Introduction
Ecological intensification seeks to increase crop productivity using ecological processes more intensively in a sustainable manner (Bommarco et al., Reference Bommarco, Kleijn and Potts2013). One way to maximize yield through ecological intensification is to promote biodiversity by maintaining natural habitats next to agricultural lands, thus providing ecosystem regulation such as pest control in the crops (Gaba et al., Reference Gaba, Bretagnolle, Rigaud and Philippot2014). For example, implementation of off-field measures such as hedgerows may encourage beneficial organisms within agroecosystems because of the resulting high biodiversity (Pollard & Holland, Reference Pollard and Holland2006; Hannon & Sisk, Reference Hannon and Sisk2009; Batary et al., Reference Batary, Matthiesen and Tscharntke2010; Moradin & Kremen, Reference Moradin and Kremen2013; Haenke et al., Reference Haenke, Kovács-Hostyánszki, Frund, Batary, Jauker, Tscharntke and Holzschuh2014; Moradin et al., Reference Moradin, Long and Kremen2014; Park et al., Reference Park, Blitzer, Gibbs, Losey and Danforth2015; Dainese et al., Reference Dainese, Inclán-Luna, Sitzia, Sigura and Marini2015, Reference Dainese, Montecchiari, Sitzia, Sigura and Marini2016). Biodiversity can enhance the survival of natural enemies (NEs) of pests and thereby improve their efficiency as pest-control agents by top–down effects, providing them with food (pollen, nectar and alternative prey) as well as favourable micro-climates (Landis et al., Reference Landis, Wratten and Gurr2000; Bianchi et al., Reference Bianchi, Booij and Tscharntke2006). Top–down control can lead to a positive relationship between biodiversity and pest control when NEs complement each other (Letourneau et al., Reference Letourneau, Jedlicka, Bothwell and Moreno2009). Greater plant species diversity may also be beneficial via a direct bottom–up effect on herbivores’ ability to locate their host (Finch & Collier, Reference Finch and Collier2000). However, although there is compelling evidence that diversified agroecosystems benefit pest control and yield (Thies & Tscharntke, Reference Thies and Tscharntke1999; Letourneau et al., Reference Letourneau, Jedlicka, Bothwell and Moreno2009; Vandermeer et al., Reference Vandermeer, Perfecto and Philpott2010; Burel et al., Reference Burel, Lavigne, Marshall, Moonen, Ouin and Poggio2013; Woltz & Landis, Reference Woltz and Landis2014; Henri et al., Reference Henri, Jones, Tsiattalos, Thébault, Seymour and van Veen2015; Gurr et al., Reference Gurr, Lu, Zheng, Xu, Zhu, Chen, Yao, Cheng, Zhu, Catindig, Villareal, Chien, Cuong, Channoo, Chengwattana, Lan, Hai, Chaiwong, Nicol, Perovic, Wratten and Luen Heong2016), there are also examples where biodiversity fails to support biological pest control in crops. For instance, top–down control can be dampened by intra-guild predation or balanced by functional redundancy (Straub et al., Reference Straub, Finke and Snyder2008; Snyder & Tylianakis, Reference Snyder, Tylianakis, Gurr, Wratten, Snyder and Read2012). In a recent study, Tscharntke et al. (Reference Tscharntke, Karp, Chaplin-Kramer, Batáry, DeClerck, Gratton, Hunt, Ives, Jonsson, Larsen, Martin, Martínez-Salinas, Meehan, O'Rourke, Poveda, Rosenheim, Rusch, Schellhorn, Wanger, Wratten and Zhang2016) have identified five hypotheses for when and why a more natural habitat does not always lead to more beneficial insects and reduced pest populations. It maintains that some alternative management approaches on local scales (pesticide avoidance, implementing habitat patches, replacing invasive plants with native flora) as well as on a landscape scale (increasing habitat availability and crop diversity) may be bolster biocontrol.
