Summations
∙ Analysing group effects and responses to manipulations and treatments is the mainstay of modelling diseases, including affective disorders. Such work had major contribution to scientific exploration but is repeatedly criticised for problems with reproducibility and validity.
∙ Attention to individual variability in animal models of affective diseases and their treatments may provide an important tool to gain further understanding into the underlying biology of disease and treatment and provide better predictive validity for novel treatments.
∙ Some relatively simple procedures may indicate situations where it is advantageous to emphasise individual variability.
Considerations
∙ It is possible to suggest that in the context of psychiatric disorders, the best we can study in animals is variation in levels of normal adaptive behaviours rather than clinically diagnosed pathologies.
∙ Considering that the underlying biology of psychiatric disorders is not clear, the models lack construct or even aetiological validity. Consequently, even attention to individual variability may not result in significantly better understanding of the mechanisms related to the diseases.
∙ Whereas individual variability in behaviour is clear and easy to examine and measure, there is a major question regarding the consistency or stability of the variability and its relationship with any consistent physiological changes.
Introduction
General
Individual differences in emotional behaviour in people and animals are well known and their underlying physiology is studied for years using many approaches. For example, detailed exploration of brain areas related to emotion was performed in animal models (Reference Phelps and LeDoux1) and humans (Reference van der Werff, van den Berg, Pannekoek, Elzinga and van der Wee2). Such studies had a significant contribution to the understanding of the neural substrates of emotion. Genetic and molecular studies explored interesting aspects of individual temperaments of people (Reference Cloninger, Svrakic and Przybeck3) with findings such as the involvement of the DRD4 receptor in novelty seeking or the serotonin transporter with anxiety-related traits or neuroticism (Reference Ebstein, Zohar, Benjamin and Belmaker4).
In that context, the aim of the present manuscript is to emphasise the need to examine individual differences between animals while using animal models related to affective disorders and their treatment. We will present some examples from previous studies and suggest some future directions.
Approaches to develop better animal models for affective disorders
One important approach to study mechanisms of disease in general and to develop better treatments is through the utilisation of appropriate animal models. Such models are also used in the study of major depression disorder (MDD) and bipolar disorder (BD) and their treatment (Reference Cryan and Slattery5,Reference Einat6). However, animal models in the context of affective disorders are repeatedly criticised as not being helpful enough in deciphering the underlying mechanisms of these complex diseases and not predictive enough to accurately anticipate drug effects in patients (Reference Agid, Buzsaki, Diamond, Frackowiak, Giedd, Girault, Grace, Lambert, Manji, Mayberg, Popoli, Prochiantz, Richter-Levin, Somogyi, Spedding, Svenningsson and Weinberger7–Reference Nestler and Hyman10). A number of approaches were suggested in the last decade to improve the validity, predictability and consequently the usefulness of animal models of affective disorders. These important approaches include (1) better emphasis on homology between models and diseases (Reference Insel11). For example, Kronfeld-Schor and Einat repeatedly suggest that considering the relevance of circadian rhythms to affective disorders it is advantageous to use diurnal and not nocturnal rodents as model animals for these disorders (Reference Kronfeld-Schor and Einat12,Reference Bilu, Einat and Kronfeld-Schor13). Another interesting idea within the same approach is the suggestion of Hendrie and Pickles that depression in humans might be an adaptive process in an environment with social organisation and selective pressures and that rats and mice are therefore not the ideal animals to model such issues (Reference Hendrie and Pickles14). (2) Stronger connections between models and biological hypotheses (Reference Cryan and Slattery5). (3) Modelling endophenotypes or domains of disease rather than attempting to model the entire scope of a disorder (Reference Einat6,Reference Gould and Gottesman15), an approach that fits well with the new concepts of research domain criteria (Reference Cosgrove, Kelsoe and Suppes16), and the matching idea to develop more tests that could have stronger relevance to any specific disease phenotype or possibly better translational value (Reference Malkesman, Scattoni, Paredes, Tragon, Pearson, Shaltiel, Chen, Crawley and Manji17,Reference Perry, Minassian, Paulus, Young, Kincaid, Ferguson, Henry, Zhuang, Masten, Sharp and Geyer18). Such tests can also be used together as a battery that will evaluate different domains of a disorder in one cohort of animals (Reference Cryan and Holmes19,Reference Stukalin and Einat20). Yet, one critical factor that was only lightly touched and needs more attention is individual variability.
Individual variability in affective disorders
Considering the immense variability in affective states and emotional behaviour in people, it is not surprising that significant variability is also described in individuals afflicted with affective disorders including MDD as well as BD. This group of disorders constitutes a major public health concern and is among the most debilitating diseases worldwide (21). A great degree of heterogeneity is shown between individuals in the course and symptoms of both MDD and BD as well as in the patterns of drug response. This significant variance in symptoms contributes to relatively high rates of misdiagnosis (Reference Vohringer and Perlis22). Moreover, individual differences account at least in part for the deficit in sound scientific data concerning the underlying pathophysiology of affective disorders as well as accurate prediction of response to treatment or identification of the optimal treatment strategy for each patient (Reference Fountoulakis, Kasper, Andreassen, Blier, Okasha, Severus, Versiani, Tandon, Moller and Vieta23,Reference Schumann, Binder, Holte, de Kloet, Oedegaard, Robbins, Walker-Tilley, Bitter, Brown, Buitelaar, Ciccocioppo, Cools, Escera, Fleischhacker, Flor, Frith, Heinz, Johnsen, Kirschbaum, Klingberg, Lesch, Lewis, Maier, Mann, Martinot, Meyer-Lindenberg, Muller, Muller, Nutt, Persico, Perugi, Pessiglione, Preuss, Roiser, Rossini, Rybakowski, Sandi, Stephan, Undurraga, Vieta, van der Wee, Wykes, Haro and Wittchen24). Recent research coming from clinical work suggests that important goals for future studies are the identification of neurobiological markers for the risk of developing affective disorders as well as markers predicting the response to therapy that can lead towards tailored interventions to specific subtypes of a disorder and specific individuals (Reference Schumann, Binder, Holte, de Kloet, Oedegaard, Robbins, Walker-Tilley, Bitter, Brown, Buitelaar, Ciccocioppo, Cools, Escera, Fleischhacker, Flor, Frith, Heinz, Johnsen, Kirschbaum, Klingberg, Lesch, Lewis, Maier, Mann, Martinot, Meyer-Lindenberg, Muller, Muller, Nutt, Persico, Perugi, Pessiglione, Preuss, Roiser, Rossini, Rybakowski, Sandi, Stephan, Undurraga, Vieta, van der Wee, Wykes, Haro and Wittchen24,Reference Hasler and Wolf25).
