Historically, coronary arterial anomalies have been difficult to diagnose by non-invasive methods. With improvement in the acquisition of images, and the technology used to display them, the ability to identify the origins and courses of the coronaries has markedly improved over the last decade. Identification of coronary arterial origins is now a routine component of the standard paediatric echocardiogram.Reference Lai, Geva and Shirali1 Anomalies of origin account for just over 1% of all congenital cardiac malformations.Reference Yamanaka and Hobbs2 These diagnoses include anomalous origin from the pulmonary trunk, congenital atresia of the coronary artery, and anomalous aortic origin. Despite improved echocardiographic techniques, invasive investigation is still sometimes required for diagnosis. In this review, we discuss the value and limitations of echocardiographic diagnosis.
Development
Before the development of the coronary arteries, the loosely packed myocardium of the embryonic heart is nourished from the cavities by sinusoids. As the myocardium becomes more compact, arteries and veins develop within the epicardial layer, with the intramyocardial circulation developing within the myocardium itself. The key feature in development, however, is the growth of the arterial channels into two of the developing aortic valvar sinuses. It used to be thought that endothelial buds sprouted from the base of the developing arterial trunks to make contact with the epicardial network,Reference Gittenberger–de Groot and Poelmann3–Reference Hackensellner5 itself derived from the pro-epicardial organ.Reference Hutchins, Kessler-Hanna and Moore6 It was the elegant study of Bogers et al,Reference Bogers, Gittenberger-de Groot, Poelmann, Peault and Huysmans7 however, which showed that the arterial sprouts grew into the developing aortic sinuses, rather than growing out from the arterial roots. This concept of ingrowth provides a much more rational basis for understanding the myriad patterns taken by anomalous coronary arteries.
Anomalous coronary artery from the pulmonary arteries
Anomalous origin of the left coronary artery from the pulmonary trunk
The anomalous origin of a coronary artery from the pulmonary trunk was first described by Brooks in 1886, followed by a case report by Abbott in 1908. It was the description given by Bland et al,Reference Bland, White and Garland8 however, which provided the eponym for the now well-recognised clinical syndrome. This anomaly comprises approximately 0.5% of congenital cardiac anomalies.Reference Keith9 It is typically an isolated defect, but can be seen in association with other congenital cardiac malformations, such as ventricular septal defect, tetralogy of Fallot, and patent arterial duct. Although either coronary artery may arise from the pulmonary trunk, it is most commonly the left coronary artery that takes such an anomalous origin, branching in its usual manner to form the left anterior descending and circumflex arteries.
The description of clinical presentation given in 1933 by Bland et al was extremely accurate. At approximately 6–10 weeks of age, concomitant with the natural decrease in pulmonary vascular resistance, there is reversal of flow in the left coronary artery, such that the blood flows from the myocardium into the pulmonary trunk, the so-called “steal” phenomenon. This results in decreased myocardial perfusion, and is the impetus for the development of myocardial ischaemia and infarction. The typical clinical presentation includes respiratory distress, tachypneoa, diaphoresis, pallor, and failure to thrive. One of the pathognomonic features is colic, which occurs during feeds rather than after them. This finding is thought to be the result of angina-like chest pain that occurs with the stress and exertion of oral feeding. Some infants have brief syncopal episodes associated with exertional activities including feeding and defaecation. In addition to tachypnoea and tachycardia, which are present on physical examination, there is often a holosystolic murmur of mitral regurgitation. The papillary muscles are supplied by end arteries, and are vulnerable to ischaemia and infarction, resulting in mitral valvar incompetence. Occasional patients with the anomalous pulmonary origin of the left coronary artery will survive the neonatal period without significant symptoms because they have developed early collateral circulation from the right coronary artery, and thus have adequate flow to the myocardial territories supplied by the left coronary artery. In addition, the rare patient with associated congenital cardiac disease may have systemic pulmonary arterial pressures, and hence may not develop retrograde flow in the coronary artery.
The typical presentation of the isolated lesion in childhood or adulthood is a continuous murmur on physical examination, the murmur produced by the flow through collateral coronary arteries. Some patients complain of chest pain with exertion or exercise. Such a diagnosis may be made in a patient being assessed for an unrelated reason. Unfortunately, the first symptom can also be sudden or near sudden death.
Echocardiography
The diagnosis can be confirmed by using transthoracic echocardiographyReference Schmidt, Cooper, Silverman and Stanger10 albeit that this diagnosis can also easily be missed, even with a thorough and detailed examination. The anomaly, therefore, can be ruled in, but never be ruled out, by echocardiography. There are inherent limitations to the resolution of ultrasound that reduce the ability to distinguish the origin of the coronary artery with confidence, specifically because there can be ultrasonic dropout of the wall of the aorta adjacent to the transverse sinus and the wall of the left coronary artery. Since the advent of Doppler colour flow technology, this has been less of an issue. Importantly, if the coronary artery appears to arise from the aorta, but all other factors point towards anomalous origin from the pulmonary trunk, a different modality such as cardiac catheterisation, magnetic resonance imaging, or computerised tomographic imaging should be used to determine the origin of the left coronary artery. This is essential, since the anomalously arising coronary artery can be treated surgically, while aside from cardiac transplantation, dilated cardiomyopathy cannot.
Many echocardiographic features are not directly related to the visualisation of the coronary arterial origin. Infants usually present with a markedly dilated and poorly contractile left ventricle (Fig 1). The right ventricle often has better systolic performance. This finding can be confused with a myopathic process, such as cardiomyopathy or myocarditis. The timing of onset of dysfunction is a key feature. Any infant presenting with a dilated cardiomyopathy should be considered to have an anomalous origin of the left coronary artery from the pulmonary trunk until proven otherwise. There are many echocardiographic hints to the diagnosis. The myocardium often shows evidence of ischaemia, with a “shiny” appearance to the endocardium and papillary muscles. This can be readily seen in all views but particularly in the apical four-chamber (Fig 1) and parasternal views. Significant mitral regurgitation is almost invariable.