The Mediterranean region of Europe, particularly the province of Almería in south-eastern Spain, has one of the largest concentrations of protected crop production in the world with around 30,000 ha of greenhouse vegetable production. This intensive horticulture creates a landscape characterized by crops under high pressure of pests and diseases (Glass & González, Reference Glass and González2012), with non-crop areas dominated by non-native weedy species and little remaining native vegetation (Mendoza et al., Reference Mendoza-Fernández, Martínez-Hernández, Pérez-García, Garrido-Becerra, Benito, Salmerón-Sánchez, Guirado, Merlo and Mota2015). The whitefly Bemisia tabaci (Gennadius) (Homoptera: Aleyrodidae), namely tobacco whitefly, and the thrips Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae), commonly known as western flower thrips are the most abundance insect pest species in this horticultural system. They are effective vectors of viruses and this is deemed the direst risk of these pests (Gilbertson et al., Reference Gilbertson, Batuman, Webster and Adkins2015). To encourage less insecticide use and promote non-chemical pest-management practices, the EU obliged all Member States to apply the general principles of integrated pest management (IPM) by 2014 (Directive 2009/128/EC). Adoption of IPM has been particularly successful in greenhouse horticulture of Almería, where IPM decreased the need for pesticides considerably through a greater reliance on commercial augmentative biological control, and managing pest pressure through cultural practices (Lozano et al., Reference Lozano, Diánez and Camacho2010; Pérez-Mesa & Galdeano-Gómez, Reference Pérez-Mesa and Galdeano-Gómez2010; Calvo et al., Reference Calvo, Knapp, van Houten, Hoogerbrugge and Belda2015). Therefore, in the current context of pesticides reduction, promoting specific habitats, with emphasis on planting hedgerows containing native perennial plants among greenhouses, may boost biodiversity, encouraging NEs and thus pest control.
Native perennial vegetation has demonstrated value in horticultural systems by showing less risk for hosting vegetable pests than non-native weedy plants, and by harbouring predators and parasitoids of pests (Rencken, Reference Rencken2006; Stephens et al., Reference Stephens, Schellhorn, Wood and Austin2006; Fiedler & Landis, Reference Fiedler and Landis2007a, Reference Fiedler and Landisb; Schellhorn et al., Reference Schellhorn, Glatz and Wood2010; Bianchi et al., Reference Bianchi, Shellhorn and Cunningham2013). The native flora of Almería is typical of Mediterranean semi-arid environments and is rich in shrubs adapted to the harsh and variable climate (Mendoza et al., Reference Mendoza-Fernández, Martínez-Hernández, Pérez-García, Garrido-Becerra, Benito, Salmerón-Sánchez, Guirado, Merlo and Mota2015). Similarly, terrestrial arthropod fauna in semi-arid areas of SE Spain are highly diverse (Piñero et al., Reference Piñero, Tinaut, Aguirre-Segura, Miñano, Lencina, Ortiz-Sánchez and Pérez-López2011). The most suitable perennial plants for hedgerows among greenhouses have been already selected and include shrubs that have become commercially available. These include nectar- and pollen-rich species with overlapping bloom periods and varied habits (Rodríguez et al., Reference Rodríguez, Schwarzer, van der Blom and González2012). On the other hand, previous results have demonstrated that the main viruses affecting greenhouse crops in Almería are not found on native perennial plants (Rodríguez et al., Reference Rodríguez, van der Blom, González, Sánchez, Janssen, Ruiz and Elorrieta2014). Thus, replacing non-native weeds by perennial plants may decrease the sources of viruses at a farming scale (Schellhorn & Bianchi, Reference Schellhorn, Bianchi, Koizumi, Okabe, Thompson, Sugimora, Toma and Fujita2010). However, although biological control programmes have been successfully implemented in Almería horticulture, and perennial plants host fewer vegetable viruses, it remains unclear whether the selected shrubs for hedgerows may provide benefits to horticulture production in terms of pest suppression (top–down and bottom–up effects). It is unknown how pests and their NEs can use newly planted vegetation in highly simplified settings. Therefore, the aim of this study was to identify which perennial plants may harbour pests (tobacco whitefly and western flower thrips) and their NEs, in a set of Mediterranean shrubs newly planted within the greenhouse landscape. This knowledge is much in demand from growers and local authorities and it is a starting point for horticultural management aimed at natural pest prevention.
Material and methods
Site description
Field surveys were conducted in an experimental field at the Experimental Station ‘Las Palmerillas’, in the agricultural region of Almería Province, Spain (36°48′N, 2°3′W and ≈155 m a.s.l.). In December 2010, we established a semi-arid shrub patch (17 m × 10 m in size), that was hand weeded during the study, composed of 3-year-old individuals of 22 plant species pooled, belonging to 13 different botanical families. The experimental design included nectar-rich plants (eight species), pollen-rich plants (six species) and pollen- and nectar-rich plants (seven species) (table 1). The design was meant to simulate natural plant species associations in a spatially explicit semi-arid environment. Each plant species was replicated at a different ratio according to its size. This experimental field was located in the centre of the Campo de Dalías, which is the largest region of greenhouses in Europe and an area of year-round intensive horticulture. Since 2008, all the greenhouses in the experimental area were managed under an IPM regimen with emphasis on augmentative biological control, guaranteeing that native plant–arthropod interactions were not affected by the impact of pesticides.