Attention to factors that induce variability
Scientists working with animal models of disease are well aware of the variability of behaviour between individual animals even when formal variables such as sex, age, weight, housing and so on are well controlled for. Such differences appear in baseline measures and when testing response to manipulations or treatments. However, individual variability within groups is usually considered a disturbing factor and efforts are invested to minimise it by different means such as standardisation of procedures and increasing the number of animals in groups (Reference Crabbe, Wahlsten and Dudek26,Reference Lewejohann, Zipser and Sachser27) or alternatively, meta-analysis studies (Reference Kara, Stukalin and Einat28). However, it might also be possible that instead of finding ways to overshadow heterogeneity, it can be utilised to get better insights into the underlying biology responsible for differential responses.
In the context of neuropsychiatric-related models, attention to individual variability in animal models can be of significant importance in at least two aspects, the variability in response to a variety of stimuli that are used to induce a disease-like state and the variability between animals in response to treatments.
Clear sources for variability
Whereas attention to individual variability in animal models research of affective disorders is relatively rare, some studies across the years were focused on individual animal performance. This increased attention towards the individual animal develops in parallel to the increasing interest in the individual patient and the introduction of personalised medicine in general and stratified medicine in affective disorders (Reference Hasler and Wolf25).
Strain
One approach that received significant attention was exploring differences between strains (Reference Flaisher-Grinberg and Einat29–Reference Sugimoto, Kajiwara, Hirano, Yamada, Tagawa, Kobayashi, Hotta and Yamada32) [for reviews see (Reference Einat6,Reference Jacobson and Cryan33,Reference Kara and Einat34)].
Sex
An important issue that was mostly ignored for many years but receives more attention in the last decade is the issue of sex differences (Reference Ene, Kara and Einat35–Reference Warner, Libman, Wooten and Drugan37) [for reviews see (Reference Bale and Epperson38–Reference Simpson, Ryan, Curley, Mulcaire and Kelly41)].
Origin of animals
An additional direction that received some attention but clearly needs further exploration is differences between animals of the same strain but from different colonies or suppliers. Such differences were demonstrated in the literature (Reference Juetten and Einat42–Reference Sparks, Sciascia, Ayorech and Chaudhri44). These differences are usually attributed to dissimilarity in the origin of the strain (Reference Juetten and Einat42) or other genetic changes across generations (Reference Pena-Oliver, Sanchez-Roige, Stephens and Ripley45) although phenotypic differences between mice of the same strain but different colonies may also stem from environmental factors.
Cycling hormones
Another important factor that induces individual variability is cycling hormones and hormonal state of the animal. In the same cohort of animals, we may find individuals that at a certain testing point are at different hormonal states and this may have significant effects on behaviour. The most obvious example is of course the oestrous cycle in females where levels of oestrogen and progesterone are significantly different across the cycle and these hormones are well known to influence behaviours in model animals (Reference Becker and Cha46,Reference Milad, Igoe, Lebron-Milad and Novales47). However, other hormones also have significant effects on behaviour and biology and can contribute to individual variability. For example, corticosterone in mice and rats, as well as cortisol in humans, have a circadian secretion pattern and therefore testing animals at different hours of the day (even if within the same phase of the light/dark cycle) may have influence on behaviour (Reference Verma, Hellemans, Choi, Yu and Weinberg48). The issue of hormonal state should therefore be considered when trying to evaluate individual variability in model animals.
Selection by breeding
One more approach that utilises individual differences is selection and breeding of subgroups of animals based on a specific response. There are some examples within the area of modelling affective disorders including the Flinders sensitive line (Reference Overstreet, Friedman, Mathe and Yadid49), stress reactivity mice (Reference Kara, Flaisher-Grinberg, Anderson, Agam and Einat50,Reference Touma, Bunck, Glasl, Nussbaumer, Palme, Stein, Wolferstatter, Zeh, Zimbelmann, Holsboer and Landgraf51); Rouen mice (Reference Bougarel, Guitton, Zimmer, Vaugeois and El Yacoubi52) and other important and interesting sub-strains [for partial review see (Reference Wegener, Mathe and Neumann53)]. Whereas such work is certainly valuable, we would like to suggest that the breeding approach is conceptually different from what we suggest below in the present paper.
