophose calthrops; (G) candelabra. SC, subdermal inhalant cavities. Scale bars: A, 5 cm; B, 200 µm; C, D, 100 µm; E, 20 µm; F, 10 µm; G, 10 µm.
MATERIAL EXAMINED
Holotype: RBINS POR 85, Martinique, Le Diamant, Grotte du Fer à Cheval, 14°28′04.72″N 61°00′59.37″W, 22 m depth, coll. Ph. Willenz and Brad Rosenheim, 7 June 2003.
Paratypes: RBINS POR 86 and RBINS POR 87, same locality, same collectors, 9 June 2003.
COMPARATIVE MATERIAL EXAMINED
Corticium candelabrum Schmidt, Reference Schmidt1862 (SME AE 011), NE Mediterranean Sea (Marseille region), underwater cave of Maire Island.
DIAGNOSIS
Corticium leaf-like, thin, partly encrusting. Colour in vivo light brown, preserved specimens are dark brown. Loosely attached to the substrate, dense ectosome with abundant regular calthrops, rare tetralophose calthrops and candelabra with equally ramified (2–4 rays) conical actines (the fourth one basally ramified in 4–5 microspined rays).
DESCRIPTION
Sponge thin, ear- or leaf-shaped, lobate. Size up to 9 × 13 cm wide by 1.5 cm thick, Colour in vivo light brown. Preserved samples are darker brown. Sponge adhering to the substrate punctually, easily removed. Surface uneven, thin exhalant canals organized in an irregular mesh, slightly rough to touch. Oscula short and discrete, at the convergence of swelled exhalant canals, 1–3 mm in diameter, located on the margin of the sponge and forming short oscular chimneys up to 1–5 mm high.
GENERAL ORGANIZATION
Ectosome with a well defined cortex 200–350 µm thick. Subectosomal cavities well developed from 150 to 400 µm in diameter (Figure 12B). Aquiferous system leuconoid, with ovoid choanocytes chambers. Consistency firm but flexible, cartilaginous in alcohol.
SKELETON (FIGURE 12B–D)
Confused, with spicules scattered between choanocyte chambers. Candelabra and tetralophose calthrops concentrated at the surface and bordering canals.
SPICULES (FIGURE 12E–G)
Calthrops regular, non-lophose, in one size-class. Actines: 15–31.5–33/3–5.2 µm (N = 20; Figure 12E). Tetralophose calthrops very rare with a ramification pattern in which lophose actines have only a single proximal ramification point which gives rise to 3–4 conical, smooth rays (ramification pattern 1p, conical). Total length: 34.7–36.8–41.3 µm (N = 6; Figure 12F). Candelabra with equally ramified actines bearing conical ramification pattern in 2(rare)–4 rays. Fourth actine ramifies basally in 4–5 microspined rays. Total length: 28.4–30.6–41.1 µm long (N = 15; Figure 12G).
HABITAT
Sciaphilous, occurring on vertical walls of reef caves between 22 and 28 m depth.
REPRODUCTION
Sponges, collected in June, have well developed typical hollow cinctoblastula larvae about 320–340 × 200–220 µm.
DISTRIBUTION
Caribbean: Martinique (present study).
ETYMOLOGY
The specific name refers to the collecting locality, Le Diamant, Martinique.
TAXONOMIC REMARKS
So far, only one species of the genus Corticium was described from both the Atlantic Ocean and the Caribbean basin: C. quadripartitum Topsent, Reference Topsent1923. Corticium diamantense sp. nov. mainly differs from C. quadripartitum by the presence of abundant calthrops and by ramified candelabra with 2(rare)–4 rays, whereas C. quadripartitum possesses candelabra with 7–10 rays. Corticium diamantense sp. nov. differs from the type species C. candelabrum Schmidt, Reference Schmidt1862 by the presence of tetralophose calthrops and absence of monolophose calthrops. Corticium diamantense sp. nov. also differs from all other known Corticium. Corticium acanthastrum Thomas, Reference Thomas1968 from India has a unique blood red colour, with presence of triods, absence of non-lophose calthrops and lophose calthrops of small size (21–33 µm). Corticium bargibanti Lévi & Lévi, Reference Lévi and Lévi1983 from New Caledonia has a yellowish-grey colour, with absence of non-lophose calthrops, large lophose calthrops (73–75 µm) and candelabra (62–68 µm). Corticium niger Pulitzer-Finali, Reference Pulitzer-Finali1996 from New Guinea from C. diamantense sp. nov. is black, and characterized by large non-lophose calthrops (37–160 µm), and absence of lophose calthrops. Finally, Corticium simplex Lendenfeld, Reference von Lendenfeld1907 from N Australia differs from the new species by the absence of non-lophose and lophose calthrops.