Figure 1 Off-axis apical four-chamber view in colour-compare mode demonstrating marked left ventricular dilation with echobright endocardium and papillary muscle. Colour flow mapping demonstrated significant mitral regurgitation from the papillary muscular dysfunction; LV = left ventricle; LA = left atrium.
The parasternal short-axis plane is one of the best views to visualise the origins of the coronary arteries. Should it arise from the pulmonary trunk, the left coronary artery then follows its normal path, often coursing quite close to the aorta, and sometimes even taking an intramural course through the aortic wall. Thus, it often appears to have a normal aortic origin (Fig 2). Cross-sectional imaging in isolation, on this count, may be inadequate to rule out the diagnosis. Colour flow mapping is quite helpful, but also has limitations. Lowering the Nyquist limit permits greater sensitivity for imaging the coronary arteries. When the colour scale is lowered to 18–50 centimetres per second, it is possible to assess the direction of coronary arterial flow. With pulmonary arterial origin, it is often possible to identify reversal of flow from the myocardium towards the pulmonary trunk, at least during part of the cardiac cycle. This is one of the most important findings suggestive of the diagnosis. In addition, as the lesion is physiologically a fistula, and there is increased collateral flow from the right to the left coronary arterial systems, a substantial increase is usually detected in the amount of flow across the minor coronary arteries. This flow, detected by the lowered Nyquist limit, is an important indirect diagnostic feature. In ideal circumstances, interrogation reveals the anomalous origin of the left coronary artery from the pulmonary trunk (Fig 3). This is more feasible when the arterial origin is located anteriorly from the pulmonary trunk. Typically, however, the origin is posterior or leftwards, just adjacent to the normal origin from the aorta. In some cases, off-axis parasternal short-axis views help identify the anomalous origin, and colour flow mapping reveals a jet emptying into the pulmonary trunk.

Figure 2 Parasternal short-axis view in colour-compare mode demonstrating the “fake out” that can occur when trying to visualise the origin of the left coronary artery. In this frame, the left coronary artery appears to arise from the aorta, and colour flow even suggests antegrade flow in at least part of the cardiac cycle. In fact, the artery arises from the pulmonary trunk. Still frame images should not be used to assess the origins of the coronary arteries; Ao = aorta; LCA = left coronary artery.

