Introduction
The genus Ericthonius has a complicated taxonomic history, principally due to the lack of clear interspecific differences in females, and to intraspecific variability of male gnathopod 2 according to growth stage. As a result, Ericthonius species have undergone a series of erroneous synonymizations and consequent mis-identifications (Myers & McGrath, Reference Myers and McGrath1984; Chapman, Reference Chapman and Carlton2007; Beermann & Franke, Reference Beermann and Franke2011; Krapp-Schickel, Reference Krapp-Schickel2013; Marchini & Cardeccia, Reference Marchini and Cardeccia2017). Some species within this genus, namely E. brasiliensis (Dana, Reference Dana1853), E. difformis H. Milne Edwards, Reference Milne Edwards1830, E. pugnax (Dana, Reference Dana1852), E. punctatus (Spence Bate, Reference Spence Bate1857) and E. rubricornis (Stimpson, Reference Stimpson1853), have been reported to have a very wide geographic distribution. In many instances, these are likely cases of ‘pseudocosmopolitanism’ (sensu Darling & Carlton, Reference Darling and Carlton2018), i.e. illusory effect of synonymization/mis-identification, but some geographically disjunct records of Ericthonius species can also be attributed with a high degree of confidence to human-mediated dispersal (Marchini & Cardeccia, Reference Marchini and Cardeccia2017).
Due to the complexity and the similarities between the species, the genus Ericthonius has been widely revisited (Myers, Reference Myers and Ruffo1982; Myers & McGrath, Reference Myers and McGrath1984; Lowry & Berents, Reference Lowry and Berents1996). Many misidentifications have occurred, and some disagreement exists between authors on the validity of some species. Fundamentally, 21 Ericthonius species are considered valid globally (Horton et al., Reference Horton, Lowry, De Broyer, Bellan-Santini, Coleman, Corbari, Costello, Daneliya, Dauvin, Fišer, Gasca, Grabowski, Guerra-García, Hendrycks, Hughes, Jaume, Jazdzewski, Kim, King, Krapp-Schickel, LeCroy, Lörz, Mamos, Senna, Serejo, Sket, Souza-Filho, Tandberg, Thomas, Thurston, Vader, Väinölä, Vonk, White and Zeidler2019), nine of those occurring in European waters: E. argenteus Krapp-Schickel, Reference Krapp-Schickel1994 (not 1993), E. brasiliensis, E. didymus Krapp-Schickel, Reference Krapp-Schickel2013, E. difformis, E. fasciatus (Stimpson, Reference Stimpson1853), E. megalops (G.O. Sars, Reference Sars1879), E. punctatus, E. rubricornis, E. stephenseni Myers & McGrath, Reference Myers and McGrath1984. The present paper deals with the first records of E. didymus outside its type locality, with additional taxonomic descriptions (accessory flagellum and epimeral plate), and provides an identification key for all Ericthonius species with a focus on three closely related species: E. didymus, E. convexus Ariyama, Reference Ariyama2009 and E. pugnax.
Materials and methods
Study area: Arcachon Bay
Arcachon Bay (Figure 1) is a 180 km2 macrotidal coastal lagoon, connected to the Atlantic Ocean by a narrow channel and receiving freshwater inputs in its south-eastern part (Leyre River). This lagoon is characterized by large intertidal flats (115 km2) covered by Zostera noltei Hornemann, Reference Hornemann1832 seagrass beds (70 km2) (Plus et al., Reference Plus, Dalloyau, Trut, Auby, de Montaudouin, Emery, Noel and Viala2010). The lower parts of the tidal flats are mainly occupied by oyster farms (10 km2) (Pacific oysters, Magallana gigas (Thunberg, Reference Thunberg1793) introduced from Japan). In the inner lagoon, tidal channels represent 71 km2, with 1.02 km2 occupied by eelgrass beds (Zostera marina Linnaeus, Reference Linnaeus1753) (Plus et al., Reference Plus, Dalloyau, Trut, Auby, de Montaudouin, Emery, Noel and Viala2010). Tidal channels present soft bottoms, the few hard bottoms not very well studied are represented by constructions of anthropogenic origin (bunkers, wrecks, rockfills). In ‘Thiers’ station, temperature and salinity ranged between 5.6–25.1°C and 20.8–34.2 PSU respectively between 2014 and 2016 (data Ifremer LER Arcachon).

Fig. 1. Records of Ericthonius didymus in European waters. (1) Lagoon of Venice – Italy, type locality; (2) São Miguel – Portugal; (3) Port Camargue – France; (4) Arcachon Bay – France. Sampling stations for Ericthonius didymus in Arcachon Bay (see code legend in Table 1). Shaded areas indicate intertidal zone. Intertidal ( ) and subtidal (
) and subtidal ( ) stations.
) stations.
Table 1. Examined material of Ericthonius didymus in Arcachon Bay (France, stations: see Figure 1), and E. convexus and E. pugnax in Japan

Material examined
Ericthonius didymus was collected during benthic surveys in four different areas in European waters (Figure 1): (1) Lagoon of Venice – Italy, 9 and 10 December 2012 (collectors: Anna Occhipinti and Jasmine Ferrario; coordinates: 37°44′N 25°39′W; marina pontoons, about 1 m depth); (2) São Miguel – Azores, Portugal, summer 2014 (collectors: Joana Micael and Ana C. Costa; coordinates: 37°44′N 25°39′W; marina pontoons, about 1 m depth); (3) Port Camargue – French Mediterranean coast, between 16 and 28 May 2015 (collector: Aylin Ulman; coordinates: 43°30′N 4°07′E; one boat hull and marina walls/pontoons, about 1 m depth); and (4) Arcachon Bay – French Atlantic coast, between 2013 and 2017 (collector: Benoit Gouillieux; Table 1 for coordinates, depth and substrate).