Molecular analysis of the new and revised species
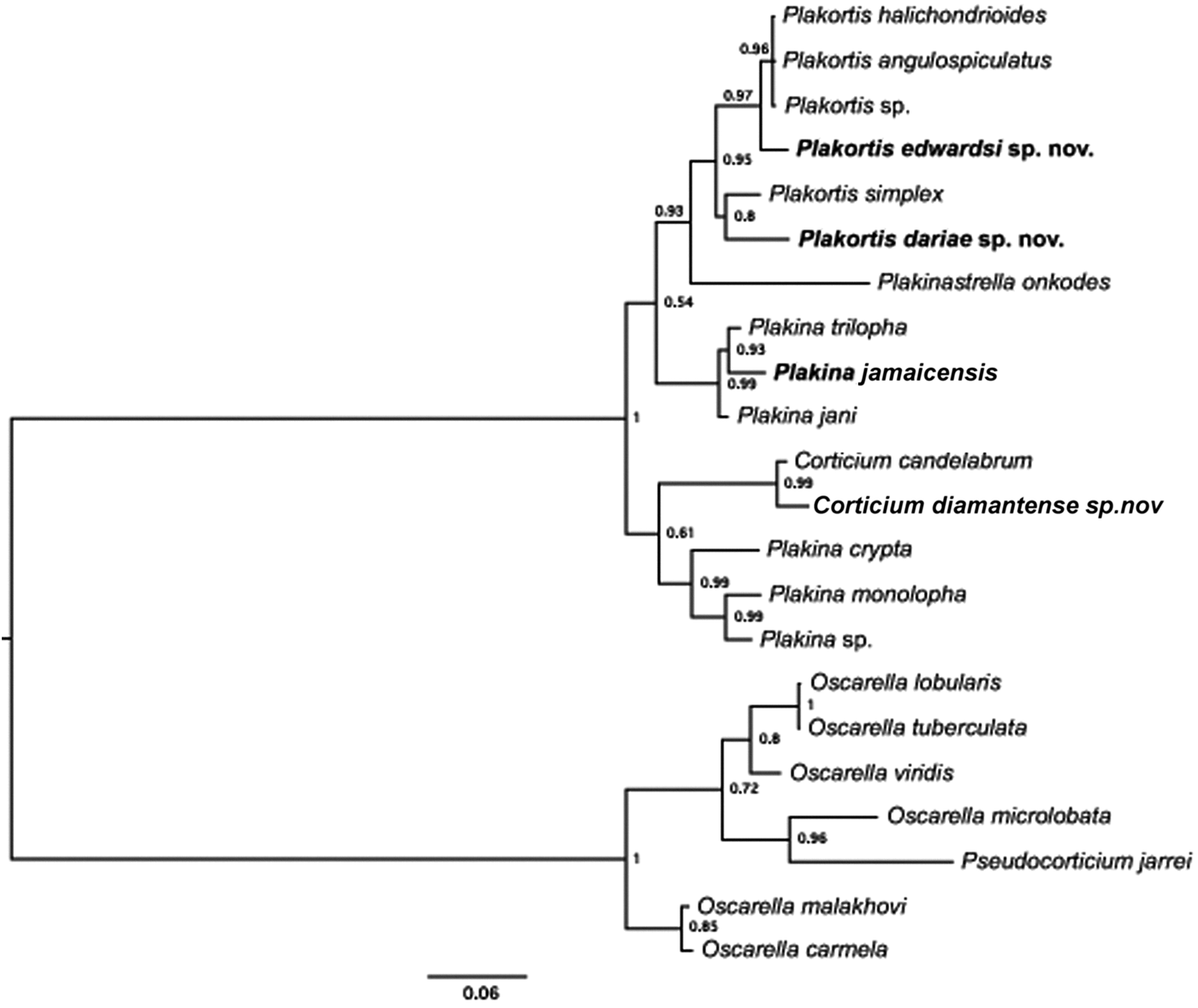
In addition to the morphological description, we determined partial Cytochrome b gene (cob) sequences for three new and the revised species of Homoscleromorpha described in this study: Plakortis edwardsi sp. nov. and P dariae sp. nov., Plakina jamaicensis Lehnert & van Soest, Reference Lehnert and van Soest1998 (two individuals) and Corticium diamantense sp. nov. (three individuals). In addition, Plakortis angulospiculatus collected at the Smithsonian marine station Bocas del Toro (S107-108) was also sequenced for comparison. Phylogenetic analysis of these sequences confirmed the genetic distinctiveness of the newly described species and revealed its affinities to other species of Homoscleromorpha (Figure 13). Plakortis edwardsi sp. nov. was closely related to Plakortis halichondrioides (96% sequence identity) while Plakortis dariae sp. nov. was most closely related to P. simplex (95% sequence identity, Figure 13). Interestingly, the sequence of P. angulospiculatus was 100% identical to that of P. halichondrioides reported in our previous study (Gazave et al., Reference Gazave, Lapébie, Renard, Vacelet, Rocher, Ereskovsky, Lavrov and Borchiellini2010). The latter result was confirmed by comparison of two barcoding sequences from P. angulospiculatus collected in Belize (Porifera barcoding database ## 142, 137) which were also 100% identical to cox1 sequence from P. halichondrioides (Gazave et al., Reference Gazave, Lapébie, Renard, Vacelet, Rocher, Ereskovsky, Lavrov and Borchiellini2010), suggesting the conspecificity of both species. Plakina jamaicensis was most closely related to the Plakina trilopha/P. jani clade (97% average sequence identity, Figure 13). Corticium diamantense sp. nov. was most closely related to C. candelabrum from NW of Mediteranean Sea (98% sequence identity, Figure 13).