Figure 3 High parasternal short-axis view in colour-compare mode of the right ventricular outflow tract and pulmonary trunk demonstrating the leftward and anterior origin of the left coronary artery from the pulmonary trunk. Colour flow demonstrates flow from the artery filling the pulmonary trunk in retrograde fashion; RVOT = right ventricular outflow tract; MPA = pulmonary trunk; LCA = left coronary artery.
In contrast to those presenting as infants, late presentation is not usually associated with significant left ventricular dysfunction. It is perhaps because of a better-developed collateral arterial system, but also because of some narrowing of the left coronary arterial orifice as it enters the pulmonary root. In addition, any elevation of pulmonary arterial pressure allows better perfusion of the myocardium, such as is the case with patency of the arterial duct. Any diminution of pulmonary arterial pressure may cause acute symptoms.
Children presenting late are typically referred for an echocardiogram because of the finding of a continuous murmur, or a holosystolic murmur of mitral regurgitation on physical examination or during screening for an unrelated symptom. The most striking echocardiographic features in such patients are the dilation of the right coronary artery and the collateral arterial flow seen in the ventricular septum (Figs 4 and 5). The collateral flow is often misdiagnosed as multiple muscular ventricular septal defects. Distinction from ventricular septal defects can be made by pulsed-wave interrogation, which reveals continuous flow in collateral arteries as opposed to systolic flow across ventricular septal defects. In addition, no colour flow is seen entering the right ventricle in the presence of collateral arteries. When the right coronary artery is dilated, it is usually easily identified in the parasternal short-axis view, but can also be seen in other views, such as the apical four-chamber view. The right coronary artery has connections to the anomalously arising left coronary artery similar to fistulous connections, and therefore is often tortuous in its appearance. In those presenting at this age, the lesion may easily be misinterpreted as a coronary arterial fistula because, in the era of colour flow mapping, it is an easy matter to visualise the collateral arteries. In addition, the enlarged right descending coronary artery may have the appearance of a fistula. There is variable mitral regurgitation on presentation, and often there is no significant evidence of left ventricular or papillary muscular ischaemia.
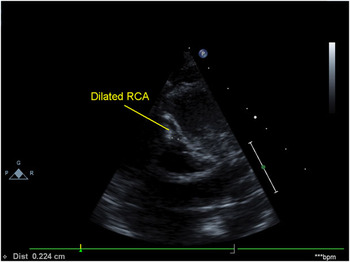
Figure 4 Parasternal short-axis view demonstrating a dilated right coronary artery in a patient with the anomalous origin of the left coronary artery from the pulmonary trunk; RCA = right coronary artery.

Figure 5 Subxiphoid sagittal view in colour-compare mode demonstrating significant coronary collateral flow in the ventricular septum in a child with a late presentation of the anomalous origin of the left coronary artery from the pulmonary trunk; RV = right ventricle;, LV = left ventricle.
Surgical intervention
In the past, the anomalous coronary artery was often ligated, with the hope that the collateral circulation would provide adequate oxygenation to the myocardium. This had variable results, with high early and late rates of death.Reference Bunton, Jonas, Lang, Rein and Castaneda11 In more recent years, the anomalous left coronary artery is usually either re-implanted, provided with a bypass graft, or transposed to the aorta using the Takeuchi procedure. The latter procedure entails creation of an aortopulmonary window, thus tunnelling the artery to the aorta through the pulmonary trunk without needing to translocate the artery itself (Fig 6). This surgical procedure is advantageous if patients have a very anterior origin of the left coronary artery from the pulmonary trunk.

Figure 6 Parasternal short-axis view in a patient who has had the Takeuchi procedure. The tunnel from the pulmonary trunk to the aorta is seen in cross-section in this view; Ao = aorta; PA = pulmonary trunk.
Immediate post-operative imaging is sometimes warranted using transoesophageal echocardiography. Even as the patient is separated from the cardiopulmonary bypass, the calibre of the coronary arteries may change. Whereas the right coronary artery is usually larger than the left prior to the operation, once the left coronary artery has been connected to the aorta, it often becomes the larger vessel. This relates to the changing volumes of flow in the dependent myocardium.
In the long term, echocardiography after surgical repair is needed to assess the recovery of left ventricular performance, as well as the resolution of mitral regurgitation. In some, the mitral regurgitation remains severe, and valvoplasty or valvar replacement may be required. Diastolic function may not recover because of ischaemia and fibrosis (Fig 7), and should be assessed over the long term using tissue Doppler and newer modalities such as strain and strain rate imaging. The Takeuchi procedure may result in unique complications, including leaks across the baffle, and supravalvar pulmonary stenosis due to the baffle obstructing the pulmonary trunk. The flow of coronary arterial blood can be assessed after re-implantation or the Takeuchi procedure using colour and pulsed-wave Doppler echocardiography.

