Introduction
Previously, there was no practical way to count fetal movements for many hours at a time. Recently, a fetal movement acceleration measurement recorder (FMAM recorder, http://e-mother.co-site.jp) was developed. It was designed to detect oscillations of the maternal abdominal wall caused by fetal movement during overnight sleep at home. Gross fetal movement and abdominal oscillation were observed by ultrasonography and FMAM recorder, respectively, and almost perfect agreement has been shown between the two. Reference Ryo, Nishihara, Matsumoto and Kamata1
Thus, the FMAM recorder has enabled fetal movement counting in clinical settings. In a case report of a pregnant woman, decreased fetal movement was recorded with the recorder 8 d before emergent cesarean delivery due to non-reassuring fetal status. Reference Ryo, Kamata and Seto2 In another study using the recorder, fetuses with a longer umbilical cord showed more fetal movement than the other fetuses. Reference Ryo, Kamata, Seto, Morita and Yatsuki3 Furthermore, normal reference values for gross fetal movement count at home have been made with the FMAM recorder, and the values are similar to those made by ultrasonography. Reference Ryo, Kamata and Seto4
Fetal movement is an important index of fetal well-being and development; however, little is known about the factors related to fetal movement. With the use of the FMAM recorder, it has been reported that small-for-gestational-age infants are associated with decreased gross fetal movement even when they are not hypoxic. Reference Morita, Ryo, Kamata, Seto and Yatsuki5 However, there has been little reported about the relationship between size and movement of fetus.
The hypothesis of the study was there was relationship between fetal movement and newborn size, and the purpose of this study was to investigate that.
Methods
Counting fetal movements
The FMAM recorder was explained in detail in our past studies. Reference Ryo, Nishihara, Matsumoto and Kamata1–Reference Morita, Ryo, Kamata, Seto and Yatsuki5 It weighs 290 g and is appropriate for use at home. It has two acceleration sensors: one is a fetal movement sensor that attaches to the mother’s abdomen, and the other is a mother’s movement sensor that attaches to her thigh. The sensitivities of the fetal and mother’s sensors are 700 and 120 mV/0.1 G, respectively. The fetal movement sensor detects oscillations of the mother’s abdominal wall caused by gross fetal movement. However, the mother’s body movements themselves also cause oscillations. The recorder is unsuitable when the mother moves frequently and is best used only during night sleep. However, mothers move occasionally even during night sleep. In principle, when the mother’s movement sensor detects no movement and the fetal movement sensor detects oscillations, gross fetal movement is judged to have occurred.
We accepted a record as valid only when data could be obtained for more than 4 h per night. Each record was analyzed, and fetal movements were counted using a software system (NoruPro, version 1.05; Light Systems Inc., Tokyo, Japan) developed especially for the FMAM recorder. Reference Nishihara, Ohki, Kamata, Ryo and Horiuchi6 In brief, the system was set up as follows: (1) the low acceleration signals were filtered and changed to absolute integral values per 50 ms; (2) when the integral values were greater than twice the average amplitude during the 3 s just before and after measurement, they were judged to be positive for acceleration; (3) any period in which the mother’s movement sensor detected positive accelerations more than four times per minute was deleted from the data because this usually indicated that the mother was active or awake; (4) characteristic regular accelerations at 15–20 beats/min detected by the fetal movement sensor were a sign of fetal hiccups Reference Kamata, Ryo, Seto, Morita and Nagaya7 and not counted.
The recording was divided into intervals (epochs) of 10 s, and an epoch that had a fetal movement was defined as a positive epoch. The ratio of positive epochs to all epochs during one night was calculated as the positive epoch ratio, which was the index of fetal movement used in this study.
Subjects
A total of 90 pregnant women recorded fetal movement for more than 4 h per night and delivered a singleton newborn at term between April 2010 and August 2017 at Teikyo University Hospital. None of the mothers had any medical complications, and none of the newborns had any anomalies or neurological problems. Table 1 shows the characteristics of the mothers and the newborns. The FMAM recorder was used at the mother’s home. In principle, the mothers were asked to record once a week only after 28 weeks, because the accuracy of the FMAM recorder is limited before 28 weeks. Reference Ryo, Nishihara, Matsumoto and Kamata1
Table 1. Characteristics of the mothers and newborns
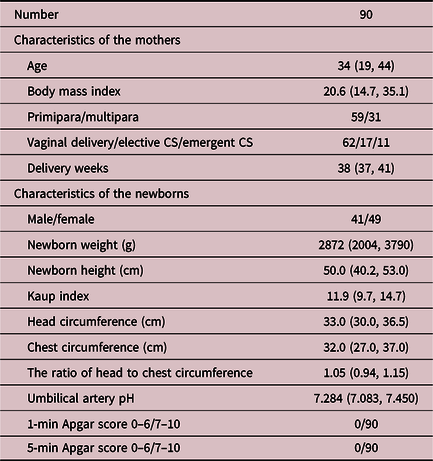
CS, cesarean section.
Data are expressed as number or median (minimum, maximum).
The newborns’ body weight, height, head circumference, and chest circumference were measured just after delivery. Newborn weight was measured with an electronic scale until two same four-digit values in grams were obtained. Head and chest circumference and height were measured by an experienced midwife using a measure that gave a two-digit-point-one-digit length in centimeters. The Kaup index and the ratio of head to chest circumference were calculated with those values. As a result, six data points were obtained for each newborn (Table 1).
Analyses
First, independent explanatory variables for the fetal movement index were selected from eight possibilities, that is, maternal age, gestational week, and the six physical measures of the newborn, based on the stepwise regression procedure (PIN = 0.05, POUT = 0.05). Second, with the selected variables, the effect of the physical measures of the newborn on the positive epoch ratio of fetal movement was analyzed using multiple regression analysis.