Table 1. Shrub species selected and sampled for habitat management in semi-arid Mediterranean greenhouse areas.
Collecting pests and NEs
After 18 months, when the perennial plants were well established, the insects were sampled monthly in 162 plants from June 2012 to June 2013 at a patch scale (17 m × 10 m in size). The insects were collected by vacuuming each shrub for 40 s (Stihl® SH 85C), time enough to vacuum the entire surface area of the shrub (Fiedler & Landis, Reference Fiedler and Landis2007a). Insects from each shrub were collected in a fine mesh bag inserted into the vacuum nozzle. Bags were labelled and kept on ice until identification in the laboratory. Particularly, the pest species identified were the western flower thrips and the tobacco whitefly. As reproduction habitats can be identified by the presence of wingless and less mobile immature stages, and the feeding/resting habitats can be identified by the presence of mobile adults (Bianchi et al., Reference Bianchi, Shellhorn and Cunningham2013), data from the pests were sorted into immature stages and adults. The major NEs identified included the whitefly parasitoid Eretmocerus ssp. (Hymenoptera: Aphelinidae), the whitefly predator Nesidiocoris tenuis (Reuter) (Hemiptera: Miridae), the thrips parasitoid Ceranisus ssp. (Hymenoptera: Eulophidae) and the thrips predator Orius spp. (Hemiptera: Anthocoridae). Most of these, including Eretmocerus ssp., N. tenuis and Orius spp., are being mass reared and used for augmentative releases in the study area.
To assess whether seasonal abundance of pests in crops (indoors) was similar to that in perennial plants (outdoors), a comparison was made. The data from crops are shown as the percentage of plants damaged by western flower thrips and tobacco whitefly, and were provided by the Andalusia's Alert and Phytosanitary Information Network (RAIF). Particularly, the information used in this study refer to tomato, pepper, eggplant and cucumber grown in greenhouses that surrounded the experimental field during the sampling. A key point in this network is the availability of up-to-date real data, taken in the field on a regular basis by the agricultural technicians of the different associations (http://www.tragsa.es/_layouts/GrupoTragsa/Ficha-Proyecto.aspx?param=ENG.0000000187).
Flowering
To relate the occurrence of pests and NEs with plant-food resources, we assessed the flowering of each native plant each month of the sampling year (from June 2012 to June 2013), with dates of day before arthropod collection. Flowering was calculated by estimating the overall flower abundance per plant (scale 0–1).
Data analysis
To assess differences in the abundance of the different insects, we first pooled all the data referring to a single plant of each species and then performed a generalized linear model with a Poisson error structure. Next, we analysed the results of the model outputs with a Tukey's post hoc evaluation to determine significant differences between the plant species tested. To separate the plants with a higher risk of harbouring pests from plants with a lower risk, we show significant differences from the Tukey's test only for those plants that surpassed the average pest abundance. A set of generalized linear mixed models were built to identify the resource (blooming or host) that best explained the abundance of pests and NEs in each plant species, as appropriate. For the two pests (tobacco whitefly and western flower thrips), we built the models by including as a fixed factor the plant species and their percentage of flowering. Sampling date was included as a random factor. A set of eight models was generated by combining fixed and random factors. For NEs, we built models in the same way as for pests but we included the variable prey representing the abundance of the above-mentioned pest species associated with their NE. Thus, for N. tenuis and Eretmocerus spp., we used the abundance of tobacco whitefly, and for Orius spp. and Ceranisus spp., we used the abundance of western flower thrips. Thus, for NEs, we generate 12 models by combining the fixed factors plant species, flowering and prey together with the random factor sampling date. Similarly, only the plants that showed higher abundance than the average established are shown.
We chose the model-selection procedure as an alternative to traditional hypothesis testing (Johnson & Omland, Reference Johnson and Omland2004; Canham & Uriarte, Reference Canham and Uriarte2006). Alternative models were compared using the Akaike information criterion (AICc) corrected for small sample sizes (Burnham & Anderson, Reference Burnham and Anderson2002). Models with a difference in AICc > 2 indicate that the worse model has virtually no support and can be omitted from further consideration. Models were tested for validation by analysing residuals using the DHARMa package (Hartig, Reference Hartig2016). For the best model, we calculated the R 2 to account for the variability supported by the best models. Two components of R 2 can be calculated for generalized linear mixed models: (1) a marginal R 2m that takes into account only the variability explained by fixed effects; and (2) a conditional R 2c that accounts for the variability supported by both the fixed and random effects (Nakagawa & Schielzeth, Reference Nakagawa and Schielzeth2013). Analyses were made with the packages ‘lme4’ (Bates et al., Reference Bates, Maechler, Bolker and Walker2014) and ‘multcomp’ (Hothorn et al., Reference Hothorn, Bretz, Westfall, Heiberger, Schuetzenmeister and Scheibe2016) written for the R environment (R Development Core Team, 2014). Based on these two indicators, i.e. R 2 and DHARMa outputs, we chose those response variables to be considered for further discussion, as stated in the following section.