Fig. 13. Phylogenetic analysis of homoscleromorph relationships using mitochondrial cob sequences. Posterior majority-rule consensus tree obtained from the analysis under the HKY model in the MrBayes 3.2 program is shown. Two runs each with four independent chains were run for 5.0 million generations. The first 20% of these trees were discarded as burn-in. Convergence among the chains was monitored by comparison of maximum standard deviation of split frequencies for tree samples. The number at each node represents the Bayesian posterior probability. New species are indicated in bold.
DISCUSSION
The five new species reported here belong to worldwide distributed genera previously known, or at least mentioned as far as Oscarella nathaliae sp. nov. is concerned, from the Caribbean basin (Muricy & Diaz, Reference Muricy, Diaz, Hooper and van Soest2002).
The genus Oscarella includes 16 valid species known from different oceans around the world. Three additional indeterminate Oscarella sp. have been registered on the Brazilian coasts (Muricy & Moraes, Reference Muricy and Moraes1998; Muricy & Hajdu, Reference Muricy and Hajdu2006). Several authors mentioned the occurrence of Oscarella in the Caribbean without further details (Díaz & van Soest, Reference Díaz, van Soest, van Soest, van Kempen and Braekman1994; Rützler et al., Reference Rützler, Diaz, van Soest, Zea, Smith, Alvarez and Wulff2000; Díaz et al., Reference Díaz, Smith and Rützler2004; Díaz, Reference Díaz2005; Díaz & Rützler, Reference Díaz and Rützler2009). Oscarella nathaliae sp. nov. occurring in southern Martinique, northern Jamaica and Guadeloupe was already illustrated from the Bahamas but remained non-described (Zea et al., Reference Zea, Henkel and Pawlik2009). This species remarkably differs from other Oscarella species by its leaf-like thinly encrusting, flat body, loosely attached to the substrate and a perforated, not lobate surface. Oscarella nathaliae sp. nov. contains also two bacterial morphotypes and is characterized by two particular mesohylar cell types with inclusions.
Identification of Oscarella at the species level has always been tricky as this genus has no skeleton and as histological characters are generally unvaried (Boury-Esnault et al., Reference Boury-Esnault, Solé-Cava and Thorpe1992; Diaz & van Soest, 1994; Muricy et al., Reference Muricy, Boury-Esnault, Bézac and Vacelet1996; Muricy & Diaz, Reference Muricy, Diaz, Hooper and van Soest2002; Muricy & Pearse, Reference Muricy and Pearse2004; Ereskovsky, Reference Ereskovsky2006; Ereskovsky et al., Reference Ereskovsky, Borchiellini, Gazave, Ivanišević, Lapebie, Pérez, Renard and Vacelet2009a, Reference Ereskovsky, Sanamyan and Vishnyakovb; Pérez et al., Reference Pérez, Ivanišević, Dubois, Pedel, Thomas, Tokina and Ereskovsky2011). Several new species could recently be described thanks to ultrastructural and genetic methods. However, complementary tools appear necessary to further investigate Oscarella species diversity and phylogeny as a combination of molecular markers, biochemical fingerprints, symbiotic microbes investigation, and new morphological characters.