Ericthonius didymus from Portugal and French coasts were compared with specimens from Italy (Venice lagoon specimens – present paper, and museum specimens from the type locality – data in Krapp-Schickel, Reference Krapp-Schickel2013). Due to the possibility of a non-indigenous species introduced with the Pacific oysters such as Aoroides (Gouillieux et al., Reference Gouillieux, Lavesque, Leclerc, Garrec, Viard and Bachelet2015), they were also compared with E. convexus and E. pugnax from Japan (Table 1). Specimens were observed with a Nikon SMZ 1500 stereomicroscope and a Nikon Eclipse E400 microscope with up to 112.5 and 400× magnifications (and transmitted light) respectively, and photographed with a Nikon DS-Fi2 camera. Drawings were carried out from pictures using Inkscape software (v.0.92). Body length (BL) was measured with NIS-Elements Analysis software from the anterior margin of head to the posterior end of telson. For SEM studies, specimens were dehydrated in a graded ethanol series, critical point dried, sputter coated with gold and examined with a Hitachi TM3030Plus scanning electron microscope. Some specimens were deposited in the Muséum National d'Histoire Naturelle (MNHN, Paris, France) (Appendix 1).
DNA isolation, amplification and sequencing
Sub-samples for DNA analysis were removed from live specimens, placed in ethanol 96% and frozen at −20°C. Extraction of DNA was done with QIAamp DNA Micro Kit (QIAGEN) following protocol supplied by the manufacturers. About 340 bp of 16S genes were amplified using primers 16STf (Forward, 5′- GGTAWHYTRACYGTGCTAAG-3′) (Macdonald et al., Reference Macdonald, Yampolsky and Duffy2005) and 16Sbr-H (Reverse 5′- CCGGTTTGAACTCAGATCATGT-3′) (Palumbi et al., Reference Palumbi, Martin, Romano, McMillan, Stice and Grabowski1991). The PCR (Polymerase Chain Reaction) was done with Gotaq G2 Flexi DNA Polymerase (PROMEGA), with 50 μl mixtures containing: 10 μl of 5× Colorless GoTaq® Reaction Buffer (final concentration of 1×), 1.5 μl of MgCl2 solution (final concentration of 1.5 mM), 1 μl of PCR nucleotide mix (final concentration of 0.2 mM each dNTP), 0.5 μl of each primer (final concentration of 1 μM), 0.2 μl of GoTaq® G2 Flexi DNA Polymerase (5U μl−1), 1 μl template DNA and 33.8 μl of nuclease-free water. The temperature profile was as follows: 4 min denaturation hold at 94°C; 45 cycles of 1 min at 95°C, 1 min at 45°C, 2.5 min at 72°C; and 10 min extension hold at 72°C. Amplified PCR products were analysed by electrophoresis in a 1% p/v agarose gel stained with ethidium bromide, and were sent to GATC Biotech Company for complete double strain sequencing, using the same set of primers as used for the PCR. Overlapping sequence (forward and reverse) fragments were merged into consensus sequences and aligned using Clustal Omega. The minimum length coverage was around 334 bp for 16S. Sequences of one specimen for each species obtained in this study have been deposited in GenBank (http://www.ncbi.nlm.nih.gov/genbank/).
Pairwise Kimura 2-parameter (K2P) genetic distance and maximum likelihood tree using K2P model and non-parametric bootstrap branch support (1000 replicates) were performed using MEGA version 7.0.26. to validate the identity of our material and in order to compare different specimens. The 16S sequence of another ischyrocerid amphipod Jassa slatteryi Conlan, Reference Conlan1990 collected from Arcachon Bay was included as outgroup.
Results
Systematics
Order AMPHIPODA Latreille, 1816
Suborder SENTICAUDATA Lowry & Myers, 2013
Infraorder COROPHIIDA Leach, 1814 (sensu Lowry & Myers, 2013)
Parvorder CAPRELLIDIRA Leach, 1814 (sensu Lowry & Myers, 2013)
Superfamily PHOTOIDEA Boeck, 1871
Family Ischyroceridae Stebbing, 1899
Genus Ericthonius H. Milne Edwards, Reference Milne Edwards1830
Species Ericthonius didymus Krapp-Schickel, Reference Krapp-Schickel2013
A total of 686 specimens of Ericthonius didymus were collected and examined: 40 in the Lagoon of Venice, four in São Miguel, two in Camargue (=Ericthonius cf. pugnax in Ulman et al., Reference Ulman, Ferrario, Occhpinti-Ambrogi, Arvanitidis, Bandi, Bertolino, Bogi, Chatzigeorgiou, Çiçek, Deidun, Ramos-Esplá, Koçak, Lorenti, Martinez-Laiz, Merlo, Princisgh, Scribano and Marchini2017) and 640 in Arcachon Bay. Specimens from Arcachon Bay were collected between September 2013 and July 2017 (see Table 1), mainly in hydrozoa (Amphisbetia operculata (Linnaeus, 1758)) which may be partially attributed to a higher sampling effort on this habitat.
DESCRIPTION (Figures 2 & 3)
Based on adult male (BL: 5.2 mm), Arcachon Bay.