Results
Seasonal distribution of pests in horticultural crops (indoors) and in perennial plants (outdoors)
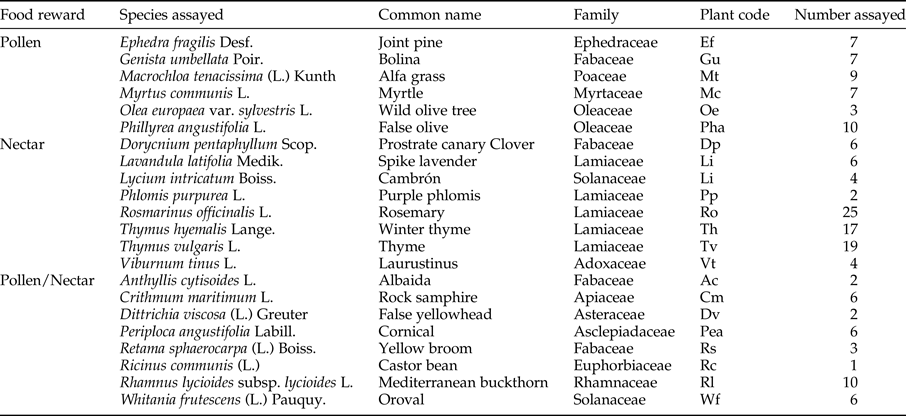
Tobacco whitefly and western flower thrips showed similar abundance patterns (fig. 1) in horticultural crops within greenhouses (indoors) and in perennial plants (outdoors). Particularly, tobacco whitefly abundance tended to be low in the perennial plants, registering a peak only in October with 9.9 whiteflies per plant. In crops, around 10% of the plants were damaged and the pest reached a peak of abundance with 18% of the plants damaged 1 month earlier than outdoors (fig. 1a). Therefore, the period when tobacco whitefly was present within the greenhouses was longer than outside (fig. 1a). The highest population of whitefly was recorded at 29.9°C indoors and 25.7°C outdoors for temperature, respectively. Western flower thrips gradually increased in abundance outdoors during late January, when temperatures were relatively low (12–13°C), peaking with 35.23 thrips per plant in late March with a considerable population decline throughout April and May. Within the greenhouses, where temperatures were higher than indoors (16–25°C), this pest was active longer than in perennial plants, peaking earlier than outdoors in mid-October (18% of plants damaged), and two sharper spikes during January and April (31 and 45% of the plants damaged, respectively) (fig. 1b). As with tobacco whitefly, the abundance of western flower thrips was delayed outdoors compared with indoors.
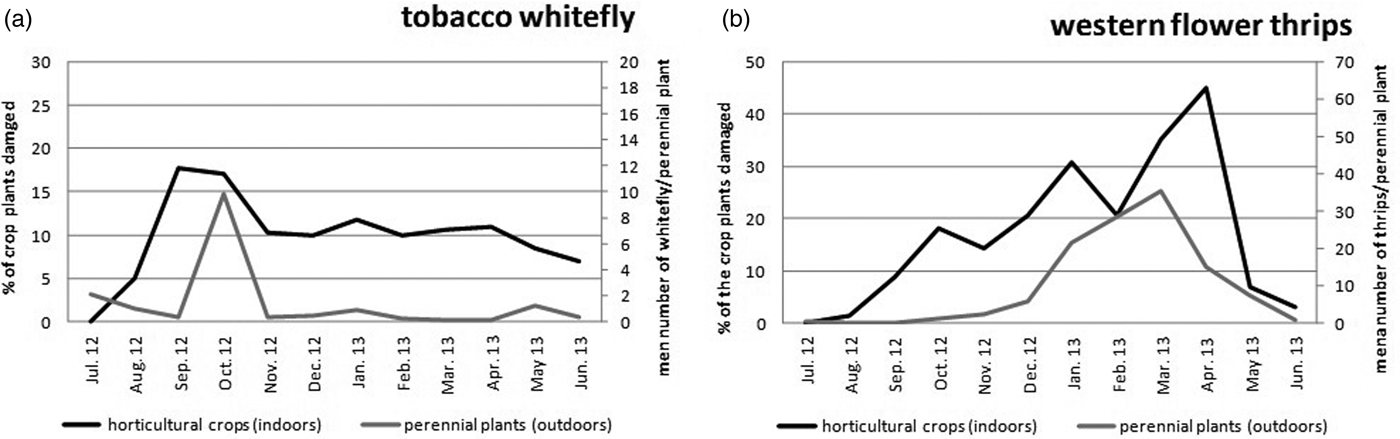
Fig. 1. Monthly mean numbers per perennial plant of the two major horticultural pests: tobacco whitefly (Bemisia tabaci) (a) and western flower thrips (Frankliniella occidentalis) (b), and percentage of the horticultural crop plants damaged during the growing season 2012/2013 in Almeria (south-eastern Spain).