Nineteen species of the genus Plakortis are known throughout the world, four of which occur in the Caribbean Sea and more precisely in the Jamaican region: P. angulospiculatus, P. zyggompha, P, halichondrioides and P. simplex (Carter, Reference Carter1879; Schulze, Reference Schulze1880; de Laubenfels, Reference Laubenfels1934; Hechtel, Reference Hechtel1965; Boury-Esnault, Reference Boury-Esnault1973; Wiedenmayer, Reference Wiedenmayer1977; Pulitzer-Finali, Reference Pulitzer-Finali1986; Zea, Reference Zea1987; Mothes & Bastian, Reference Mothes and Bastian1993; Diaz & van Soest, 1994; Lehnert & van Soest, Reference Lehnert and van Soest1998; Moraes & Muricy, Reference Moraes and Muricy2003).
In his recent work on the genus Plakortis, Muricy (Reference Muricy2011) distinguished three groups of species based on spicule types and size: (1) P. simplex species group, including the nominal species and all other species with only diods and triods, with diods smaller than 190 µm long; (2) P. angulospiculatus species group, also with a nominal species complex and other species with only diods and triods, but with the largest diods varying between 190 µm and 300 µm long; and (3) P. lita species group, including species with microrhabds. Plakortis myrae sp. nov. is part of the P. lita species group because it is characterized by the presence of microrhabds. Plakortis edwardsi sp. nov. and P. dariae sp. nov. should be placed in the P. simplex species group, however, such placement is not supported by molecular data. Instead, P. edwardsi sp. nov. is grouped with P. halichondroides/P. angulospiculatus while P. dariae sp. nov. is grouped with P. simplex (Figure 13). Spicule size appears then to be a dubious character for species classification in the Plakortis genus and other characters are to be defined. As a side note, we originally identified P. dariae sp. nov. as P. angulospiculatus and its genetic distinctiveness lead to the ‘posterior’ recognition of morphological uniqueness (see also Blanquer & Uriz, Reference Blanquer and Uriz2007). Finally, our observation that P. halichondrioides and P. angulospiculatus share identical cob sequences supports the idea that at least some sponges assigned to P. angulospiculatus and P. halichondrioides are in fact conspecific (Diaz & van Soest, 1994).
In summary, the characteristics of the three new species found in Jamaica are consistent, and differ enough from the previously known Plakortis species to consider them as three different new species. A molecular phylogenetic analysis encompassing more Plakortis species will be necessary to investigate phylogenetic relationships inside of this large and complex genus.
Recent evidences based on nuclear ribosomal (18S and 28S) and mitochondrial coding sequences strongly support Plakina as a paraphyletic genus composed from at least two clades: B3 (including Plakina jani and P. trilopha) and B4 (including Plakina monolopha and P. crypta) (Gazave et al., Reference Gazave, Lapébie, Renard, Vacelet, Rocher, Ereskovsky, Lavrov and Borchiellini2010). The clade B3 is characterized by its convoluted brain-like surface, the presence of a well developed mesohyl, well-differentiated ectosome, large subectosomal cavities and a tetralophose calthrops, whereas all these characters are absent in the clade B4. The two clades were also recovered in phylogenetic analysis conducted for this study based on partial mitochondrial cob sequences. Furthermore, our molecular and morphological data show that Plakina jamaicensis is most closely related to the Plakina trilopha/P. jani clade.
The genus Corticium Schmidt, Reference Schmidt1862 includes six species with a wide-range distribution, from NW Mediterranean to Indian Ocean, Western Pacific (van Soest et al., Reference Soest, Boury-Esnault, Hooper, Rützler, de Voogd, Alvarez de Glasby, Hajdu, Pisera, Manconi, Schoenberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann and Díaz2013). Only one species was described from both the Atlantic Ocean and the Caribbean basin: C. quadripartitum Topsent, Reference Topsent1923. Corticium diamantense sp. nov. differs from C. quadripartitum and from other Corticium species by the presence of regular non-lophose calthrops of one size-class, very rare tetralophose calthrops and candelabra with the fourth actine ramified basally in 4–5 microspined rays.
Our results based only on a few exploratory dives show that the sponge biodiversity in cryptic habitats of the Caribbean Sea is still poorly known, particularly for Homoscleromorpha that deserve more investigations.
The low diversity of this group in the Caribbean might only be a consequence of the lack of exploration, among other in cryptic habitats, like underwater caves and tunnels. This is confirmed by a more complete study of the Caribbean sponges that we have in preparation.
IDENTIFICATION KEY FOR THE WESTERN ATLANTIC SPECIES OF HOMOSCLEROMORPHA
1. Inorganic (spicular) skeletal complement present…………2
— Inorganic (spicular) skeletal complement absent…………Oscarella nathaliae sp. nov.