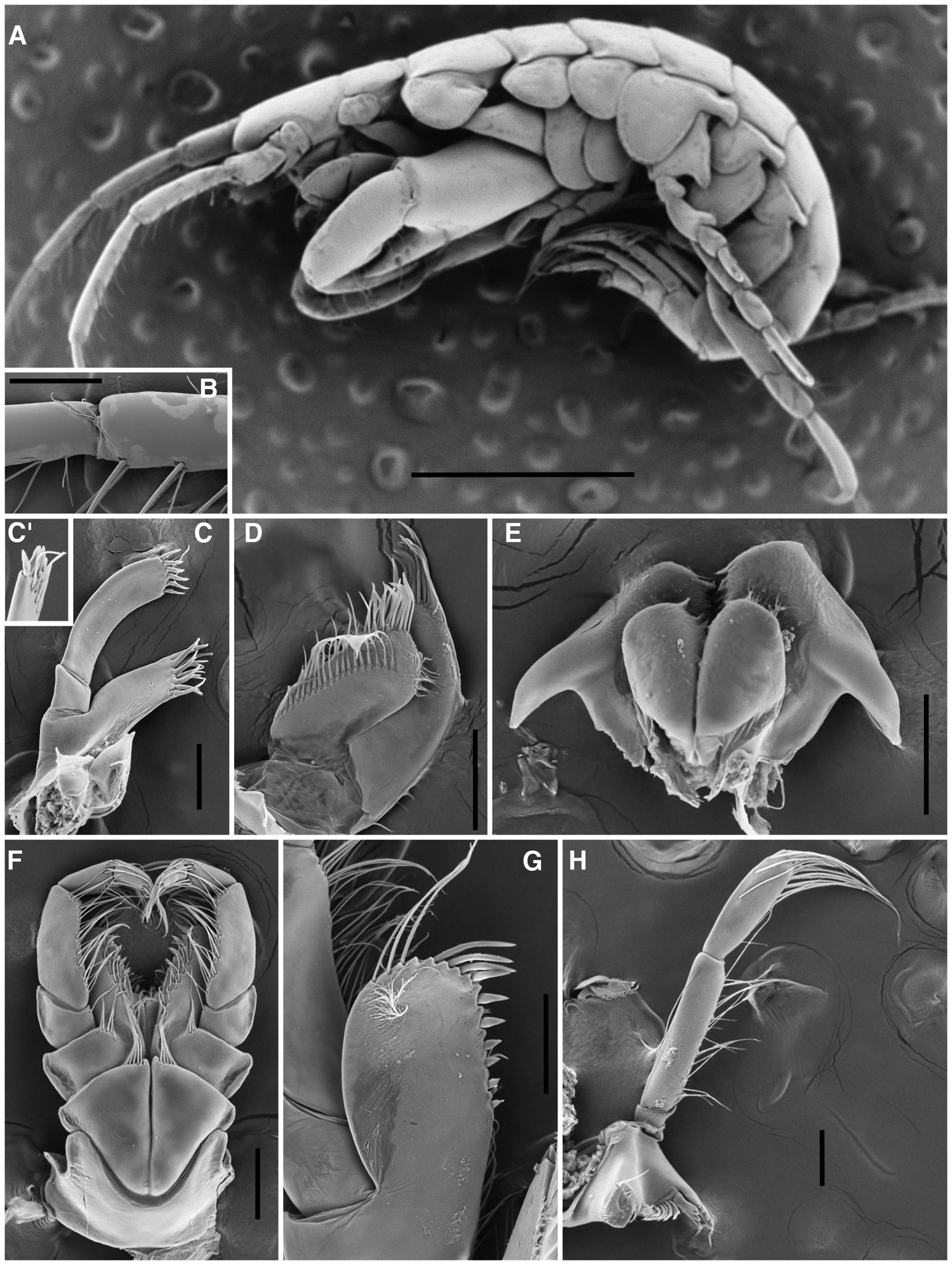
Fig. 2. Adult males (BL: 3.93–4.43 mm) of Ericthonius didymus from Arcachon Bay. (A) whole specimen; (B) accessory flagellum; (C) maxilla 1; (C’) maxilla 1 palp article 2 anterior surface; (D) maxilla 2; (E) lower lip; (F) maxilliped; (G) maxilliped outer plate; (H) left mandible. Scale bars: (A) 1 mm; (B–H) 0.1 mm.
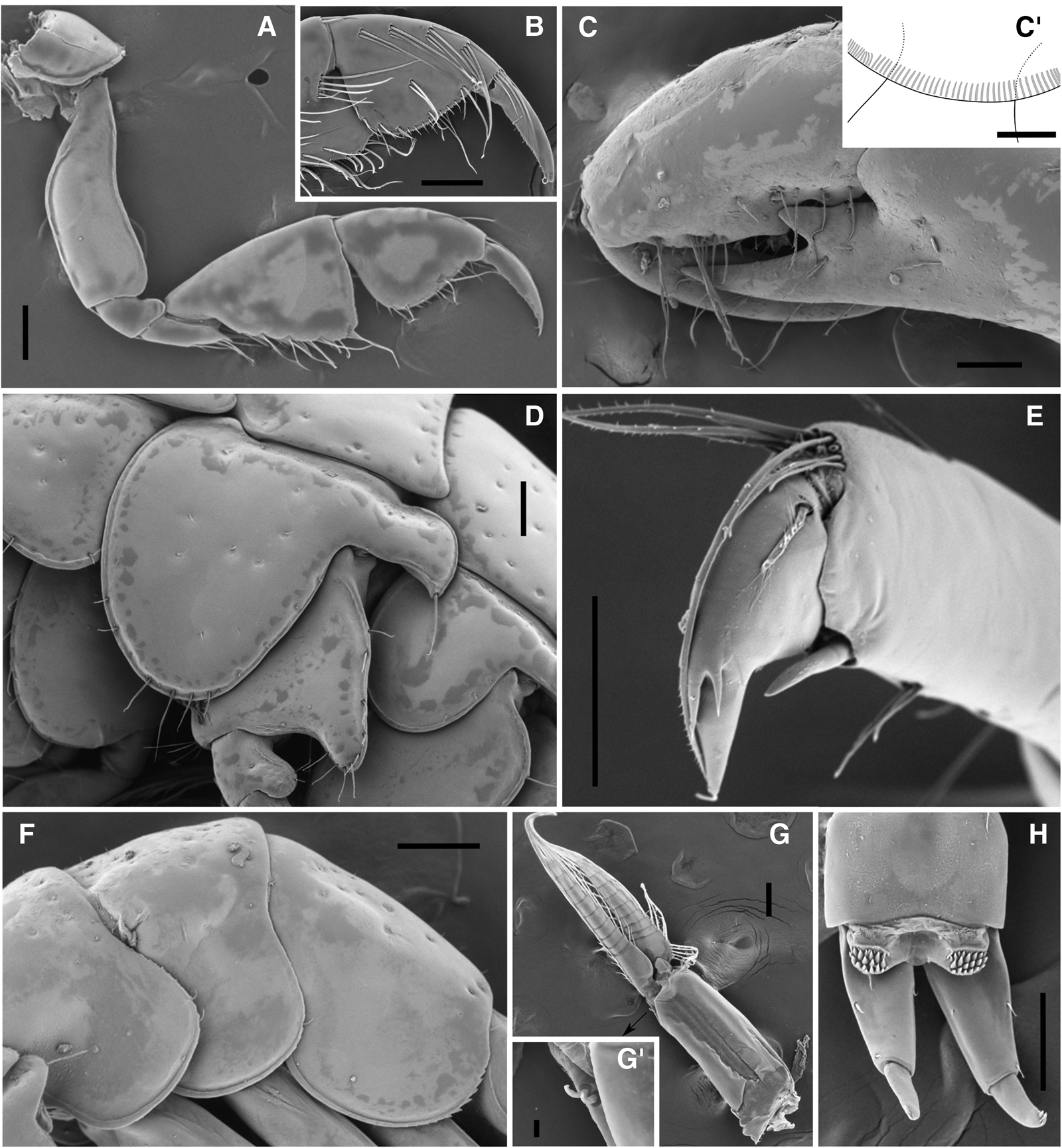
Fig. 3. Adult males (BL: 3.93–4.43 mm) of Ericthonius didymus from Arcachon Bay. (A) gnathopod 1, lateral view; (B) gnathopod 1, medial view of carpus (in part), propodus and dactylus; (C) gnathopod 2, lateral view of carpus (in part), propodus and dactylus; (C’) gnathopod 2 coxa with stridulating ridges; (D) pereopod 5 coxa, basis and ischium; (E) pereopod 5 propodus (in part) and dactylus; (F) left epimeral plates 1–3; (G) pleopod 2; (G’) coupling hooks of pleopod 2; (H) uropod 3 and telson, dorsal view. Scale bars: (A–D), (F–H) 0.1 mm; C’: 0.05 mm; E: 0.5 mm; G’: 0.01 mm.
Body. Slender, relatively depressed. Head. Ocular lobes projected. Eyes round, reddish alive, black when conserved. Antenna 1 subequal in length to antenna 2; peduncular article 1 smallest, with robust seta on posterodistal margin; article 3 0.8× as long as article 2; accessory flagellum small, scale-like. Antenna 2 peduncular article 3 with robust seta on posterodistal margin; article 4 smaller in length to article 5. Upper lip distally rounded with many setules, epistome strongly projected anteriorly. Mandibles in left and right with 4 cusps on incisor; accessory setal row with 6 serrulate setae; palp 3-articulate; article length ratio 1:3.6:2.8. Lower lip with many small setae on apical and medial margins of inner and outer plates; mandibular lobes short. Maxilla 1 inner plate pointed ventrally, with 2 long and many thin lateral simple setae; outer plate with 10 distal cuspidate stout setae; palp article 2 with 5 simple subdistal and 6 robust distal setae. Maxilla 2 with both plates heavily setose. Maxilliped inner plate with 1 ventrodistal and 3 distal robust setae and many plumose setae, outer plate with 10 mediodistal robust setae and 3 long plumose distal setae; palp heavily setose.