Identifying perennial plants harbouring pests
Model estimation displayed a mean value for tobacco whitefly abundance in perennial plants of 16.6 ± 22.7 whiteflies per plant. This pest was significantly higher on Dodonaea viscosa, Withania frutescens, Thymus vulgaris, Thymus hyemalis and Dorycnium pentaphyllum (fig. 2a). Particularly, the maximum estimated value for tobacco whitefly exceeded 50 individuals in two cases. However, excepting these five plant species, the estimated whitefly abundance on the perennial plants tested remained below eight, with 7.4 ± 3.6 whiteflies per plant. For immature stages of the pest, the estimated value was below two on all perennial plants (fig. 2b). However, the value of immature pests was significantly higher in some plant species including D. viscosa and T. vulgaris (with 13.5 and 11.3 15 immature per plant, respectively), followed by T. hyemalis and W. frutescens (with <3 immature per plant) (fig. 2b). Western flower thrips were found in plant species with an estimated mean value of 101.39 + 105.9 thrips per plant. This pest reached the highest values in six of those species: D. pentaphyllum, Grimpoteuthis umbellata, Rosmarinus officinallis, Anthyllis cytisoides, W. frutescens and T. hyemalis (fig. 2c). The first two plants exceeded 300 estimated individuals per plant and about 50% of plants tested exceeded 50 individuals of maximum estimated value (fig. 2c). As in the case of whitefly, the plant species that supported higher abundance values of adult stages of western flower thrips also recorded the higher abundance of immature stages of this pest. The highest number of immature pest was found in Rubus occidentalis (72 immature/plant), followed by D. pentaphyllum, T. hyemalis (44 and 41 immature/plant, respectively), W. frutescens, G. umbellata, A. cytisoides (29, 27 and 24 immature/plant, respectively) and T. vulgaris (16 immature/plant) (fig. 2d). Next, a set of three plant species, including Phanera purpurea, Lycium intricatum and Lavandula latifolia, supported a lower level of both pests compared with the previous plant species. Finally, a set of ten plant species was identified for supporting the lowest levels of both of the two horticultural pests. These plants were: Prunus angustifolia, Crithmum maritimum, Retama sphaerocarpa, Rhamnus lyciodes, Marsdenia tenacissima, Olea europaea var. sylvestris, Ephedra fragilis, P. angustifolia, Macrozamia communis and Viburnum tinus (fig. 2). The lowest values of immature stages were also observed for such plants species.
Fig. 2. Estimated abundance value per plant of adults + immature stages of tobacco whitefly (Bemisia tabaci) (a), immature of tobacco whitefly (b), adults + immature stages of western flower thrips (Frankliniella occidentalis) (c) and immature of western flower thrips (d). Small letters denote significant pairwise Tukey's differences between perennial plants species (P < 0.05). Significant differences were shown only when estimated abundance was equal to or higher than the mean of the estimated value. For plant species code, see table 1.
Pests, NEs and flowering
For both pests, the best model that predicts their occurrence in perennial plants included the plant species and their corresponding flowering period (table 2). In the case of tobacco whitefly, the model predicted that, when T. vulgaris was blooming, it supported more whiteflies than other blooming plants such as T. hyemalis and W. frutescens. However, in D. viscosa and D. pentaphyllum, no more whiteflies were collected even though abundant flowers were present (fig. 3a). Flowering also significantly influenced the abundance of western flower thrips (fig. 3b). Overall, the model predicted that the bloom period of the solanaceous plant W. frutescens coincided with the highest abundance of western flower thrips. However, the abundance of thrips did not necessarily correspond to the availability of floral resource on G. umbellata, one of the plants most preferred by this pest.

Fig. 3. Model estimations for the best model selected showing the relationship between flowering and abundance of the pests, tobacco whitefly (Bemisia tabaci) (a) and western flower thrips (Frankliniella occidentalis) (b) in the most preferred perennial plants.
