Antisocial behavior during childhood and adolescence can lead to serious harmful behavior against others, adult crime, and psychopathology (Farrington, Reference Farrington1995; Kokko, Pulkkinen, Huesman, Dubow, & Boxer, Reference Kokko, Pulkkinen, Huesman, Dubow and Boxer2009) later in development. Antisocial behavior herein refers to externalizing behavior problems (e.g., aggression and delinquent behavior; Achenbach & Rescorla, Reference Achenbach and Rescorla2001), conduct disorder (e.g., aggression that causes physical harm), and oppositional defiant symptoms (e.g., disobedient and hostile behavior toward parents). Antisocial behavior and its relation to reactivity to stressors in early adolescence is an especially important problem given that stress and aggression are linked (Holz et al., Reference Holz, Boecker, Buchmann, Blomeyer, Baumeister, Hohmann and Laucht2014). Going back decades stress was considered to rise with the neuroendocrine hormone changes of puberty, but virtually no longitudinal studies assess the simultaneous trajectory of the stress hormone cortisol and a gonadal steroid marker of pubertal progression, testosterone. Although heavily speculated that testosterone is a mechanism involved in aggressive behavior (Archer, Reference Archer2006; Holtz et al., Reference Holz, Boecker, Buchmann, Blomeyer, Baumeister, Hohmann and Laucht2014; van Goozen, Fairchild, Snoek, & Harold, Reference van Goozen, Fairchild, Snoek and Harold2007), the pubertal rise and daily rhythms in testosterone secretion (Grumbach & Styne, Reference Grumbach, Styne, Larsen, Kronenberg, Melmed and Polonsky1998) rarely are measured directly in relation to cortisol reactivity and antisocial behavior, especially in young adolescents or girls. The current study was designed to answer two important questions. Is there a correspondence between cortisol reactivity and diurnal testosterone across 1 year in young adolescents? Does cortisol reactivity and diurnal testosterone interact to predispose boys and girls toward antisocial behavior over time? Identifying the interaction between cortisol reactivity and diurnal testosterone is of critical importance given the prevalence (e.g., Centers for Disease Control and Prevention, 2014) of antisocial behavior in early adolescence. Currently, there are insufficient longitudinal data of high methodological rigor to confirm that changing testosterone levels during puberty are significantly associated with mood and behavior (Duke, Balzer, & Steinbeck, Reference Duke, Balzer and Steinbeck2014). The findings have the potential to advance the field by identifying an important hormone mechanism involved in the interaction between stress and antisocial behavior and perhaps the design of preventive interventions to reduce antisocial behavior.
The rationale and importance for examining these questions considers, first, the longitudinal changes in cortisol reactivity and diurnal testosterone during early adolescence. Cortisol reactivity rarely has been examined longitudinally during early adolescence, with some exceptions (Gunnar, Wewerka, Frenn, Long, & Griggs, Reference Gunnar, Wewerka, Frenn, Long and Griggs2009; Stroud et al., Reference Stroud, Foster, Papandonatos, Handwerger, Granger, Kivlighan and Niaura2009) and is virtually never examined in relation to puberty-related testosterone increases, especially in girls, in spite of research showing that both cortisol reactivity and testosterone are related to antisocial behavior (Duke et al., Reference Duke, Balzer and Steinbeck2014; Susman, Dockray, et al., Reference Susman, Dockray, Granger, Blades, Randazzo, Heaton and Dorn2010). Gunnar et al. (Reference Gunnar, Wewerka, Frenn, Long and Griggs2009) showed that cortisol reactivity increased with age from age 11 to 13 years in boys. There was also a significant increase in cortisol from age 13 to 15 years in girls. Cortisol reactivity is expected to rise during puberty in the current sample given the fluctuating hormone environment and multiple social stressors of young adolescence (Gunnar et al. Reference Gunnar, Wewerka, Frenn, Long and Griggs2009; Susman, et al., Reference Susman, Inoff-Germain, Nottelmann, Loriaux, Cutler and Chrousos1987). A related question is whether the change in cortisol reactivity is unique to puberty and its multiple endocrine changes or is part of a developmental trend from childhood to adulthood. If testosterone does play a role in increases in reactivity to stressors there should