Figure 7 Off axis apical four-chamber view demonstrating persistent infarct of the papillary muscle. Note the outline of fibrosis; LV = left ventricle.
Anomalous origin of the right coronary artery from the pulmonary trunk
Anomalous pulmonary origin of the right coronary artery is much less common, and typically has a more benign course. It is often diagnosed later, typically in childhood or early adulthood after a cardiac murmur has been recognised. Patients are usually asymptomatic, or have other associated congenital diseases requiring surgical treatment. It is easier to recognise the pulmonary truncal origin of the right coronary artery, since retrograde flow into the pulmonary trunk is typically seen (Fig 8). The left coronary artery is usually enlarged and tortuous (Fig 9) because of the collateralisation to the right coronary artery, and collateral arteries are seen in the ventricular septum as with anomalous origin of the left coronary artery. Left ventricular performance is usually normal, and right ventricular performance may be diminished or normal. Significant tricuspid regurgitation is not a common feature. Surgical intervention usually entails re-implantation of the anomalous artery.
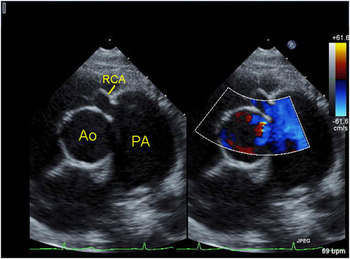
Figure 8 Parasternal short-axis view in colour-compare mode demonstrating the anomalous origin of the right coronary artery from the pulmonary trunk. The right coronary artery is seen entering the pulmonary trunk just above the pulmonary valve. Colour flow mapping demonstrates that the flow is from the artery into the pulmonary trunk, which is dilated as a result of this flow; Ao = aorta; PA = pulmonary trunk; RCA = right coronary artery.

Figure 9 Parasternal short-axis view in the same patient with the anomalous origin of the right coronary artery from the pulmonary trunk demonstrating a dilated left coronary artery that provides collateral flow to the right coronary artery; Ao = aorta; PA = pulmonary trunk; RCA = right coronary artery; LCA = left coronary artery.
Congenital coronary arterial atresia
Congenital atresia of the left coronary artery is extremely rare. In this anomaly, the left coronary arterial system has a normal epicardial course, but ends blindly, usually in close proximity to the aorta. The left coronary arterial bed is supplied by the right coronary artery. This disorder, ironically coined CALM syndrome for Congenital Absence of the Left Main, must be distinguished from a single right coronary artery, the latter arrangement carrying a more benign course in most cases. Almost uniformly, patients with atresia of the arterial orifice develop cardiac symptoms similar to the infantile presentation of the Bland–White–Garland syndrome, in other words, colic with feeds, tachypneoa, and tachycardia. Rarely, the patients do not present until adolescence or adulthood, when they can suffer syncope, tachyarrhythmias, or symptoms of myocardial infarction. Unfortunately, sudden death may be the presenting feature. Associated lesions are unusual, but may include supravalvar aortic stenosis and ventricular septal defect.Reference Musiani, Cernigliaro, Sansa, Maselli and De Gasperis12
The echocardiographic findings are very similar to those of anomalous origin of the left coronary artery from the pulmonary trunk, except that the left coronary artery does not arise from the pulmonary trunk. Again, it must be emphasised that the artery may appear to arise from the aorta, especially when its atretic end is in close approximation to the left coronary aortic sinus (Fig 10). Demonstration of flow in the coronary artery is extremely helpful, when retrograde flow in at least part of the cardiac cycle should arouse suspicion (Fig 11). The myocardium may also have the abnormal appearance of non-compaction (Fig 12). If other findings suggest an anomalous origin, but the sinusal origin cannot be confirmed, cardiac catheterisation may be necessary to make the diagnosis.

Figure 10 Parasternal short-axis view in a patient with left coronary ostial atresia. The left coronary artery appears to originate normally from the aorta; Ao = aorta; RVOT = right ventricular outflow tract; LCA = left coronary artery.

Figure 11 Parasternal short-axis view in colour compare mode in the same patient with left coronary ostial atresia. The colour flow in the artery suggests retrograde flow, which should alert the echocardiographer that it may not be connected to the aorta; Ao = aorta; RVOT = right ventricular outflow tract.