Table 2. Comparison of alternative models (using AICc) for the two pests tested in this study (i.e. Bemisia tabaci and Frankliniella occidentalis) to identify flowering-dependent effects of pest occurrence in native plants. According to the rule that ∆AIC < 2 suggests the best parsimony in a group of candidate models, only one model (marked in bold) was selected to likely explain the pests’ abundance in flowering-native plants. The marginal (m) and conditional (c) R 2 refer to the best model.
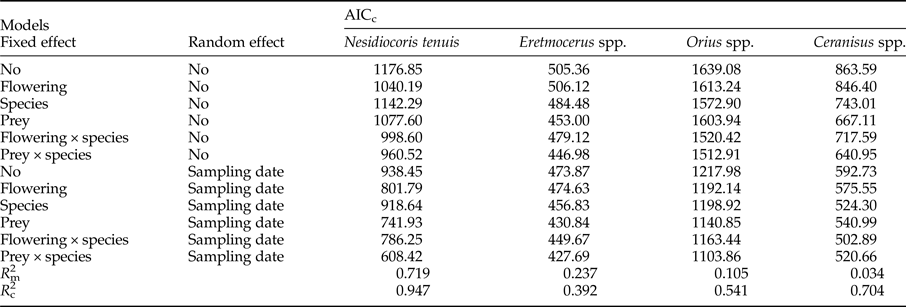
Regarding NEs, the models indicated that, excepting the parasitoid of thrips Ceranisus spp., host/prey availability was better than flowering as a predictor of the occurrence of NEs in perennial plants (table 3). With regard to NEs of tobacco whitefly, two plants species, D. viscosa and P. purpurea, harboured more predators (N. tenuis) and parasitoids (Eretmocerus spp.), respectively, than did other plants (fig. 4). In the case of western flower thrips, the abundance of the predator Orius spp. was higher in the aromatic species T. vulgaris than in the other plants (fig. 4). In the particular case of the parasitoid Ceranisus spp., its occurrence was greater in D. pentaphylllum and R. officinalis when these plants were in bloom (fig. 4).

Fig. 4. Model estimations for the best model selected for each of the natural enemies tested in this study showing the relationship between their abundance and the predictors of occurrence (flowering vs. host/prey availability) in different plant species (see table 3).
Table 3. Comparison of alternative models (using AICc) for the natural enemies tested in this study to identify their occurrence on native plants. According to the rule that ∆AIC < 2 suggests the best parsimony into a group of candidate models, only one model (marked in bold) was selected to likely explain the natural enemies’ abundance. The marginal (m) and conditional (c) R 2 refer to the best model.
Discussion
Despite boosting the abundance of major NEs, native flora also can also reportedly increase the abundance of some potential pest species for nearby crops (Fiedler & Landis, Reference Fiedler and Landis2007a, Reference Fiedler and Landisb; Danne et al., Reference Danne, Thomson, Sharley, Penfold and Hoffmann2010; Winkler et al., Reference Winkler, Wäckers, Termorshuizen and van Lenteren2010). Thus, the identity of perennial plants and the timing of pest attacks on them have significant relevance for habitat management (Lavandero et al., Reference Lavandero, Wratten, Didham and Gurr2006). In this study, the seasonal distribution of the two pests, tobacco whitefly and western flower thrips, in crops such as tomato, pepper, eggplant and cucumber, as well as in perennial plants proved very similar. Moreover, the pests occurred for less time in perennial plants than in crops, and later outdoors than indoors. The warm conditions and abundant food in a greenhouse is likely to provide a stable environment for pest development. The results indicate that perennial plants did not represent an initial pest source for crops. In this sense, Schellhorn et al. (Reference Schellhorn, Glatz and Wood2010) found that crops support higher densities of thrips than do perennial plants, and Bianchi et al. (Reference Bianchi, Shellhorn and Cunningham2013) showed that crops usually act as sources of pest species much more than do shrubs.