be parallel (correlated) growth in both cortisol reactivity and testosterone. Second, consistent with the framework used by Eisenberg et al. (Reference Eisenberg, Sulik, Spinrad, Edwards, Eggum, Liew and Hart2012) on the interaction between physiological reactivity, the exogenous environment, and adjustment problems, two theoretical perspectives were integrated herein to conceptualize the interaction between cortisol reactivity and diurnal testosterone as a moderator of cortisol reactivity: diathesis–stress (Zuckerman, Reference Zuckerman1999) and biological sensitivity to context (BSC; Boyce & Ellis, Reference Boyce and Ellis2005) and differential susceptibility (DS; Belsky & Pluess, Reference Belsky and Pluess2009). BSC and DS share similarities in that both emphasize the importance of contexts. Individual differences in cortisol reactivity (hypo- and hypercortisol) to contextual stressors are considered a reflection of a vulnerability or susceptibility to endogenous and exogenous-contextual stressors.
The diathesis–stress hypothesis assimilates well with the BSC and DS perspectives. The diathesis–stress framework proposes that some individuals are disproportionately vulnerable to adversity (Monroe & Simmons, Reference Monroe and Simmons1991; Richters & Weintraub, Reference Richters, Weintraub, Rolf, Masten, Cicchetti, Nuechterlein and Weintraub1990; Zuckerman, Reference Zuckerman1999). The theoretical model proposed here suggests that children who are vulnerable to exogenous stressors also will be vulnerable to endogenous stressors like changes in the endocrine milieu at puberty. Specifically, children who are more reactive to exogenous stressors also were expected to be more reactive to endogenous stressors like testosterone given testosterone's mood- and behavior-altering capabilities (Duke et al., Reference Duke, Balzer and Steinbeck2014). Nonetheless, individual differences in response to testosterone changes will vary across adolescents and across age.
The BSC perspective proposes that some children are more sensitive to environmental stressors (exogenous and contextual) than others during childhood and they may be positively or negatively affected by these sensitivities. Children who are more reactive (sensitive) to environmental stressors may exhibit dysregulated behavior such as aggression (Belsky & Pluess, Reference Belsky and Pluess2009; Boyce & Ellis, Reference Boyce and Ellis2005; Del Guidice, Ellis, & Shirtcliff, Reference Del Giudice, Ellis and Shirtcliff2011; Pluess & Belsky, Reference Pluess and Belsky2011). Scientists have conceptualized individual differences in sensitivity to the environment in slightly different ways. Boyce and Ellis (Reference Boyce and Ellis2005) introduced the notion of BSC based on empirical observations of differences in children's autonomic and adrenocortical reactivity to challenge and proposed a context-sensitive endophenotype. Some children are proposed to be unusually susceptible to the risk-enhancing environmental influences of negative early social environments. Using evolutionary terminology Belsky and Pluess (Reference Belsky and Pluess2009) hypothesized that children should differ in their susceptibility to rearing environments as a strategy against an uncertain future. Both perspectives are built on evolutionary explanations of why children vary systematically in their sensitivity to experiential and contextual influences on development and health (Ellis, Boyce, Belsky, Bakermans-Kranenburg, & van IJzendoorn, Reference Ellis, Boyce, Belsky, Bakermans-Kranenburg and van IJzendoorn2011). Specifically, DS to stressors will be reflected in individual differences in hypothalamus–pituitary–adrenal (HPA) axis activation. Further, BSC will be reflected in the endogenous context of changing testosterone concentrations. In brief, we expanded the notion of stress–diathesis, DS, and BSC social and familial contextual influences by considering the neuroendocrine context, that is, the hypothalamus–pituitary–gonadal (HPG) activated hormone testosterone and propose that children with a preexisting susceptibility/sensitivity to the influence of exogenous stressors will be especially sensitive to endogenous, testosterone increases (neuroendocrine context); thus, testosterone will be a potent moderator of cortisol reactivity and antisocial behavior given the energizing and mood altering qualities of testosterone (Brain & Susman, Reference Brain, Susman, Stoff, Breiling and Maser1997).