Figure 12 Subxiphoid left anterior oblique view demonstrating abnormal myocardium in the left ventricle suggestive of non-compaction in a patient with left coronary ostial atresia; RA = right atrium; RV = right ventricle; LV = left ventricle, Ao = aorta.
Surgical intervention is indicated once the diagnosis is made, because of the high risk of sudden death. Revascularisation may be achieved by coronary arterial bypass grafting, or in the case of most children, by reconstructing a left coronary arterial orifice within the aortic sinus. Recovery of ventricular performance is determined by the severity of infarction that has already occurred. Systolic and diastolic dysfunction may persist after revascularisation.
Anomalous aortic origin of the coronary arteries
Anomalous origin of the left coronary artery from the right coronary sinus
The anomalous origin of the main stem of the left coronary artery from the right coronary sinus is rare, but of great clinical importance, because it has been associated with sudden cardiac death in children and adolescents.Reference Roberts13–Reference Barth and Roberts16 After its anomalous origin, the left main coronary artery may take one of the four different routes. It can run anterior to the pulmonary trunk, posterior to the aorta, through the tissue plane between the arterial trunks, or through the crest of the muscular ventricular septum.
It is those in whom the artery takes a course between the arterial trunks who are at greatest risk for sudden death. The mechanism of such sudden death is acute myocardial ischaemia, produced by reduced flow in the anomalously arising artery. This occurs most often during exercise, when myocardial demand for oxygen is at its highest, and the increased stroke volume causes outward expansion of the roots of both arterial trunks. The mechanism of obstruction to flow seems to be a combination of compression of the intramural portion of the coronary artery itself, compression of the artery between the aorta and the pulmonary trunk, and worsening of the intrinsic narrowing or kinking at the anomalous ostium.Reference Roberts13
The most common presenting symptoms of individuals with anomalous left main coronary artery arising from the right coronary sinus are syncope, pre-syncope, and chest pain associated with exercise. Unfortunately, as has been found even in professional athletes, the first real symptom may be sudden death. In fact, coronary arterial anomalies, including anomalous origin of the main stem of the left coronary from the right coronary aortic sinus, join hypertrophic cardiomyopathy as the most common cardiac anomalies associated with sudden cardiac death in adolescents and young adults. Exercise stress testing of patients with this anomaly may show myocardial ischaemia, but also may be completely normal. Thus, any evaluation of exercise-related syncope, pre-syncope, or chest pain must include a definition of coronary arterial anatomy, and echocardiography is most often the imaging modality of choice.Reference Chu and Cheitlin17
The definition of the origins and proximal courses of the anomalous coronary artery may often be achieved with echocardiography. The parasternal short-axis view at the base of the heart is the best view to appreciate both the relationship of the origin of the left coronary artery to the zone of apposition between the aortic valvar leaflets guarding the coronary aortic sinuses, its often intramural course, and its relationship to the right ventricular outflow tract. On account of the extreme translation of the coronary arterial origins during the cardiac cycle, the excellent temporal resolution of echocardiography, compared with that of other non-invasive techniques, makes this the diagnostic technique of choice in most cases. However, this diagnosis is often difficult to make in real time. Thus, obtaining a loop that is one to several heartbeats in duration, and then playing this loop back slowly, is often of great help in visualising the anomalous coronary artery, as well as discerning its relationship to the zones of apposition between the aortic valvar leaflets. Colour Doppler should be used to identify any intramural segment of the main stem. In fact, an abnormal jet seen during diastole over the space between the aorta and the pulmonary trunk is often the first indication of an abnormal coronary arterial course (Fig 13). The inter-arterial course can often be seen to advantage in the leftwardly angled parasternal long-axis view.