Tobacco whitefly and western flower thrips were collected from all plant species. However, three plant species, W. frutescens, D. pentaphyllum and T. hyemalis, harboured more pests than did all other plants. More specifically, two plant species, D. viscosa and T. vulgaris, supported more whiteflies, while another three species, G. umbellata, R. officinalis and A. citysoides, had more western flower thrips. In general, adults and immature stages of pests were more abundant in the same set of plant species, indicating that reproduction and feeding took place on the same host plants. However, it should be noted that immature stages of tobacco whitefly were found at low densities in perennial plants, and this could have been influenced by the sampling, which was carried out by vacuum, which likely biased samples towards sessile stages, such as immature stages of tobacco whitefly. This pest is an enormously polyphagous insect associated with almost 600 different species of plants, including cultivated and non-cultivated annuals and perennials worldwide, although it prefers Asteraceae, Cruciferae, Cucurbitaceae, Euphorbiaceae, Fabaceae, Labiatae, Malvaceae and Solanaceae families (Inbar & Gerling, Reference Inbar and Gerling2008; Shah et al., Reference Shah, Zhang and Liu2015). In the present study, this pest was more abundant in perennial species from Asteraceae, Labiateae, Fabaceae and Solanaceae. Its abundance was not clearly associated with flowering in all plant species, signifying that floral resources themselves had no strong impact on the occurrence of the pest, though its abundance was higher in some plants when they were in bloom, such as T. vulgaris. Similarly, Fiedler & Landis (Reference Fiedler and Landis2007b) found that herbivore abundance in perennial plants, including sap-sucking insects, increased with particular floral traits although the pests responded more weakly to these traits than did the NEs. Our result is difficult to interpret because whiteflies are phloem-feeding insects and do not feed on floral resources; thus, the positive impact of flowering on tobacco whitefly abundance is probably related to some quality changes linked to blooming of each perennial plant species (Rebek et al., Reference Rebek, Sadof and Hanks2005). Hence, tobacco whitefly abundance was limited to a very specific, short period of time, and occurred in high abundance only in a few plant species, suggesting that plant diversity may provide relatively few acceptable host plants for this pest. Implications for practice of these results are of relevance, since providing plant diversity among greenhouses may be a promising option to reduce pest pressure outdoors by a bottom–up effect, operating via diversification of the first trophic level (Finch & Collier, Reference Finch and Collier2000; Gurr et al., Reference Gurr, Wratten and Luna2003; Aguilar-Fenollosa et al., Reference Aguilar-Fenollosa, Ibáñez-Gual, Pascual-Ruiz, Hurtado and Jacas2011). On the other hand, seasonal abundance of western flower thrips in perennial plants was greater than that of tobacco whitefly, and also western flower thrips were supported by a broad spectrum of perennial plants than tobacco whitefly. Thus, although western flower thrips appear to prefer some plant species over others, it was prevalent on almost all the plant species studied; therefore, the polyphagous nature of these thrips creates a more difficult pest-control situation outdoors. A total of 244 species of plants belonging to 62 different plant families have also been found to host western flower thrips, including open agricultural crops, ornamentals and protected crops (Tommasini & Maini, Reference Tommasini, Maini, Loomans, van Lenteren, Tommasini, Maini and Riudavets1995; Lewis, Reference Lewis1997). Few studies have reported western flower thrips occurrence on non-crop plant species, but our results coincide with some reports from the USA (Cockfield et al., Reference Cockfield, Beers and Zack2007; Miliczky & Horton, Reference Miliczky and Horton2011), Chile (Ripa et al., Reference Ripa, Funderburk, Rodríguez, Espinoza and Mound2009), South Africa (Allsopp, Reference Allsopp2010) and South Australia (Schellhorn et al., Reference Schellhorn, Glatz and Wood2010), where this pest is usually collected in large numbers from a number of weed species growing in and around the fields and also from several perennial plants. Furthermore, our results show that flowering positively influenced western flower thrips abundance in perennial plants. Adults of this pest are highly mobile and feed primarily by piercing plant cells but also consume floral parts such as petals and pollen, so that adults are often found in large numbers in flowers of several plants and crops (Tommasini & Maini, Reference Tommasini, Maini, Loomans, van Lenteren, Tommasini, Maini and Riudavets1995; Lewis, Reference Lewis1997). Therefore, the overlapping bloom period of the perennial plant species in the experimental field might also ensure continuous availability of floral (food) resource for western flower thrips adults, this explaining why this pest was more polyphagous and ubiquitous in perennials than was tobacco whitefly. However, Schellhorn et al. (Reference Schellhorn, Glatz and Wood2010) showed that crop proximity influenced the probability of density of western flower thrips in flowers of exotic weeds and native plants species, but the effect appears to be less so when the adjacent vegetation is native. Particularly, our results show that flowers of the solanaceous plant W. frutescens were highly attractive to the pest. Strikingly, the flowering of the leguminous plant G. umbellata, which supported the highest level of the pest, had a negligible influence on western flower thrips abundance. In this case, pest peak coincided with G. umbellata bud break (data not shown), suggesting that the pest was present in pre-bloom inflorescences. The attraction of floral buds to western flower thrips has been reported in plant hosts (Ripa et al., Reference Ripa, Funderburk, Rodríguez, Espinoza and Mound2009; Allsopp, Reference Allsopp2010). On this basis, flowering on W. frutescens and pre-bloom inflorescences on G. umbellata could be significant predictors of western flower thrips occurrence outdoors, this opening the opportunity for researching the use of these perennial plants as traps for controlling this pest outdoors.