Cortisol Reactivity as an Index of Sensitivity to the Neuroendocrine Context
The relation between cortisol reactivity and antisocial behavior is reported to be negative (Fairchild et al., Reference Fairchild, van Goozen, Stollery, Brown, Gardiner, Herbert and Goodyer2008; Gordis, Granger, Susman, & Trickett, Reference Gordis, Granger, Susman and Trickett2009; Lopez-Duran, Olsen, Hajal, Felt, & Vazquez, Reference Lopez-Duran, Olson, Hajal, Felt and Vazquez2009, van Goozen et al., Reference van Goozen, Fairchild, Snoek and Harold2007), positive (e.g., Lopez-Duran et al., Reference Lopez-Duran, Olson, Hajal, Felt and Vazquez2009; Susman, Dorn, Inoff-Germain, Nottelmann, & Chrousos, Reference Susman, Dorn, Inoff-Germain, Nottelmann and Chrousos1997), or have no relation (Klimes-Dougan, Hastings, Granger, Usher, & Zahn-Waxler, Reference Klimes-Dougan, Hastings, Granger, Usher and Zahn-Waxler2001). In a meta-analysis, aggression was related to baseline cortisol but not cortisol reactivity to a stressor (Alink et al., Reference Alink, van IJzendoorn, Bakermans-Kranenburg, Mesman, Juffer and Koot2008). Studies since the 2008 review similarly are inconsistent regarding whether cortisol reactivity is positively or negatively related to aggression. These inconsistencies are explained by dysfunctions of the serotonergic system, brain developmental differences, composition of the sample, outcome measures (van Goozen et al., Reference van Goozen, Fairchild, Snoek and Harold2007), and variations in exposure to violence (Peckins, Dockray, Eckentode, Heaton, & Susman, Reference Peckins, Dockray, Eckenrode, Heaton and Susman2012). An additional explanation is that adverse conditions that produce elevated cortisol early in life are hypothesized to contribute to the development of hypocortisolism in adulthood (Gunnar & Vazquez, Reference Gunnar and Vazquez2001; Susman, Reference van Bokhoven, van Goozen, van Engeland, Schaal, Arseneault, Séguin and Tremblay2006). Alternatively, Gunnar and Vazquez (Reference Gunnar and Vazquez2001) suggest that hypo- and hyperreactivity can reflect normal variations in cortisol reactivity in children. Finally, reduced or enhanced cortisol reactivity reflects the presence of internalizing or externalizing problems, respectively (e.g., Han, Miller, Cole, Zahn-Waxler, & Hastings, Reference Han, Miller, Cole, Zahn-Waxler and Hastings2015). Additional longitudinal studies are needed to resolve this issue.
Contextual changes also can explain differences in cortisol reactivity. Children showed different patterns of cortisol reactivity prior to (hypoarousal) and after (hyperarousal), a school transition (Shirtcliff & Essex, Reference Shirtcliff and Essex2008). Hypo- or hypercortisol reactivity may reflect early, prior to puberty, psychological vulnerabilities (i.e., diathesis–stress) secondary to rearing experiences, genetics, and exposure to adversity such as maltreatment (Heim & Nemeroff, Reference Heim and Nemeroff2001). In addition to early experiences, hypo- or hypervariation in cortisol reactivity also is considered a reflection of adaptation of the stress system in current, novel, and challenging situations otherwise considered a susceptibility index to environmental challenges (Belsky & Pluess, Reference Belsky and Pluess2009; Pluess & Belsky, Reference Pluess and Belsky2011). There is mounting evidence that lower cortisol reactivity is typically associated with negative affect or aggression (e.g., Haltigan, Roisman, Susman, Barnett-Walker, & Monahan, Reference Haltigan, Roisman, Susman, Barnett-Walker and Monahan2011; Koss et al., Reference Koss, George, Cicchetti, Davis, Cummings and Sturge-Apple2013; Laurent, Ablow, & Measelle, Reference Laurent, Ablow and Measelle2012). However, given the nature of the acute laboratory stressor, pubertal-age children who are highly cortisol sensitive to the laboratory challenge are expected to increase in cortisol reactivity whereas others may decrease in cortisol reactivity.