Pereon. Gnathopod 1 smaller than gnathopod 2, subchelate; coxa small, anteroventral corner with seta; basis marginally bare except seta on middle part of anterior margin and 2 posterodistal setae; ischium anterodistal corner roundly produced; merus with acute process on posterodistal corner, distal edge of medial surface setose; carpus longish triangular, posterior margin and posterior half of medial surface with many setae; propodus short, palmar margin slightly convex, minutely serrate; dactylus minutely serrate along posterior margin, except distal part. Gnathopod 2 extremely large, carpochelate; coxa widely separate from coxae 1 and 3, rounded, bearing short seta anteroventrally, ventral margin with >60 stridulating ridges; basis relatively wide, anterior margin with sparse short setae, posterior margin bare except posterodistal seta; anterodistal corner of ischium roundly protruded; merus extended along posterior margin of carpus, with cluster of short setae on posterior margin; carpus greatly enlarged, posterodistal corner bearing large process with 2 teeth, separated by V-shaped incision, posterior margin of outer tooth setose, middle part of distal margin roundly inflated; propodus anterior and posterior margins undulate and setose; dactylus stout, anterior and posterior margins with setae increasing in length distally, tip of dactylus with cluster of long setae. Pereopods 3 and 4 similar; coxae protruded posterodorsally, each bearing 2 ventral setae; bases wide, ovoid, anterior margins with several setae, posterior margins with seta on distal third and 2 posterodistal setae; meri, carpi and propodi posterior margins with setae increasing in length distally; meri projected anterodistally; dactyli narrow, slightly curved, with pappose seta on proximal outer margins. Pereopod 5 short; coxal plate wide, anterior lobe broad, with several short and long setae ventrally, posteroventral corner of posterior lobe with robust seta, many very small setae on all coxal surface; basis roundish, posterodistal corner strongly produced, distal half of anterior and posterior margins sparsely setose; ischium posterodistal corner roundly produced; posterodistal corner of merus projected; anterodistal and posterodistal corners of carpus setose; propodus with robust seta and several long setae on distal edge; dactylus short, strongly curved, with pappose seta proximally and 3 accessory teeth on outer margin. Pereopod 6 and 7 similar, pereopod 6 slightly shorter than 7; coxae posterodistal corners with robust seta, surface of coxa 6 with some very small setae; posterior margins of bases with a few setae; meri, carpi and propodi sparsely setose; propodi with anterodistal robust seta and several long posterodistal setae; dactyli short, strongly curved, with pappose seta proximally, 5 accessory teeth and simple seta on outer margins, minutely serrate on proximal halves of inner margins.