Individual differences within group
Variability in baseline behaviours
Animals within a group that show higher activity levels or higher risk taking behaviour (reduced anxiety) when exposed to a novel environment or demonstrate higher reward seeking behaviours when they get access to rewarding stimuli may represent some facets of manic-like behaviour. It is possible that if we used only such animals when attempting to model mania (and not the whole cohort of animals) we will have a more representative and homologous model. Moreover, in the same way that the control population in clinical trials is composed of people that are not afflicted with the disorder, it may be possible to use animals that do not show ‘manic-like’ spontaneous tendencies as a control group. For example, a study by Cervantes and Delville (Table 1) (Reference Cervantes and Delville54) found individual variability in aggressive behaviour of hamsters and used this variability to explore some of the underlying neural bases of impulsive aggression by comparing the more aggressive to the less aggressive animals (Reference Cervantes and Delville54). Variability of responses to stimuli can also serve to select animals that have higher resemblance to the modelled disease. A slightly more complex approach is taken by Cohen and her colleagues who examine individual variability of delayed responses to acute stress (exposure to cat odor) in mice and rats and based on these responses are able to distinguish between susceptible and resilient animals and develop a highly useful model for post traumatic stress disorder (Table 1) (Reference Cohen, Zohar and Matar55,Reference Matar, Zohar and Cohen56). This possibility is demonstrated in studies from different areas. For example, an interesting and detailed demonstration of individual variability that might be relevant to depression was reported by Cohen and Kronfeld-Schor (Reference Cohen and Kronfeld-Schor57) (Table 1) that examined the circadian rhythms of golden spiny mice in different laboratory conditions. Whereas these rodents show diurnal activity patterns in the wild, the study showed that their adaptation to laboratory conditions is highly individualised with some animals showing diverse phase shifts (Reference Cohen and Kronfeld-Schor57). Large individual variability was also observed in reaction to a defeat stress in C57BL/6 mice (10 defeats over 10 days), as 24 h. after the last defeat (day 11) 40–50% of the population were found to be resilient (not displaying social avoidance) and the other mice being susceptible (Table 1) (Reference Krishnan, Han, Graham, Berton, Renthal, Russo, Laplant, Graham, Lutter, Lagace, Ghose, Reister, Tannous, Green, Neve, Chakravarty, Kumar, Eisch, Self, Lee, Tamminga, Cooper, Gershenfeld and Nestler62). Only susceptible mice showed increase in brain-derived neurotrophic factor in the nucleus accumbens as well as of downstream signalling molecules (Akt, Gsk-3beta, ERK1/2). Furthermore, in the same study, a genome-wide microarray analysis of gene expression in the ventral tegmental area (VTA) revealed strong differences among vulnerable and resilient mice: for example, in the VTA, 104 genes were downregulated in the resilient mice and 35 genes were upregulated in the vulnerable mice, but only two of these genes overlapped in the two populations (Reference Krishnan, Han, Graham, Berton, Renthal, Russo, Laplant, Graham, Lutter, Lagace, Ghose, Reister, Tannous, Green, Neve, Chakravarty, Kumar, Eisch, Self, Lee, Tamminga, Cooper, Gershenfeld and Nestler62). Such individual variability can seem rather surprising among groups of inbred strains, as they theoretically display minimal genetic variability (they are supposed to be homozygotes at all loci). However, animals are subjected to differences in their early environment, which can induce permanent epigenetic changes and thus behavioural variability. For example, rodents can receive high or low level of mothering behaviour. High level of mothering behaviour induces high levels of transcription factor nerve growth factor-inducible protein A in the hippocampus. This change in turn decreases methylation of the glucocorticoid receptor (GR) gene and thus induces higher expression, leading to low corticosterone in stressful situation as well as low anxiety behaviour and high mothering behaviour (Reference Feder, Nestler and Charney70).
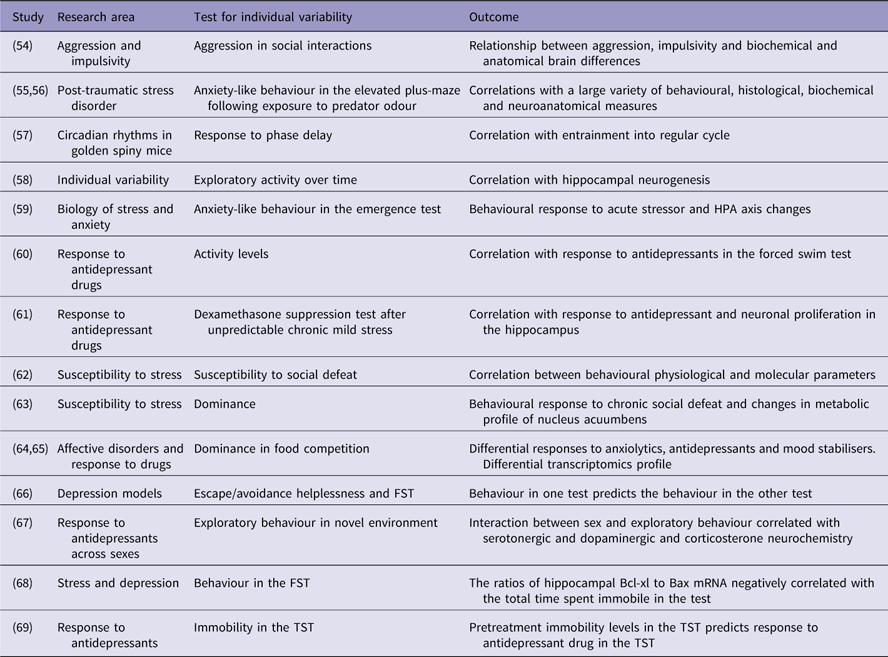
Table 1 Examples of studies that utilised individual variability
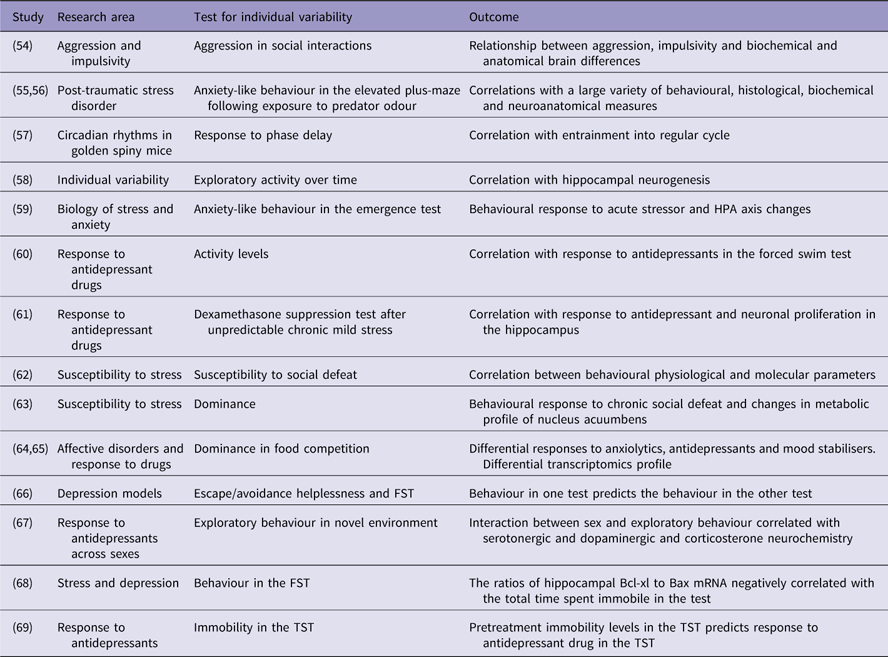
FST, forced swimming test; TST, tail suspension test.