Adverse conditions that produce elevated cortisol levels early in life are hypothesized to contribute to the development of hypocortisolism in adulthood. Briefly, adverse conditions, such as the trauma of maltreatment, or even less severe traumas (e.g., child care circumstances; Haltigan et al., Reference Haltigan, Roisman, Susman, Barnett-Walker and Monahan2011) that produce elevated cortisol levels early in life may contribute to hypocortisolism later in life. The hypothesized mechanism is that over time the HPA axis adapts to heightened cortisol by not reacting to acute stressors (Susman, Reference Susman2006). Alternatively, hypercortisolism may reflect acute or less severe or infrequent exposure to trauma in early life. The inconsistencies in cortisol reactivity in a lab-based stress paradigm can reflect life history events that cannot be controlled. Given the ubiquity of the findings of hyporeactivity and hypereactivity in comparable laboratory settings, it is essential that more long-term studies be done to test adverse events as a precursor to antisocial or aggressive behavior. Adverse conditions that produce elevated cortisol levels early in life are hypothesized to contribute to the development of hypocortisolism in adulthood. Therefore, the inconsistent findings may reflect the possible confounds of unknown historical incidences affecting both antisocial behavior and cortisol.
Testosterone as a Neuroendocrine Context
Testosterone is considered a mechanism important for the regulation of aggression in vertebrate species, including birds, hamsters, and humans (Soma, Reference Soma2006), and is also an endogenous marker of degree of pubertal maturation, particularly in boys. The activation of the HPG axis at puberty is responsible for the increase in testosterone, reproductive capability of the gonads, and secondary sex characteristics, and may have an important effect on antisocial behavior via correlated brain changes (Paus et al., Reference Paus, Nawaz-Khan, Leonard, Perron, Pike, Pitiot and Pausova2010). Testosterone increases in both sexes across puberty, and testosterone and antisocial behavior have been related in both observational and clinical trials (Archer, Reference Archer2006; Brooks-Gunn & Warren, Reference Brooks-Gunn and Warren1989; Finkelstein et al., Reference Finkelstein, Susman, Chinchilli, Kunselman, D'Arcangelo, Schwab and Kulin1997; Susman et al., Reference Susman, Inoff-Germain, Nottelmann, Loriaux, Cutler and Chrousos1987). Strong support for the influence of testosterone on aggressive behavior is demonstrated by a randomized clinical trial wherein increasing levels of exogenously administered testosterone was accompanied by increases in self-reported aggressive behavior in delayed puberty boys (Finkelstein et al., Reference Finkelstein, Susman, Chinchilli, Kunselman, D'Arcangelo, Schwab and Kulin1997). We propose that diurnal testosterone is a reflection of the degree to which peripheral and perhaps brain tissue is exposed to testosterone and, in turn, is a potential potent influence on antisocial behavior. It is known that testosterone levels rise even during childhood prior to puberty and continue to rise across puberty (Mitamura et al., Reference Mitamura, Yano, Suzuki, Ito, Makita and Okuno1999). Recent important findings show that testosterone increases are related to decreases in brain volume, particularly during early puberty (Herting, Gautam, Spielberg, Dahl, & Sowell, Reference Herting, Gautam, Spielberg, Dahl and Sowell2014). Thus, it seems that testosterone has a relationship with brain development and perhaps behavior especially during puberty when brain changes are pervasive.