All data were analyzed with JMP Pro 13 (SAS Institute Japan Ltd., Tokyo, Japan). The statistically significant difference was set at a P-value of less than 0.05.
This study was approved by the ethics committee of Teikyo University. All women gave written informed consent before participating in the study.
Results
A total of 2812.95 h from only 423 night records were available for this study, because not all the mothers did recording every week, and record exceeding 4 h could not be always obtained because of several kinds of technical errors. Gestational weeks and the weight of the newborns were selected as the two significant independent variables for the fetal movement index. Table 2 shows the results of our multiple regression analysis. Gestational weeks had a negative effect (P < 0.0001) on the fetal movement index, and newborn weight had a positive effect (P < 0.0001). Fig. 1 shows the relationships between fetal movement and newborn weight. The multiple regression equation to predict fetal movement index from gestational weeks and newborn weight was “The fetal movement index (%) = 34.9989−0.9088 × gestational weeks + 0.0033 × newborn weight (g).”
Table 2. Results of multiple regression analysis for fetal movements

PRC, partial regression coefficient; SE, standard error.

Fig. 1. Relationship between fetal movement and newborn weight.
Discussion
The results of this study showed that gestational weeks had a negative effect on the fetal movement index. Fetal movements were reported to decrease as pregnancy progressed, Reference Ryo, Kamata and Seto4 and this study confirmed that report. A new finding from this study was that full-term newborn weight has a positive correlation with the fetal movement index. Recently, Huang et al. Reference Huang, Han and Fan8 reported that pregnant women who reported increased/excessive fetal movement had a higher probability of giving birth to a large-for-gestational-age newborn. Our results are consistent with theirs.
The reason why bigger fetuses move more frequently is not fully understood; however, this correlation does not seem surprising. The intrauterine environment, including the placental function, is important for fetal physical growth and motor development. Sufficient supplies of nutrition and oxygen likely increase both fetal weight and movement. Another possibility is that more fetal movements might increase muscle volume and bone density, which lead to fetal weight gain. Moreover, bigger fetuses tend to have more amniotic fluid volume, Reference Tomoda, Brace and Longo9,Reference Fuchs, Aouinti and Souaied10 which might lead to more space for gross movement.
Additionally, there has been a series of studies that show a positive correlation between birth weight and physical ability later in life. Moura-Dos-Santos et al. Reference Moura-Dos-Santos, Almeida, Manhães-De-Castro, Katzmarzyk, Maia and Leandro11 reported that birth weight was positively correlated with handgrip strength and negatively correlated with 20-m sprint times in 7- to 10-year-old children. In a systematic review by Dodds et al., Reference Dodds, Denison, Ntani, Cooper, Sayer and Baird12 17 out of 19 studies showed that higher birth weight was associated with greater muscle strength in 7- to 18-year-old children. In the same review, a meta-analysis of 13 studies of more than 20,000 people aged from 9 to 67 years showed a 0.86 kg increase in muscle strength per additional kilogram of birth weight.
Furthermore, there is strong evidence that physical ability has a positive correlation with physical activity. Lubans et al. Reference Lubans, Morgan, Cliff, Barnett and Okely13 reviewed 21 articles and found a positive correlation between fundamental movement skills competency and physical activity in children and adolescents. Also, physical activity during the younger years may well continue as the children age. Tammelin et al. Reference Tammelin, Näyhä, Hills and Järvelin14 reported that participation in sports at the age of 14 years was associated with high levels of physical activity at the age of 31 years. Telema Reference Telema15 reviewed studies on the tracking of physical activity in all phases of life from childhood to late adulthood and supported the idea that the enhancement of physical activity in children and adolescents is of great importance for one’s entire life.
In summary, birth weight correlates with physical ability and may correlate with physical activity in adult life. It is uncertain why birth weight correlates with physical ability and later activity levels; however, if bigger fetuses have more movement, which could strengthen physical ability and promote activity, a positive correlation between fetal movement and later physical activity seems more direct and reasonable than the correlation with only birth weight. Physical ability and activity levels throughout life may well originate in activity levels during fetal life.
The Developmental Origin of Health and Disease theory is a well-known theory supported by more and more evidence. It has been established that a fetus developing in an environment of nutritional insufficiency will easily fall victim to cardiovascular and metabolic diseases in adult life. How these phenomena happen has been the purpose of many studies, mainly from the nutritional programming viewpoint, including nutrient-sensing signals, oxidative stress factors, tissue remodeling, epigenetic regulation, gut microbiota, and so on Reference Hsu and Tain16 ; however, the biological mechanisms of the theory are not yet fully understood.
It is well known that physical exercise in adult life has a great positive effect on cardiovascular and metabolic health. Considering that some degree of physical ability and activity level during a person’s entire lifetime might originate from activity during fetal development, this study might provide a new way of looking at the Development Origin of Health and Disease theory.
However, this study only showed a positive correlation between fetal movement and birth weight; we did not study a direct correlation between fetal movement and either physical ability or later activity levels. Further studies about the direct correlation between the two are needed.
Fetal movement has long been recognized as a marker of fetal well-being. Reference Lai, Nowlan, Vaidyanathan, Shaw and Lees17 In addition, it may be an important marker of development and health for a person’s entire life. Studies of fetal movement are important not only for prenatal medicine but also for health care throughout life.
In conclusion, gross fetal movement has a positive correlation with birth weight. Fetal movement may well be important for human development and an indicator of health throughout life.
Acknowledgments
We thank Professor Takuya Ayabe and Mrs. Mieko Fuse for their support, and we appreciate language help by Mr. Howard Stacey. We also appreciate the corporation of the women who participated in this study.
Financial support
This work was supported by the Japan Society for the Promotion of Science (grant number JP 16K10109).