Pleon. Epimeral plate 3 regularly rounded, posterodistal corner serrate. Pleopods relatively long, pleopod 3 shortest; peduncles each with 2 coupling hooks, peduncle of pleopods 1 with seta, peduncle of pleopod 2 bare; outer rami shorter than inner, outer rami of pleopods 1–3 each with 9 articles, inner rami with 8, 9, 8 articles, respectively, medioproximal margins of inner rami each with 3 or 4 clothespin setae. Urosomites 1 and 2 with distal dorso-median simple seta. Uropod 1, lateral margin of peduncle with 2 or 3 robust setae and laterodistal seta, medial margin serrate, with 3 robust setae; rami subequal in length, all margins minutely serrate, outer ramus with 3 lateral, 2 medial and 4 terminal robust setae, inner ramus with 3 medial and 2 terminal robust setae. Uropod 2, lateral margin of peduncle bearing 3 robust setae, mediodistal corner with long robust seta; rami with all margins minutely serrate, outer ramus slightly longer than inner, with 1 lateral and 4 terminal robust setae, inner ramus with 1 marginal and 2 terminal robust setae. Uropod 3 small, uniramous; peduncle stout, with 1 lateral, 2 laterodistal, 1 mediodistal and 1 dorsal setae; single ramus short, marginally bare, tip with 3 protuberances and 2 short setae. Telson entire, short, with pair of densely spinulose lobes each bearing 2 plumose setae; posterior edge between lobes slightly convex.
FEMALE (Figure 4A–D)
Sexually dimorphic characters, based on adult females (BL: 4.2–7.1 mm), Arcachon Bay.

Fig. 4. (A–F) Ericthonius didymus from Arcachon Bay (BL: 4.04–7.12 mm). (A) female, whole specimen; (B) female, gnathopod 2; (B’) female, gnathopod 2, distal part of carpus posterior margin and propodus; (C) female, pereopod 5 coxa, basis, ischium and merus (in part); (D) male (hyper-adult form, BL: 5.48 mm), gnathopod 2, distal part of carpus, propodus and dactylus; (E) male (BL: 4.04 mm), gnathopod 1 coxa, basis and ischium; (F) male, uropod 1 dorsal view; (G–H) adult male (BL: 6.58 mm) of Ericthonius convexus from Japan; (G) gnathopod 1 coxa, basis and ischium; (H) uropod 1 dorsal view; (I–J) adult male (BL: 5.73 mm) of Ericthonius pugnax from Japan; (I) gnathopod 1 coxa, basis and ischium; (J) uropod 1 dorsal view. Scale bars: (A) 1 mm; (B, D) 0.2 mm; B’, (C, E–J) 0.1 mm.
Marsupium present on coxae 2–5. Coxa 2 not widely separate from coxae 1 and 3. Coxae 3–7 with less than 10 simple setae; coxae 3 and 4 with less than 10 plumose setae; coxae 5–7 with more than 25 plumose setae, posteroventral corners of posterior lobes without robust seta. Gnathopod 2, carpus distal part of posterior margin with 6 robust setae increasing in length distally, posterodistal lobe without tooth; propodus palmar margin defined by 1 single distal and 2 pairs of robust setae for resting tip of dactylus; tip of dactylus without long setae. Pereopod 5, coxa posteroventral corner of posterior lobe without robust seta; basis posterodistal corner not produced; ischium posterodistal corner slightly produced. Pleopod 1 peduncle with 15 to 19 plumose setae on proximal two thirds of lateral margin; Pleopods 2 and 3 medioproximal margins of inner rami with 4 or 5 clothespin setae. Uropod 1–2 number of robust setae varying in size (see Table 2).
Table 2. Number of robust setae on uropods 1 and 2 in Ericthonius didymus from Arcachon Bay, E. convexus and E. pugnax from Japan. Distal robust setae on rami not included

a Mediodistal position.
LM: Lateral Margin; MM: Medial Margin.
COLOURATION (Figure 5)
Eyes red, becoming dark brown to preserved specimens. Body mottled brown and white, occupancy ratio of brown part higher in female, dorsal surface of pereonite 3 (sometimes pereonites 2 and 4 also, very rarely on pereonite 6) with distinct dark brown spot medially in males, absent in females.

Fig. 5. Ericthonius didymus from Arcachon Bay. Dorsal and lateral views of (A) male (BL: 4.57 mm) and (B) female preserved specimens with a focus on the eye lobe (BL: 6.25 mm); (C) male alive specimen in its tube. Scale bars: 1 mm.
VARIATION IN MALES (BL: 4.0–5.5 MM)
Antenna 2 peduncular article 3 with or without robust seta on anterodistal margin. Mandible with 5 or 6 serrulate setae. Maxilla 1 inner plate with 1 to 3 long setae. Coxae 1–7 surfaces bearing very small setae, with maximum on coxa 5. Gnathopod 1 basis anterior margin with 1 to 4 setae. Gnathopod 2 basis with 5 to 9 setae on anterior margin, 0 to 2 setae on posterior margin (posterodistal setae not included); carpus with single posterior tooth in hyper-adult form (Figure 4E), size of specimens with hyper-adult form of gnathopod 2 larger than 5.5 mm BL. Pereopod 5 coxa with 3 or 4 plumose setae; basis sometimes with small setae; ischium with 0 to 2 setae on posterodistal corner. Pleopod 1 peduncle with or without seta; outer ramus with 9 or 10 articles, inner ramus with 8 to 10 articles. Pleopod 2 peduncle 0 or 2 lateromedial setae; inner and outer rami with 9 or 10 articles. Uropod 1–2 number of robust setae varying in size (see Table 2). Uropod 3 ramus with or without lateral seta.