2. Skeleton mainly composed of diods, triods, and/or calthrops in one size-class…………3
— Skeleton mainly composed of diods, triods and/or calthrops with a large size variation…………9
3. Lophose diods, triods, or calthrops complement the main skeleton of non-lophose spicules…………4
— Lophose spicules absent, microscleres (microrhabs) present in some species…………11
4. Heterolophose calthrops (candelabra) complement the main skeleton of non-lophose spicules present…………5
— Lophocalthrops with one to four homogeneously ramified actines complement the main skeleton of non-lophose spicules present; candelabra absent…………6
5. Non-lophose calthrops present…………Corticium diamantense sp. nov.
— Non-lophose calthrops absent…………Corticium quadripartitum
6. Diods absent…………7
— Diods present…………8
7. Mono- and dilophose calthrops absent, tertalophose calthrops present…………Plakina tetralopha
— Monolophose calthrops present, tertalophose calthrops absent…………Plakina versatilis
8. Di-, tri- and tetralophose calthrops absent.Plakina elisa
— Di-, tri- and tetralophose calthrops present…………Plakina jamaicensis
9. Skeleton composed of non-lophose diods, triods and/or calthrops in three size-classes…………10
10. Massive or thick encrusting, light brown, with large diods (22–230 µm) and large calthrops (33–152 µm)…………Plakinastrella onkodes
— Encrusting, black or grey externally, with small diods (15–130 µm) and small calthrops (10–50 µm)…………Plakinastrella microspiculifera
11. Microscleres vermiform microrhabds present…………12
— Microscleres absent…………13
12. Triods present…………Plakortis myrae sp. nov.
— Triods absent…………Plakortis microrhabdifera
13. Diods of two size-classes…………14
— Diods of one size-class…………15
14. The ectosomal skeleton has a loose confused arrangement…………Plakortis edwardsi sp. nov.
— The ectosomal skeleton is distinctly reticulate…………P. dariae sp. nov.
15. Sponge thinly encrusting (15 mm thick or less), diods less than 150 µm long…………16
— Sponge massively encrusting (more than 15 mm thick), diods large (up to 220 µm long)…………17
16. Diods up to 150 µm long, consistency compressible but resistant…………Plakortis zyggompha
— Diods smaller than 100 µm, consistency very soft…………Plakortis insularis
17. Colour black or dark brown externally and internally, releases a dark exudate in alcohol…………Plakortis halichondrioides
— Colour variable, light or dark brown, often with dark patches or with greenish tinges, but never black; no dark exudate released in alcohol…………Plakortis angulospiculatus
ACKNOWLEDGEMENTS
We express our gratitude to Professors M. Jangoux and P. Flamang, Laboratoire de Biologie Marine of the University Mons Hainaut, for their cordial reception in the TEM facilities under their care. Professors E. Pays and D. Pérez-Morga are also thanked for welcoming us in the Centre for Microscopy and Molecular Imaging of the Université Libre de Bruxelles. Professor J. Billen, Laboratory for Entomology, Katholieke Universiteit Leuven gave Ph.W. the opportunity to work in his TEM facility as well. We are indebted to L. Despontin and J. Cillis, Royal Belgian Institute of Natural Sciences, for their assistance, respectively, with sample preparation and SEM technical support. P. Gayle and T. Edwards are thanked for buddy diving assistance. A. Ross gave us permission to dive and collect samples in the Montego Bay Marine Park. The material from Guadeloupe has been collected during the KARUBENTHOS Expedition (Principal Investigator Philippe Bouchet), conducted in May 2012 by the Muséum national d'Histoire naturelle, Paris, and the Parc National de la Guadeloupe, Université des Antilles et de la Guyane and Université Pierre et Marie Curie. We also thank R. van Soest, N. de Voogd (Naturalis Biodiversity Center, The Netherlands) and A. Baldinger (Museum of Comparative Zoology, Harvard University, USA) for the kind loan of type specimens. This is contribution no. 733 of the Discovery Bay Marine Laboratory.
FINANCIAL SUPPORT
The Discovery Bay Marine Laboratory supported A.E. and made all necessary facilities available. The Belgian Federal Science Policy Office funded the work of A.E. at the Royal Belgian Institute of Natural Sciences (S & T Grant for collaboration with oriental and central Europe) as well as fieldwork in Martinique (CALMARS I-contract EV/03/04B). A travel grant from the Fonds Léopold III pour l'Exploration et la Conservation de la Nature to A.E. made possible our joint field work in Jamaica.