Using individual variability to predict behaviour
Clearly, animals show large variability in their responses to stimuli but it is not clear how constant this variability is across and within animals. Can we expect the mouse that shows high activity levels in the home cage will also show high activity levels in a novel environment? Can we predict the behaviour of an animal in a behavioural test based on its behaviour in a different situation? Moreover, can we expect that there are sets of behavioural, physiological and biochemical variables that are related to each other in the variability of individual responses? Some data suggest that at least for a number of studies and variables this is indeed possible.
Selecting subgroups
Not many studies analyse behaviour of individual animals but there is some research based on the division of animals to subgroups based on a single overt feature. One interesting attempt related to research of affective disorders was done by Eva Malatynska and her colleagues who tested animals (rats and later mice) in a food competition paradigm and selected the most submissive and the most dominant subgroups (Table 1) (Reference Malatynska and Knapp64). Studies with these animals showed that behavioural differences are not just expressed in one specific test but that the submissive animals demonstrate a whole set of depression-like behaviours whereas the dominant animals exhibit a number of manic-like behaviours (Reference Malatynska and Knapp64). Moreover, some biochemical factors were found to be associated with the dominant and submissive subgroups suggesting possible new understanding of the underlying biology of bipolar disorder (Reference Pinhasov, Ilyin, Crooke, Amato, Vaidya, Rosenthal, Brenneman and Malatynska71). For example, the expression of Synapsin II isoform b (Syn IIb) is upregulated in the hippocampus and striatum of submissive mice (Table 1) (Reference Nesher, Koman, Gross, Tikhonov, Bairachnaya, Salmon-Divon, Levin, Gerlitz, Michaelevski, Yadid and Pinhasov65). This body of work represents the type of exploration that is possible when utilising initial variability within cohorts of animals. The selection of extreme dominant and submissive animals resulted in subgroups that could represent a better model for depression or for mania compared with the initial group that includes all animals. This new model based on selection allows better screening of possible treatments as well as mechanistic exploration of the differences between the subgroups that could be related to the disorders (Reference Malatynska and Knapp64,Reference Nesher, Gross, Lisson, Tikhonov, Yadid and Pinhasov72). Interestingly, a recent study that examined the dominant animals within a group of mice showed that against expectations, dominant mice demonstrate more anxiety-like behaviours compared with non-dominant animals (Table 1) (Reference Larrieu, Cherix, Duque, Rodrigues, Lei, Gruetter and Sandi63). Moreover, this study also demonstrates that the metabolic profile in the nucleus accumbens relates to social status and to the vulnerability to stress (Reference Larrieu, Cherix, Duque, Rodrigues, Lei, Gruetter and Sandi63).
In a different context, a group of researchers led by Hagit Cohen developed a model that is based on identifying subgroups within a cohort of rats or mice that are a unique model for susceptibility or resilience to the development of long-term effects following acute environmental stress (Table 1) (Reference Matar, Zohar and Cohen56). Animals are briefly exposed to an inescapable cat odor situation. When tested 10 days later, only about 1/3 of the animals show clear anxiety-like behaviours. These animals are defined as susceptible and can be compared with the ~1/3 of animals that show minimal anxiety-like behaviours and are defined as resilient (Table 1) (Reference Cohen and Zohar73). This model, established on subgrouping animals based on their initial response, was demonstrated to have great advantages in the exploration of the underlying mechanisms of chronic response to acute stress and was heavily used especially in the study of susceptibility and resilience to the development and expression of post-traumatic stress disorder (Table 1) (Reference Matar, Zohar and Cohen56,Reference Cohen, Kozlovsky, Alona, Matar and Joseph74). The behavioural phenotype was associated with biological differences. Gene expression in the periphery (blood) as well as in corticolimbic brain areas (amygdala and hippocampus) enabled to strongly differentiate vulnerable and resilient mice, particularly regarding transcription factors and GR-related signalling (Reference Daskalakis, Cohen, Cai, Buxbaum and Yehuda75).
Examination of individual animals: prediction of behaviour
Whereas the division of initial groups of animals into subgroups based on a behavioural or physiological response was already demonstrated to be helpful in the development of better and more homologous models, the possibility of examining individual animals is still relatively unexplored. However, a few studies that observed individual animals suggest that this may be an advantageous approach and that it might be possible to predict the response of a specific animal to a challenge based on its response to other challenges. For example, working with NIH-HS rats, researchers examined helplessness-like escape deficits in a shuttle box and behaviour in the forced swimming test (FST) and showed that at least for the extreme animals, the behaviour in one test predicts the behaviour in the other model (Table 1) (Reference Palencia, Diaz-Moran, Mont-Cardona, Canete, Blazquez, Martinez-Membrives, Lopez-Aumatell, Tobena and Fernandez-Teruel66).
Such predictions are not only related to behaviour but also to physiological, biochemical and molecular parameters. For example, anxiety-like responses in the elevated plus-maze (EPM) were found to be in correlation with hippocampal mRNA levels of the mineralocorticoid and glucocorticoid receptors as well as with corticosterone plasma levels (Table 1) (Reference Jakovcevski, Schachner and Morellini59) and rats that were relatively resilient in the FST (exhibited less immobility time) had significantly higher Bcl-xl/Bax ratios in the hippocampus (Table 1) (Reference Shishkina, Kalinina, Berezova, Bulygina and Dygalo68).
An interesting recent study further demonstrated that individual variability in inbred animals can also develop across time, even when housed in the same conditions (Table 1) (Reference Freund, Brandmaier, Lewejohann, Kirste, Kritzler, Kruger, Sachser, Lindenberger and Kempermann58). Moreover, one measure where differences evolved across time was complex exploration and the amount of such exploration in individual mice correlated with adult neurogenesis in the hippocampus (Reference Freund, Brandmaier, Lewejohann, Kirste, Kritzler, Kruger, Sachser, Lindenberger and Kempermann58).
Such results, showing that when examining individual animals it might be possible to predict some traits based on one trait have led to the idea that mice and rats also have ‘personality’, an individual profile of complex interactions between perception, emotion and behaviour that are related to individually unique biological factors (Reference Lewejohann, Zipser and Sachser27). Possibly, we can now introduce a new term, ‘mousality’.