MOLECULAR ANALYSES (Figure 6)
16S gene was successfully sequenced for 13 specimens belonging to the five different species. Sequences were published at NCBI GenBank for one specimen of each species: Ericthonius convexus (accession number: MK404725), E. didymus (accession number: MK404726), E. pugnax (accession number: MK404724), E. brasiliensis (accession number: MK404727) and Jassa slatteryi (accession number: MK404728).

Fig. 6. Maximum likelihood tree based on mitochondrial gene 16S rRNA sequences of the different Ericthonius species (Jassa slatteryi as outgroup species) and Kimura 2-parameters model.
The maximum-likelihood tree and interspecific pairwise genetic distances permitted to confirm the validity of the three closely related species of Ericthonius. Indeed, interspecific pairwise genetic distances were 10.8% between E. convexus and E. didymus, 10.5% between E. didymus and E. pugnax, and 15.4% between E. pugnax and E. convexus.
Discussion
Ericthonius didymus was recently described by Krapp-Schickel (Reference Krapp-Schickel2013) from the Lagoon of Venice, Italy, and was mainly compared with E. argenteus, from which it was principally distinguished by the shape of male pereopod 5 basis. Morphological characters of the Arcachon specimens almost agree with those of the original description. However, several minute characters are different: E. didymus was described with (comparison with Arcachon specimens in parentheses) maxilla 1 inner plate with 2 lateral setae (vs 1 to 4 and many thin setae), palp article 2 with 2 subdistal setae (vs row of subdistal setae); maxilla 2 outer margin of inner and outer plate bare (vs with many small setae); maxilliped outer lobe inner margin concave (vs straight); lower lip inner lobe bare (vs with many small setae); uropod 2 peduncle and rami without robust setae (vs present); telson posterior edge between spinulose lobes with 2 lobes (vs convex). Examination of E. didymus specimens from the type locality (museum and newly collected specimens) reveals the absence of these differences with Arcachon specimens. It could be explained by (1) inaccurate observations/representation or (2) mixing of two Ericthonius species (E. didymus and a new Ericthonius species not actually described) from the Italian specimens. Due to the small differences, authors consider herein the first hypothesis and assimilate Arcachon, Camargue and Azores specimens as Ericthonius didymus.
In their revision of North-east Atlantic Ericthonius species, Myers & McGrath (Reference Myers and McGrath1984) divided males in two groups (females are difficult to determine): Group 1 with male gnathopod 2 coxa with stridulating ridges, carpus posterodistal margin with 2 teeth in adults, but some species may have one tooth only in hyper-adults, coxa 2 widely separate from coxae 1 and 3; Group 2 with male gnathopod 2 coxa never with stridulating ridges, carpus posterodistal margin always with single tooth, coxa 2 more or less contiguous with coxae 1 and 3. Among European Ericthonius species, five of them belong to Group 1: E. argenteus, E. brasiliensis, E. didymus, E. difformis and E. punctatus, four of them belong to Group 2: E. fasciatus, E. megalops, E. rubricornis and E. stephenseni. But other species in the world can be included in group 1: E. convexus, E. forbesii Hughes & Lowry, Reference Hughes and Lowry2006, E. pugnax, E. rodneyi Hughes & Lowry, Reference Hughes and Lowry2006 and E. tropicalis Just, Reference Just2009. Some species have coxa 2 with stridulating ridges, carpus posterodistal margin with 2 teeth in adults, but coxa 2 more or less contiguous with coxae 1 and 3 (E. brevicarpus Vader & Myers, Reference Vader and Myers1996, E. coxacanthus Moore, Reference Moore1988, E. parabrasiliensis Just, Reference Just2009, E. tacitus Moore, Reference Moore1988). Ericthonius grebnitzkii Gurjanova, Reference Gurjanova1951, E. ledoyeri Barnard & Karaman, Reference Barnard and Karaman1991 and E. tolli von der Bruggen, Reference von der Bruggen1909 are not sufficiently described to classify them in a group, but E. ledoyeri also has coxa 2 with stridulating ridges, carpus posterodistal margin with 2 teeth in adults and E. tolli can be synonymized with E. megalops (Ariyama & Fujiwara, Reference Ariyama and Fujiwara2011). Besides the four species previously cited, no other known species in the world can be included in Group 2.
The shape of male gnathopod 2 appears to be variable, depending on the maturity of the specimen. Variability between adults and hyper-adults also occurs in other Ischyroceridae such as Cerapus, Ericthonius or Jassa (Myers & McGrath, Reference Myers and McGrath1984; Conlan, Reference Conlan1990; Lowry & Berents, Reference Lowry and Berents2005; Ariyama, Reference Ariyama2009). In Ericthonius species, adult males have 2 teeth on gnathopod 2 carpus (with outer tooth the longest); however, grown to hyper-adult form, the inner tooth is worn down to a rounded process and they regularly have only one tooth. This variability was frequently noted (Nagata, Reference Nagata1960; Ledoyer, Reference Ledoyer1969; Lincoln, Reference Lincoln1979; Myers, Reference Myers and Ruffo1982; Myers & McGrath, Reference Myers and McGrath1984; Hughes & Lowry, Reference Hughes and Lowry2006; Krapp-Schickel, Reference Krapp-Schickel2013), therefore this character must be taken with caution for identification.
The authors propose to divide Ericthonius species into three groups, based on coxa 2 and male pereopod 5 basis:
• Group 1 without stridulating ridges on coxa 2: 4 species (E. fasciatus, E. megalops ( = E. tolli), E. rubricornis and E. stephenseni)
• Group 2 with stridulating ridges on coxa 2, male pereopod 5 basis without posterodistal lobe: 12 species (E. argenteus, E. brasiliensis, E. brevicarpus, E. coxacanthus, E. difformis, E. forbesii, E. ledoyeri, E. parabrasiliensis, E. punctatus, E. rodneyi, E. tacitus and E. tropicalis)
• Group 3 with stridulating ridges on coxa 2, male pereopod 5 basis with posterodistal lobe: 3 species (E. convexus, E. didymus and E. pugnax)
Due to insufficient descriptions, E. grebnitzkii cannot be attributed to any group. Additionally, LeCroy (Reference LeCroy2007) identified a new Ericthonius species from the Western Atlantic, Ericthonius sp. A, which belongs to Group 3 but its description is insufficient to determine if it is a new or known species.