Figure 13 Parasternal short-axis view in colour-compare mode in a patient with an anomalous aortic origin of the left coronary artery from the right coronary aortic sinus. Although the origin is not well visualised by cross-sectional imaging in this still frame, colour flow mapping suggests that an artery courses from the right, between the aorta and the pulmonary trunk to the left; Ao = aorta; RVOT = right ventricular outflow tract; LCA = left coronary artery.
In older patients with slower heart rates, both magnetic resonance imaging and multislice computed tomography have proven useful as well.Reference Bunce, Lorenz and Keegan18, Reference Deibler, Kuzo and Vohringer19 Identification of the coronary arterial orifice and its anatomy can be challenging using any modality, but may be better accomplished with the latter techniques, particularly when trying to identify whether there are one or two orifices. If any question remains, coronary angiography is often recommended, although this technique does not allow for completely non-ambiguous delineation of the proximal course of the coronary artery.
Anomalous origin of the right coronary artery from the left coronary sinus
Until recently, origin of the right coronary artery from the left coronary sinus was considered a benign variant. It is reported less often than anomalous origin of the main stem of the left coronary artery from the right coronary sinus, but its true incidence is probably greater.Reference Roberts13, Reference Brandt, Martins and Marcus20 It is common for the anomalous right coronary artery to pass between the arterial trunks, although other courses are possible. Such an inter-arterial course provides the anatomic substrate for myocardial ischaemia in an identical manner to that with the anomalous origin of the left coronary artery from the right coronary aortic sinus. Although presentation with sudden cardiac death not preceded by other symptoms is less likely with this anomaly than with the anomalous origin of the left coronary artery from the right coronary aortic sinus, up to one-fourth of individuals with an anomalous right coronary artery found at autopsy died suddenly and unexpectedly, and almost one-third of them died of cardiac causes.Reference Taylor, Rogan and Virmani21 Operative intervention for the anomalous origin of the right coronary artery from the left sinus in asymptomatic patients remains controversial, but is performed in a number of institutions.Reference Frommelt, Frommelt, Tweddell and Jaquiss22, Reference Gulati, Reddy and Culbertson23
The anomaly is most often diagnosed by echocardiography, either as an unexpected finding or as part of an evaluation for a patient with chest pain or exercise-induced syncope. It is found using the same principles as for the anomalous origin of the left coronary artery from the right coronary aortic sinus, in that the parasternal short-axis view allows determination of the anomalous origin and proximal course of the right coronary artery (Fig 14). Without careful delineation, the right coronary artery can appear to take its normal origin from the right coronary aortic sinus (Fig 15). When the right coronary artery arises from the left coronary aortic sinus, it can also take an unusually high origin at or slightly above the sinutubular junction. With such a high origin, take-down of the inter-coronary commissure during an unroofing procedure is usually not necessary. Thus, identification of this feature is surgically important.Reference Taylor, Rogan and Virmani21, Reference Gulati, Reddy and Culbertson23

Figure 14 Parasternal short-axis view demonstrating anomalous aortic origin of the right coronary artery from the left coronary aortic sinus. The right coronary artery can be seen coursing between the arterial trunks; Ao = aorta; RVOT = right ventricular outflow tract; RCA = right coronary artery; LCA = left coronary artery.

Figure 15 Parasternal short-axis view in the same patient with anomalous aortic origin of the right coronary artery from the left coronary aortic sinus demonstrating that the right coronary artery can appear to have a normal origin (see crossmarks in right coronary aortic sinus); Ao = aorta, RVOT = right ventricular outflow tract.
Identifying the origin in relation to the sinutubular junction is best accomplished in the parasternal long-axis view or in a long-axis transoesophageal echocardiographic view. Transoesophageal echocardiography is the imaging modality of choice if such an anomalous aortic origin is suspected but not definitively diagnosed by transthoracic imaging.
Surgical intervention
In patients who have had an unroofing procedure for repair of the anomalous aortic origin of a coronary artery, it is necessary post-operatively to evaluate for aortic insufficiency due to potential distortion of the aortic valve. Interrogation should also demonstrate flow from the appropriate aortic sinus into the coronary artery.Reference Gulati, Reddy and Culbertson23
Important pitfalls to avoid in echocardiographic imaging of coronary arteries
One commonly performed practice that should be stringently avoided when imaging coronary arteries is that of obtaining still frames of the arterial origins, and making diagnostic decisions based upon these still images. With the anomalous aortic origin of a coronary artery from the pulmonary trunk or the aorta, the proximal coronary artery often approaches the appropriate sinus of Valsalva very closely, and resolving the thin tissue between the coronary arterial and the aortic lumens can be difficult or impossible in a still frame image. It is easy, therefore, to be falsely assured of a normal origin of the coronary artery when, in fact, the coronary artery arises anomalously from another location. This is particularly true of the right coronary artery (Fig 15).Reference Jaggers and Lodge24
Conclusions
Imaging of the coronary arteries with high-resolution ultrasound usually provides adequate imaging of their origins and proximal course. In younger patients, this can be achieved with high-resolution transthoracic echocardiography through the pre-cordial windows, whereas in older and bigger children, transoesophageal imaging may also be indicated. Whenever there is doubt as to the definition and, indeed, when there is serious clinical concern that a coronary artery has an anomalous origin, other testing, such as cine-computed tomography, magnetic resonance imaging, or cardiac catheterisation may be indicated for confirmation or to provide greater anatomic detail.

