One caveat of using one behavioural test to predict the behaviour in other tests is the need to perform more than one test on the same animal, at least one test for screening or subgrouping and another test related directly to the modelled phenomenon. It is clear that the exposure of animals to behavioural testing by itself affects the animal and needs to be considered as a factor in future work with the same animal. For example, repeated exposure to the FST results in behavioural changes not only in this specific test but also in other behavioural tests as well as to persistent changes in stress-related hormones (Reference Abel and Hannigan76). Whereas this issue of repeated testing should be considered, at least when comparing to previous work where animals experienced only one behavioural test, it is important to remember that much of the behavioural testing done today in rodent models comprised test batteries rather than individual tests. Test batteries were introduced for many reasons, conceptual as well as practical and ethical, and appear to be not less helpful than single tests, at least at the group level of data analysis (Reference Flaisher-Grinberg and Einat29,Reference Ene, Kara, Barak, Reshef Ben-Mordechai and Einat77–Reference Blanchard, Kaawaloa, Hebert and Blanchard79). It is of course important to keep this factor in mind and to be aware of the possible effects of any screening test. Some measures to minimise such effects and properly analyse repeated testing were provided in a number of papers (Reference Stukalin and Einat20,Reference Crawley80,Reference Bailey, Rustay and Crawley81).
Variability in the response to treatment
The standard approach in the utilisation of animal models to test treatments or drugs is to concentrate on group effects with less attention to individuals. This is not very different from most human studies where the initial question usually is whether the drug is better than placebo at the group level. However, in animals as well as in humans, one can see a whole range of responses within the same cohort where some respond strongly to treatment, some do not respond at all and some respond in a partial manner (Reference Schumann, Binder, Holte, de Kloet, Oedegaard, Robbins, Walker-Tilley, Bitter, Brown, Buitelaar, Ciccocioppo, Cools, Escera, Fleischhacker, Flor, Frith, Heinz, Johnsen, Kirschbaum, Klingberg, Lesch, Lewis, Maier, Mann, Martinot, Meyer-Lindenberg, Muller, Muller, Nutt, Persico, Perugi, Pessiglione, Preuss, Roiser, Rossini, Rybakowski, Sandi, Stephan, Undurraga, Vieta, van der Wee, Wykes, Haro and Wittchen24). When attention is given to individual responding, it may be possible to distinguish responders from non-responders and to explore the underlying mechanisms related to the response profile.
Hence, attention to the individual variability in response to drugs or treatments can help to gain further understanding of the underlying mechanisms of the response and of drug effects. The same approaches that were suggested above to explore variability in responses to a variety of stimuli can also be used to explore the variability of responses to drugs and treatments including exploration of different responses across strains and within strain as well as attempting to predict drug response based on undrugged traits (behavioural, biochemical or molecular).
Differences between strains and colonies in response to drugs
The dissimilarities in response to treatment across different strains of laboratory animals, rats or mice, was noted for years and explored as a practical approach to select the best strains for drug screening purposes. In the context of affective disorders and their treatment, a large study in mice compared the effects of two antidepressant drugs, the selective norepinephrine reuptake inhibitor desipramine and the selective serotonin reuptake inhibitor fluoxetine on FST behaviour in 11 common mice strains. The results show great variation in response across strains with seven strains responding to desipramine and only three strains responding to fluoxetine (Reference Lucki, Dalvi and Mayorga82). Such differences can relate to strain differences in the molecular targets of these drugs. For example, it was demonstrated that ICR, ddY and C57BL/6 mice were not sensitive to the antidepressant-like effects of the selective serotonin reuptake inhibitor fluvoxamine, while DBA/2 and BALB/c mice did respond. Interestingly, the 5-HT transporter (which is the target of fluvoxamine) enabled to distinguish these later strains from the former (Reference Sugimoto, Kajiwara, Hirano, Yamada, Tagawa, Kobayashi, Hotta and Yamada32). Some studies also compared the effects of the mood stabiliser lithium in different strains. In a study examining lithium’s antidepressant-like effect in the FST, only three out of eight inbred strains and one of three outbred strains tested responded to chronic administration of lithium (Reference Can, Blackwell, Piantadosi, Dao, O’Donnell and Gould83). Similarly, from eight strains of mice that showed amphetamine-induced hyperlocomotion, only in four strains lithium attenuated the amphetamine response (Reference Gould, O’Donnell, Picchini and Manji30).
As with spontaneous behaviours, even within strain, mice from different colonies sometimes show different response to drug treatment. Such differences is response to lithium were demonstrated in comparison between black Swiss mice from Taconic Farms and from Charles River (Reference Juetten and Einat42) as well as between ICR (CD-1®) mice from Harlan Israel compared with the same strain from Harlan USA (Reference Sade, Kara, Toker, Bersudsky, Einat and Agam84).
Individual differences within group in response to drugs
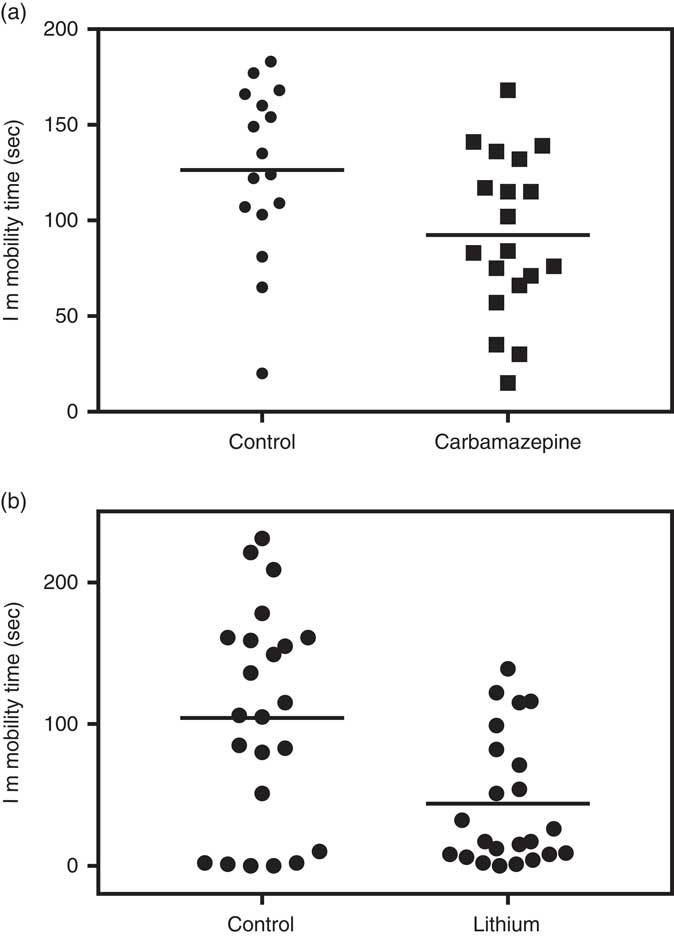
At the level of the individual animal, as with responses to environmental stimuli, response to drugs can also vary greatly within the same strain and colony. The variability of response can be easily demonstrated in most experiments testing drug effects when data are shown as a scatterplot of individual mice. For example, we previously demonstrated the group antidepressant-like effect of chronic carbamazepine administration in the FST but as shown in Fig. 1a, a scatterplot of individual animals shows a large individual variability in the response to treatment [group data previously published (Reference Kara, Karpel, Toker, Agam, Belmaker and Einat85)]. Moreover, at least for some measures, it is clear that the drug can change the heterogeneity of response to the behavioural challenge. For example, in a recent experiment in our laboratory (unpublished data) we evaluated the effects of chronic oral lithium administration in ICR male mice on some aspects of behaviour including the FST. As seen in Fig. 1b, the previously demonstrated group effect of lithium to reduce immobility was replicated but an additional effect of lithium was to reduce the variance compared with the control group (see legend for statistical analysis).