Ericthonius didymus belongs to Group 3 together with other two species: E. convexus and E. pugnax. These three closely related species can be distinguished by the following characters: E. convexus has gnathopod 1 basis anterior margin bare, uropod 1 lateral margin with 3 to 8 robust setae, telson posterior edge between lobes slightly convex, dorsal brown spot on pereonite 3 and pleonites 1–2; E. didymus has gnathopod 1 basis anterior margin with few small setae, uropod 1 lateral margin with 2 to 3 robust setae, telson posterior edge between lobes slightly convex, dorsal brown spot on pereonite 3; E. pugnax has gnathopod 1 basis anterior margin with few small setae, uropod 1 lateral margin with 3 to 6 robust setae, telson posterior edge between lobes straight to slightly concave, dorsal brown spot on pereonite 3 and pleonite 1 (Ariyama, Reference Ariyama2009; present study) (Figure 4F–K). Ulman et al. (Reference Ulman, Ferrario, Occhpinti-Ambrogi, Arvanitidis, Bandi, Bertolino, Bogi, Chatzigeorgiou, Çiçek, Deidun, Ramos-Esplá, Koçak, Lorenti, Martinez-Laiz, Merlo, Princisgh, Scribano and Marchini2017) recorded Ericthonius specimens in Port Camargue and hypothesized that E. didymus was a synonym species of E. pugnax. They assigned their specimens as E. cf. pugnax, but nevertheless they do not discount the hypothesis of the presence of a complex of cryptic species. Results of the molecular analyses herein realized on E. convexus, E. didymus and E. pugnax show differences implying that they are distinct species. But despite a first description in the Lagoon of Venice, the facts cited in Ulman et al. (Reference Ulman, Ferrario, Occhpinti-Ambrogi, Arvanitidis, Bandi, Bertolino, Bogi, Chatzigeorgiou, Çiçek, Deidun, Ramos-Esplá, Koçak, Lorenti, Martinez-Laiz, Merlo, Princisgh, Scribano and Marchini2017) may suggest that E. didymus may be an introduced species. The new data presented here further corroborate this hypothesis: all the known European records of E. didymus (Lagoon of Venice, Arcachon Bay, Port Camargue and São Miguel, Azores) are from sites of intense shellfish trade, and/or shipping and boating, which are considered the most powerful vectors of introduction of marine non-indigenous species in western Europe (Katsanevakis et al., Reference Katsanevakis, Zenetos, Belchior and Cardoso2013; Galil et al., Reference Galil, Marchini, Occhipinti-Ambrogi, Minchin, Narščius, Ojaveer and Olenin2014). And in fact, all these four sites represent ‘hotspots’ of introduction of marine species (Boudouresque et al., Reference Boudouresque, Klein, Ruitton, Verlaque, Ceccaldi, Dekeyser, Girault and Stora2011; Lavesque et al., Reference Lavesque, Sorbe, Bachelet, Gouillieux, de Montaudouin, Bonifacio, Blanchet and Dubois2013; Chainho et al., Reference Chainho, Fernandes, Amorim, Ávila, Canning-Clode, Castro, Costa, Costa, Cruz, Gollasch, Grazziotin-Soares, Melo, Micael, Parente, Semedo, Silva, Sobral, Sousa, Torres, Veloso and Costa2015; Marchini et al., Reference Marchini, Ferrario, Sfriso and Occhipinti-Ambrogi2015). The distribution restricted to sites affected by human introductions, combined with the high genetic affinity of E. didymus with species of western Pacific distribution, its scarce capabilities of natural spreading, and the invasion history of other species within this genus (Marchini & Cardeccia, Reference Marchini and Cardeccia2017), altogether suggest an exotic native origin and a human-mediated introduction of E. didymus. Additionally, similarly to the non-indigenous Pericarida Caprella scaura and Paracerceis sculpta species records in the Azores (Gillon et al., Reference Gillon, Costa and Micael2017; Marchini et al., Reference Marchini, Costa, Ferrario and Micael2018), specimens were collected from non-indigenous fouling organisms growing on artificial hard substrata, which suggests a second expansion of E. didymus from the western European coast to the Azores through shipping or recreational boating. If the exotic native origin hypothesis is correct, however, it must be acknowledged that the true native origin of E. didymus is still to be elucidated, making it a case of pseudo-indigenous species (sensu Carlton, Reference Carlton, Rilov and Crooks2009). It is possible that the putatively wide Indo-Pacific distribution of the similar species E. pugnax, reported from Papua New Guinea, Australia (Great Barrier Reef, New Caledonia and New South Wales), New Zealand, Japan, Korea, Singapore, Malaysia, Indonesia, India, Sri Lanka, Maldives, Mauritius, Madagascar and South Africa (see Marchini & Cardeccia, Reference Marchini and Cardeccia2017 and references therein) may include mis-identified populations of E. didymus.
Ericthonius didymus has probably been present for many years in European waters but was likely misidentified and confused with other Ericthonius species, partly due to the regular absence of pereopods in the majority of collected specimens, and to errors in the literature. In European keys, E. didymus might have been misidentified in Myers & McGrath (Reference Myers and McGrath1984) and Myers (Reference Myers and Ruffo1982) as E. punctatus; in Chevreux & Fage (Reference Chevreux and Fage1925) and Lincoln (Reference Lincoln1979) as E. brasiliensis (= E. punctatus, due to absence of knob-like process on posterior margin of gnathopod 1 basis and coxa 2 broader than deep). Thereby, records of E. punctatus and E. brasiliensis along the European coast must be taken with caution, especially along the Atlantic coast.