Regarding the NEs, the same plants that were hosts for tobacco whitefly and western flower thrips, also hosted their potential predators and parasitoids. Furthermore, results showed that host/prey availability was a stronger predictor of NE abundance on perennial plants than was food (floral parts). Other studies have shown that the abundance of NE on attractive plant species is explained not only by bloom, and that other important attractions include shelter, suitable micro-climate and prey/host availability. These are also essential components for conservation biological control (Rebek et al., Reference Rebek, Sadof and Hanks2005; Fiedler & Landis, Reference Fiedler and Landis2007b; Witting et al., Reference Witting, Orr and Linker2007; MacLeod & Winfree, Reference MacLeod and Winfree2011) and are especially relevant in fields that encourage biodiversity with perennial plants (Griffiths et al., Reference Griffiths, Holland, Bailey and Thomas2008; Gareau et al., Reference Gareau, Letourneau and Shennan2013). In particular, the whitefly predator N. tenuis was abundant in D. viscosa. This zoophytophagous predator maintains a close relationship with its host plants by using them not only to feed on, but also as an oviposition substrate. The relationship between this Mediterranean shrub and N. tenuis has been previously reported (Sánchez et al., Reference Sánchez, Martínez-Cascales and Lacasa2003; Cano et al., Reference Cano, Vila, Janssen, Bretones, Salvador, Lara and Téllez2009), and it has been studied for its potential role as a companion plant to control other important horticultural pests such as Tuta absoluta (Lepidoptera: Gelechiidae) in tomato crops (Biondi et al., Reference Biondi, Zappalà, Di Mauro, Garzia, Russo, Desneux and Siscaro2016). The whitefly parasitoid Eretmocerus spp., collected mainly in P. purpurea, was also a host for tobacco whitefly. It is known that host plants of tobacco whitefly may notably mediate the activity of parasitoids, since plant species and varieties have foliar attributes such as volatile compounds, presence or absence of pubescence and/or wax, etc., that affect parasitism rates (Inbar & Gerling, Reference Inbar and Gerling2008; Shah et al., Reference Shah, Zhang and Liu2015). The pirate bug, Orius spp., was found mainly in the aromatic plant T. vulgaris. Orius spp. is an important predator of thrips and other soft-bodied insects throughout the world (Lewis, Reference Lewis1997). Although they feed chiefly on prey, they also rely on different plant resources such as sap (Lundgren et al., Reference Lundgren, Fergen and Riedell2008), pollen (Kiman & Yeargan, Reference Kiman and Yeargan1985) and nectar (Bugg, Reference Bugg1987) from several plant species when prey is scarce. Thus, an association with plants species such as T. vulgaris could give Orius spp. access to both prey and plant food. The thrips parasitoid Ceranisus spp. was collected primarily in flowers of D. pentaphyllum and R. officinalis. Similarly, Lacasa et al. (Reference Lacasa, Sánchez and Lorca1996) pointed out R. officinalis as a primary plant to collect Ceranisus spp., in surveys conducted in wild plants near to greenhouses in 1996, to find potential host plants of this thrips parasitoid.
In conclusion, from all the perennial plants assessed, eight native plants were the most critical species for sustaining pest population outdoors. It has been possible to identify a subset of five plant species that supported less abundance of pests, and other subset of ten plant species that supported a very low level of pests. The tobacco whitefly was more discriminating in selecting host plants than was the western flower thrips, which were supported by a broad spectrum of perennial plants, and which showed that their abundance was favoured by blooming. NEs generally attained their highest abundance on host plants that also supported high pest abundance, suggesting that floral resource was not a strong predictor of NEs’ abundance on native plant species. Consequently, the lower host plant acceptance observed for tobacco whitefly offers a promising option to reduce this pest pressure outdoors by a bottom–up effect, operating via diversification of the first trophic level. All the perennial plants that supported low–medium pest level in the present study could be beneficial choices for conservation biocontrol in surrounding greenhouses because they might provide hosts with parasitoids and predators while not significantly benefiting the pests. Field trials in which these perennial plants can be implemented as hedgerows among greenhouses are needed to ascertain whether pest abundance outdoors is reduced.
Acknowledgements
This work was supported by ERDF (Regional Development Fund) co-financed grant RECUPERA 2020 from the CSIC (Spanish Ministry of Economy and Competitiveness), by TRANSFORMA (PP.TRA.TRA201600.9) and FEDER. E. Rodríguez held a postdoctoral contract (DOC-INIA program) granted by Spanish National Institute for Agricultural and Food Research and Technology (INIA) and the European Social Fund.