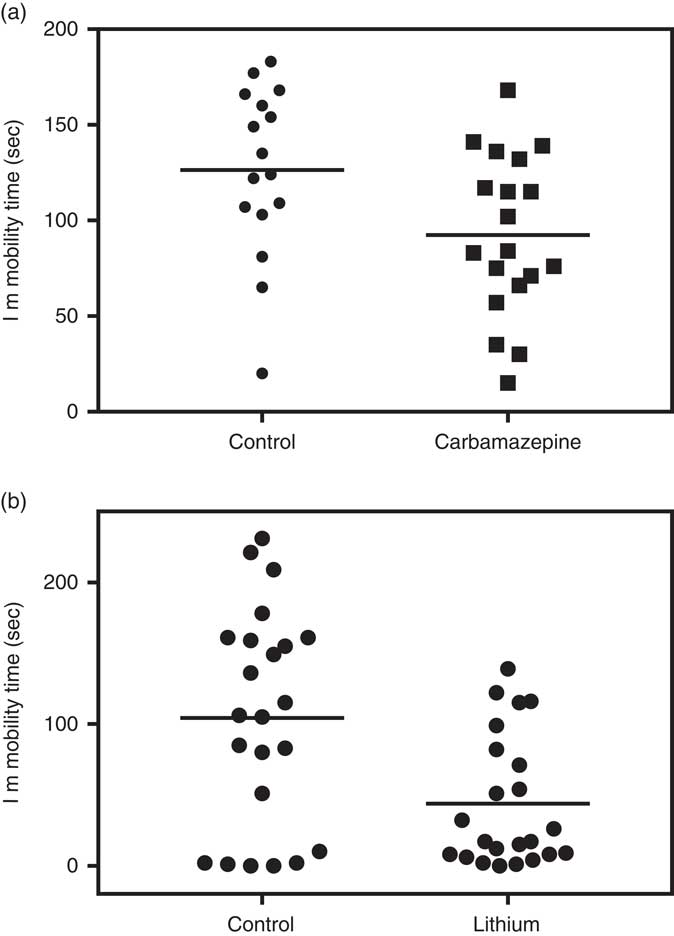
Fig. 1 Individual variability in immobility time of 12 weeks old, male ICR (CD-1®) mice in the forced swim test in the control (no treatment) groups and in mice treated with mood stabilising drugs. (a) Three weeks oral carbamazepine (10 weeks old, n=16–19/group). Whereas group effect is significant [t(33)=2.31, p=0.027], the scatterplot shows a large variety in the individual responses. (b) Three weeks oral lithium treatment (10 weeks old, n=23/group). Whereas group effect is significant [t(44)=3.26, p<0.01], the scatterplot shows a large variety in the individual responses and variance is larger in the control compared with the lithium group [Variance: Control=5804, Lithium=2131, Levene test: F(1,44)=6.26, p=0.016].
Predicting drug-response based on undrugged behaviour/biochemistry/genetics
However, as it relates to drug response, the practical question facing clinicians and scientists alike is can we find ways to predict drug response before starting treatment? Can we predict response based on undrugged behaviour or possibly biochemical or molecular biomarkers? Such findings can be of major importance in the attempts to develop personalised medicine for individuals afflicted with affective disorders.
Some work tried to predict drug response by separating animals into subgroups based on undrugged initial screening. For example, Khemissi et al. (Reference Khemissi, Farooq, Le Guisquet, Sakly and Belzung61) (Table 1) exposed mice to chronic mild stress (CMS), administered dexamethasone and subgrouped into high or low dexamethasone suppressors (HS and LS, respectively). Animals were then maintained in the CMS conditions but were now treated with fluoxetine before being evaluated in a number of tests for depression- and anxiety-like behaviours. The results of the study show that HS animals responded much better to fluoxetine compared with LS animals, suggesting that based on the dexamethasone suppression test it might be possible to predict the therapeutic-like effects of fluoxetine in models (Reference Khemissi, Farooq, Le Guisquet, Sakly and Belzung61). Interestingly, in this study, the effects of fluoxetine among the HS and LS mice were not solely different regarding the behavioural action of fluoxetine, but also regarding its effects on adult hippocampal neurogenesis. In a different study where animals were selected based on dominance or submission in a food competition test it was shown that paroxetine treatment reduced depression-like behaviour in submissive animals but induced extreme hyperactivity and complete lack of immobility in the forced swim test in dominant animals (Table 1) (Reference Nesher, Gross, Lisson, Tikhonov, Yadid and Pinhasov72). In addition, diazepam reduced anxiety-like behaviour (increased ratio between time in open and closed arms) in the EPM whereas the opposite happened in the dominant rats (Reference Nesher, Gross, Lisson, Tikhonov, Yadid and Pinhasov72). With a somewhat simpler screening system, Pitychoutis and colleagues (Reference Pitychoutis, Pallis, Mikail and Papadopoulou-Daifoti67) (Table 1) selected Sprague Dawley rats based on their rearing behaviour in a small arena, a behaviour that was suggested by the authors to represent spontaneous novelty seeking. When animals were later treated with chronic clomipramine and exposed to a number of tests for anxiety- and depression-like behaviour, the data showed that at least for males, clomipramine had stronger antidepressant – and anxiolytic-like effects in high novelty seekers (HR) compared with low novelty seekers (LR) (Reference Pitychoutis, Pallis, Mikail and Papadopoulou-Daifoti67). Using a somewhat similar approach Jama and colleagues (Reference Jama, Cecchi, Calvo, Watson and Akil60) (Table 1) selected Sprague Dawley rats based on their total activity levels in a novel environment and tested them in the FST after treatment with either desipramine or fluoxetine. Their results indicated that in the doses and schedules tested, fluoxetine had antidepressant-like effects in both high responder (active in screening test, HR) and low responder (less active in screening test, LR) rats but that treatment with desipramine was effective in LR animals only and not in HR rats (Reference Jama, Cecchi, Calvo, Watson and Akil60). Conceptually similar results were obtained in a study that separated mice to subgroups based on high versus low immobility in an undrugged tail suspension test (TST) and then re-tested the mice after treatment with either desipramine or imipramine (Table 1) (Reference Vaugeois, Passera, Zuccaro and Costentin69). The results of the study showed that drugs were effective only in the high immobility subgroup but not the low immobility mice. Moreover, the study suggests that using undrugged behaviour in the TST can also predict drugged behaviour in the FST (Reference Vaugeois, Passera, Zuccaro and Costentin69).