Identification key for adult males of Ericthonius species belonging to Group 1 (without stridulating ridges on coxa 2) – Key from Myers & McGrath (Reference Myers and McGrath1984):
1. Eye lacking, pereopods 3–4 dactyli much longer than propodus, pereopod 4 merus with brush of setae on anterior margin ……… E. stephenseni
– Eye large, pereopods 3–4 dactyli shorter than propodus, pereopod 4 merus lacking brush of setae on anterior margin ……… 2
2. Pereopod 5 basis relatively elongate, posterodistal margin with evenly rounded lobe, posterior margin weakly concave, antenna 2 peduncle elongate and slender, articles 4 and 5 longer than combined length of head and pereon segments 1–2; uropods 1–2 very elongate and slender ……… E. fasciatus
– Pereopod 5 basis stout, almost as broad as long, posterodistal margin almost a right angle, posterior margin straight; antenna 2 peduncle stout, articles 4 and 5 shorter than combined length of head and pereon segments 1–2; uropods 1–2 only moderately elongate ……… 3
3. Pereopod 3 slender, basis ovoid, length almost twice breadth; gnathopod 1 propodus greatest width situated mediodistally ……… E. megalops (= E. tolli)
– Pereopod 3 stout, basis sub-round, length about one and a half times breadth; gnathopod 1 propodus greatest width situated medially ……… E. rubricornis
Identification key for adult males of Ericthonius species belonging to Group 2 (with stridulating ridges on coxa 2, pereopod 5 basis without posterodistal lobe):
1. Gnathopod 1 basis with posterior knob-like process ……… 2
– Gnathopod 1 basis without posterior knob-like process ……… 3
2. Coxa 2 widely separate from coxae 1 and 3, longer than broad, subrectangular ……… E. brasiliensis
– Coxa 2 separate (not widely) from coxae 1 and 3, as long as broad, subquadrate ……… E. parabrasiliensis
3. Coxa 2 with anteroproximal projection ……… 4
– Coxa 2 without anteroproximal projection ……… 5
4. Gnathopod 2 coxa with a subrectangular anteroproximal projection, basis longer than broad, posterior margin with few little setae; ischium anterodistal angle without lobe ……… E. brevicarpus
– Gnathopod 2 coxa with an acute anteroproximal projection, basis as long as broad, posterior margin bare; ischium anterodistal angle lobate ……… E. coxacanthus
5. Gnathopod 1 coxa ventral margin concave, carpus distinctly larger than propodus ……… E. argenteus
– Gnathopod 1 coxa ventral margin straight to convex, carpus slightly larger than propodus ……… 6
6. Gnathopod 2 carpus with row of robust setae on posterior margin ……… 7
– Gnathopod 2 carpus without row of robust setae on posterior margin ……… 8
7. Gnathopod 2 coxa widely separate from coxae 1 and 3, uropod 1 outer ramus marginally bare, uropod 2 rami marginally bare ……… E. tropicalis
– Gnathopod 2 coxa separate (not widely) from coxae 1 and 3, uropod 1 outer ramus marginally with robust setae, uropod 2 rami with marginal robust setae ……… E. tacitus
8. Gnathopod 2 coxa longer than broad, ventral margin concave, carpus with only 1 slender simple tooth (which may have a small accessory tooth) ……… E. difformis
– Gnathopod 2 coxa longer than broad, ventral margin straight to slightly convex, carpus with 2 teeth ……… 9
9. Uropod 2 rami with marginal robust setae……… 10
– Uropod 2 rami without marginal robust setae ……… 11
10. Gnathopod 1 carpus slightly larger than propodus, gnathopod 2 basis posterior margin with few setae, uropod 2 rami with 3 lateral robust setae ……… E. forbesii
– Gnathopod 1 propodus slightly larger than carpus, gnathopod 2 basis posterior margin bare, uropod 2 rami with 1 lateral robust seta ……… E. punctatus
11. Pereopod 4 basis ovate, larger medially, uropod 1 outer ramus with marginal robust setae ……… E. rodneyi
– Pereopod 4 basis subrectangular, anterior and posterior margin parallel; uropod 1 outer ramus marginally bare ……… E. ledoyeri
Identification key for adult males of Ericthonius species belonging to Group 3 (with stridulating ridges on coxa 2, pereopod 5 basis with posterodistal lobe):
1. Uropod 1 peduncle with 2 or 3 lateral robust setae; dorsal brown spot present on pereonite 3 ……… E. didymus
– Uropod 1 peduncle with 3 to 8 lateral robust setae; dorsal brown spot present on pereonite 3 and pleonite 1 ……… 2
2. Gnathopod 1 basis anterior margin bare, posterior edge of telson between spinulose lobes convex, dorsal surface of body with a median dark brown spot on each of pereonite 3 and pleonites 1–2 ……… E. convexus
– Gnathopod 1 basis anterior margin with few small setae, posterior edge of telson between spinulose lobes straight to slightly concave, dorsal surface of body with a median indistinct brown spot on each of pereonite 3 and pleonite 1 ……… E. pugnax
Acknowledgements
The authors wish to thank Roberta Salmaso and Leonardo Latella (Verona Museum) for the loan of Ericthonius didymus specimens, Toshinori Baba (Yamaguchi Prefectural Fisheries Research Center) for Ericthonius convexus specimens from Japan, Ifremer Arcachon for temperature and salinity data, Laure Corbari and Paula Martin-Lefevre (MNHN, Direction des collections, Paris) for their help in depositing specimens in the MNHN collections. The authors also thank Horia Galea and Helmut Zibrowius for the identification of hydrozoa and the Biodiversity Platform of EPOC laboratory for the equipment made available.