Cortisol and Testosterone Interaction
Prior studies on cortisol reactivity and aggression rarely utilize a dual-axis (HPA and HPG) approach to antisocial behavior. The interaction between these two axes has recently been referred to as coupling (for a special issue on coupling see Shirtcliff et al., Reference Shirtcliff, Dismukes, Marceau, Ruttle, Simmons and Han2015). The two axes typically communicate by either inhibiting or activating the action of the other. Several central candidate anatomical structures, mainly testosterone-sensitive afferents of the HPA axis, are suggested to mediate cortisol–testosterone interaction, including the medial preoptic area, the central and medial amygdala, and the bed nuclei of the stria terminalis (Viau, Reference Viau2002). There is overlap between the structures that initiate the HPA axis and those that likely initiate the HPG axis. It is not possible to infer whether these higher order structures inhibit or activate each axis in parallel or in opposition (Han et al., Reference Han, Miller, Cole, Zahn-Waxler and Hastings2015). It is possible that some structures may activate the HPA but inhibit the HPG (i.e., amygdala), whereas other structures may not show a clear direction of effect within either axis (e.g., prefrontal cortex) or postlimbic hypothalamus and pituitary and amygdala (Han et al., Reference Han, Miller, Cole, Zahn-Waxler and Hastings2015). In the case of the interaction between HPA and HPG interactions, based on pioneering studies of Chrousos and Gold (Reference Chrousos and Gold1992), the interaction is proposed to be an adaptive process to prevent the deleterious effects of HPA activation on HPG reproductive functions.
A convincing rationale for the moderating role of testosterone on cortisol reactivity can be derived from the ontogenetic roots of testosterone and cortisol. Adrenocortical and gonadal steroidogenic cells share an embryonic origin in the coelomic epithelium and may exist as one lineage before divergence into the HPA and HPG axes (Ruiz-Cortes, Reference Ruiz-Cortiz and Ostojic2012). In spite of their similar ontogenetic roots, the interactions between cortisol and testosterone are rarely considered in human model research given the seemingly divergent functions of the HPA and HPG axes. Nonetheless stress-responsive receptors in the hypothalamic paraventricular nucleus may be responsible for the interaction between cortisol and testosterone (Handa, Burgess, Kerr, & O'Keefe, Reference Handa, Burgess, Kerr and O'Keefe1994). In brief, given a rationale based on ontogenetic roots of cortisol and testosterone, the known functional interactions between the HPA and HPG axes, the increases in puberty related changes in cortisol, and the rise in testosterone and its association with structural brain changes, we propose that cortisol reactivity will interact with diurnal testosterone so as to predispose some youth to antisocial behavior. We propose that children exhibiting hypo- or hypercortisol reactivity to endogenous and exogenous stressors, controlled by the HPA axis, are expected to be more sensitive to testosterone changes controlled by the HPG axis.
Finally, a novel aspect of the study is that both boys and girls are included. Numerous studies have examined sex differences in antisocial behavior (Archer, Reference Archer2006) and in HPA reactivity to stressors (Kirschbaum, Wüst, & Hellhammer, Reference Kirschbaum, Wüst and Hellhammer1992). Nonetheless, no identified study examined the moderating effect of testosterone, mistakenly considered a male hormone, on cortisol reactivity and antisocial behavior in girls. The prediction is that if there are within-sex differences in the interaction between cortisol reactivity and diurnal testosterone, the findings will be stronger in boys than in girls based on previous studies (e.g., Granger et al., Reference Granger, Shirtcliff, Zahn-Waxler, Usher, Klimes-Dougan and Hastings2003; Susman, Dockray, et al., Reference Susman, Houts, Steinberg, Belsky, Cuffman and DeHart2010).