Further considerations
Strain differences and background strains for targeted mutations
Attention to differences between strains of mice or rats is emphasised for nearly 30 years now, since the beginning of the molecular revolution and the development of targeted mutations techniques in animal models. The progress in the ability to produce animals with specific mutations and utilise them to explore specific functions of genes and gene products in health and disease, also brought the realisation that not all background strains of mice were equal. It was therefore suggested that before starting to develop a mutant, one needs to carefully select the background strain (or strains) for the wild type population (Reference Adams, Fitch, Chaney and Gerlai86). This issue was even more critical in neuropsychiatric research where behaviour is central to the development and evaluation of disease models (Reference Willner87,Reference Belzung88). It was also realised that when developing targeted mutation-based models it is critical to evaluate the behavioural profile of the wild type and the mutant animals in a detailed manner (Reference Crawley and Paylor89). Whereas exploring strains became a mainstay in relevant research, it is somewhat surprising that only a relatively small number of researchers took the next step to also study subgroups within a strain or explore individual animals.
Outbred and inbred strains
Whereas the issue of individual variability relates to all species of animals that are used in research, we cannot ignore the fact that much of this research is done in mice and that some strains of mice are outbred and some are inbred. Whereas individual variability can be expected in outbred mice, there is a notion that when working with inbred strains the responses within the group should be relatively homogenous as these mice carry the same genes and (unless intentionally manipulated) grow up in the same environment. Yet, data suggest that even with inbred mice there is a significant variability in behaviour. For example, a study by Lucki and colleagues (Reference Lucki, Dalvi and Mayorga82) compares different strains of mice in the FST, with or without drug treatment. Whereas the study does not examine individual variability, the range of the variability is compared across strains using the available coefficient of variance. At baseline (no drugs), all strains, inbred and outbred, show significant variance. However, the largest variance is demonstrated in an inbred strain (FVB/NJ mice with 1.62), followed by an outbred strain (Swiss-Webster mice with 0.75), another inbred strain (CH3/HeJ mice with 0.71) and so on. An additional example is the study of Ducottet and Belzung (Reference Ducottet and Belzung90) where subgroups of ‘responding’ and ‘resistant’ mice are identified in two inbred strains, C57BL/6 and BALB/c. These two are just examples from a large body of studies and data that suggest that in fact the variability of inbred mice in behavioural tests may be similar to outbred mice.
Prediction or mouse ‘personality’
One critical factor for the interpretation of individual variability within a group of animals is that the variability is consistent, meaning that it can be possible to predict a profile based on one or a few variables. In fact, in order to effectively utilise individual variability in research it is expected that mice will show a type of personality-like profile or ‘mousality’. Indeed, such traits were already described in rodents as stable phenotypes (Reference Kalueff, Keisala, Minasyan, Kuuslahti and Tuohimaa91). Further, we can define pathology-like individual animals within a larger cohort only if some animals consistently show pathology-like traits compared with the others and do that in more than one parameter. One example where such data were shown in mice is in the context of stress response where it was demonstrated that it is possible to predict behaviour after chronic mild stress by examining pre-stress exploration of the EPM (Reference Ducottet and Belzung90). Another example of such data was shown in rats where spontaneous activity levels in an open field (high responders versus low responders) was demonstrated to predict a large set of behavioural and biochemical differences related to both stress and reward seeking (Reference Kabbaj, Devine, Savage and Akil92). This last example might be especially interesting because spontaneous activity levels are not directly related to depression or anxiety and therefore might represent some kind of temperament markers of the mouse. In humans, depression trait and temperament markers are considered more stable over the life span and could be associated with heritable biomarkers much more than the fluctuating or phasic symptoms of depression. For example, an interesting study suggests that at least for girls, high negative emotionality and low positive emotionality traits may have an effect on risk for depression (Reference Shankman, Klein, Torpey, Olino, Dyson, Kim, Durbin, Nelson and Tenke93) and other studies suggest that low reward function may be a stable characteristic of people who experience depression (Reference Forbes and Dahl94). The possibility to identify such stable traits in mice might also be true.
However, it is not clear that such coherent phenotypes are always shown and it is possible that sometimes the variability across animals in a group is also variable. Whereas such data are not usually reported (as the case is with other negative data), we have seen in at least some experiments that the behavioural profile of individual mice is not always coherent. For example, in a recent study we tested ICR mice for their behaviour in the FST and the tail suspension test and found that at least in the specific conditions of that experiment, there was no correlation between measures in one test and measures in the other test (unpublished data). It is therefore important to verify that the individual variability across animals in the same group is at least in part consistent.
Potential utilisation to study mechanisms
As mentioned above, the application of such knowledge can serve in two ways. One is that using only the relevant animals from the general cohort may result in a more relevant model. For example, if we are interested in a model for depression we may want to screen animals in the EPM and use only the ones that show higher anxiety-like behaviour while using animals with lower anxiety-like behaviour as their controls. Such separation to subgroups can be based on the data showing that the behaviour in the EPM predicts the response to chronic mild stress, a well-known manipulation to induce a depression-like model (Reference Ducottet and Belzung90). The other way to apply such individual data is to explore the underlying biological differences between the subgroups. For example, examination of gene expression in high responders versus low responders rats indicated that the expression of behavioural sensitisation to amphetamine may be related to the dopamine transporter but not to tyrosine hydroxylase (Reference Dietz, Tapocik, Gaval-Cruz and Kabbaj95). In that context, it is also important to examine the possibility that the source of variability could be related to relatively simple somatic differences between the mice in the group. For example, both ICR and black Swiss mice that were mentioned above were reported to develop retinal degeneration as they mature and the degree of blindness might be different across animals even at the same age (Reference Clapcote, Lazar, Bechard, Wood and Roder96,Reference Serfilippi, Pallman, Gruebbel, Kern and Spainhour97). Such a difference could explain at least some behavioural responses of individual mice and may be unrelated to any CNS relevant mechanisms of disease or treatment.
Potential utilisation for the development and selection of treatments
In the treatment context, attention to individual variability may provide us with means to predict effects and to gain further understanding into the biology of drug response. For example, the demonstration that the function of the HPA axis (evaluated in the dexamethasone suppression test) can predict response to fluoxetine (Reference Khemissi, Farooq, Le Guisquet, Sakly and Belzung61) might have direct implications to selection of treatment in patients as the screening procedure (dexamethasone suppression) is highly translatable to humans and a variation of such tests is already used to try and predict antidepressants response in patients (Reference Ising, Horstmann, Kloiber, Lucae, Binder, Kern, Kunzel, Pfennig, Uhr and Holsboer98).
Difficulties in the utilisation of individual variability approach
Whereas the advantages of attention to individual variability might be clear, it is not surprising that such differences are ignored in most studies. First, looking at individuals and selecting subgroups means using many more animals, and issues of ethics as well as of expenses. If the research question can be answered at the group level, there might not be a need to examine individual responses. Clearly, not all experiments demand attention to individual variability. For example, a general question such as the effects of environmental factors on neuronal growth (regardless of disease state) can be examined in a regular group of mice (Reference Kempermann, Kuhn and Gage99). Such broad effects that can be tested without regard to individuals may also be related to drugs. For example, one of the main theories of lithium’s therapeutic effects is the inositol depletion hypothesis and in that context, it is important to evaluate whether valproate also affects inositol metabolism. Because the effects of lithium on inositol are independent of disease state, the work can easily be performed in regular cohorts of animals with less interest in the individuals (Reference Shamir, Shaltiel, Greenberg, Belmaker and Agam100). However, it is suggested that for many other types of studies that involve models of affective disorders, attention to individuals might be a good way to get results that are more accurate.
Further limitations
Clearly, the utilisation of individual variability can increase the homology between the models and the disorders. Yet, other issues still exist that demand additional thoughts and possibly more approaches. Two important issues in modelling affective disorders that need to be addressed and are outside the scope of the current paper are the phasic nature of both depression and bipolar disorder and the cyclic nature of bipolar disorder. Attention to individual variability in relevant studies may be a significant improvement in the predictive values of models and in the ability to dissect factors of susceptibility and resilience. Yet, they will probably not offer any advantage for the attempts to increase the homology of models related to either the phasic nature of both depression and bipolar disorder or the cyclic nature of bipolar disorder. Other approaches could be examined that might be more appropriate for this purpose. For example, a recent study demonstrated that Mice with a mutation in the circadian Clock delta-19 gene exhibit rapid mood cycling with a manic-like phenotype emerging during the day following a state of euthymic-like behaviour at night. Similar effects were demonstrated after an optogenetic stimulation paradigm that produces an increase in dopamine neuronal activity in the VTA (Reference Larrieu, Cherix, Duque, Rodrigues, Lei, Gruetter and Sandi63).
Final remarks and conclusions
Animal models for affective disorders are less than ideal and this issue was repeatedly mentioned as a major bottleneck in the understanding of these devastating disorders and in the development of better and more efficacious treatments. We suggest that one important way to advance modelling is attention to differences between animals at a number of levels including strain, sex, supplier and especially individual variability within apparently homogeneous groups. Differences at the level of strains and sex already receive significant attention but we suggest that utilising differences between individual animals within a group will provide us with critical knowledge that will significantly advance the validity and usefulness of our models.
Because of the importance of this issue, we take the liberty to provide two relatively simple and practical recommendations that could be a first step towards the utilisation of individual differences in the study of neuropsychiatric disorders and their treatment. (1) Looking at the individual results of most studies can be as simple as drafting a scatterplot of the individual data points. Even such a simple procedure can be highly informative as it can visually suggest whether the population is relatively homogeneous or that it contains distinct subgroups. When such subgroups are identified, the researcher may want to consider how to treat these results. (2) When an experiment includes more than one test or measure, it is suggested that beyond the separate analysis of each of these tests, to also examine correlations across tests and evaluate whether the behaviour of a single animal in one test can predict the behaviour in additional tests. If this is the case then possible subgroups exist across tests and may reflect individual traits in specific animals. Such knowledge can be of assistance in selecting the best animals to model the disorder of interest or to study effects of treatments.
Acknowledgement
The authors would like to apologise to all the excellent scientists whose studies were not cited in this manuscript. This paper is designed as a prospective mini-review written mostly in order to attract attention to an important issue for future work and by no means an attempt to comprehensively cover all related work in the past. Work cited in the manuscript only represents some important examples needed to explain or emphasise specific points.
Authors’ Contribution: Itamar Ezer and Nirit Z Kara did most of the literature search related to this manuscript and prepared the original draft. Catherine Belzung and Haim Einat initiated the work and worked together to write the final manuscript.
Financial Support
This work was partially supported by a Grant in Aid of Research to HE from the Tel Aviv-Yaffo Academic College Research Fund. The funding committee was not involved in the study.
Conflicts of Interest
The authors declare no conflict of interests.