In summary, the first aim was to identify the pattern of change in cortisol reactivity and diurnal testosterone within individuals across 1 year and to examine the correlation between these trajectories. Given that cortisol reactivity is expected to rise during puberty and diurnal testosterone also rises, then one would expect to see increases in cortisol reactivity parallel increases in diurnal testosterone concentrations. The second aim tested hypotheses based on the diatheses–stress/reactivity to context perspectives regarding the interaction between cortisol reactivity as a vulnerability for antisocial behavior and the moderating effects of diurnal testosterone on antisocial behavior. No specific predictions regarding the interaction were proposed; rather, the need is to fill a gap in the literature and establish whether the interactions are present in both boys and girls. Finally, we control for either age or Tanner stage in the analyses as both have been related to testosterone (Duke et al., Reference Duke, Balzer and Steinbeck2014) and cortisol reactivity (Gunnar et al., Reference Gunnar, Wewerka, Frenn, Long and Griggs2009) and antisocial behavior.
Method
Participants
Participants were 135 children and adolescents and a parent or caregiver (88% mother, 10% father, 2% grandmother). The participants were followed for 12 months at 6-month intervals. At enrollment, girls were age 8, 10, or 12 years (N = 69, M = 10.06 years, SD = 1.64), and boys were age 9, 11, or 13 years (N = 66, M = 10.94 years, SD = 1.61). The rationale for the age difference between boys and girls was to assure that boys and girls would be at similar stages of pubertal development as girls mature 18 to 24 months earlier than boys (Susman, Houts, et al., Reference Susman, Dockray, Granger, Blades, Randazzo, Heaton and Dorn2010). For girls who were postmenarcheal, assessments were done on Days 5 to 9 of their menstrual cycle when testosterone is low. Controlling for day-in-cycle eliminates the possible confound of varying hormone levels across the menstrual cycle.
The racial/ethnic composition of the adolescents is as follows: White/non-Hispanic, 89.9% girls, 87.9% boys; Hispanic, 4.3%, girls, 1.5% boys; African American/Black, 4.3% girls, 1.5% boys; Asian, 1.4% girls, 1.5% boys; other, 0% girls, 7.6% boys. The mean socioeconomic status was 50.88 (SD = ±13.50) for girls, 50.06 (SD = ±11.98) for boys (Hollingshead, Reference Hollingshead1975) with higher scores indicating higher socioeconomic status.
The recruitment strategy consisted of obtaining a list of children from designated ZIP codes from the American Student List. A list of names was generated by the American Student List from ZIP codes supplied by the investigator. The ZIP codes were chosen to include low- to middle-income neighborhoods from the county, and surrounding counties, where the lab was located. A letter was mailed to the families on the list. The remaining participants were recruited from flyers distributed throughout the community and from parental telephone response to e-mails distributed to staff (nonfaculty) at a large university. More detailed information on recruitment and the sample can be found elsewhere (Susman, Dockray, et al., Reference Susman, Dockray, Granger, Blades, Randazzo, Heaton and Dorn2010). Eligibility criteria were specified age; not on medications that affect hormones (e.g., oral steroids); and free from serious physical or mental illness. The findings were similar with and without including children on psychotropic medications. Screening interviews regarding health problems were conducted with the mother via telephone by a pediatric nurse or graduate student.
Procedures
A visit was scheduled for the adolescent and one parent or guardian at a General Clinical Research Center. The visits were scheduled at 4:00 p.m. (±1.5 hr, M = 3.38 p.m., SD = 0.58). The study protocol was approved by the institutional review board of a research university and the advisory committee of the General Clinical Research Center. Parents provided consent to the study while adolescents provided assent. Participants had been instructed not to eat, drink (except water), or vigorously exercise in the 2 hr preceding the lab visit.
Cortisol reactivity: Trier Social Stress Test for Children (TSST-C)
Cortisol reactivity was assessed using change in salivary cortisol before, during, and after completion of a modified (i.e., the stories) version of the TSST-C (Buske-Kirschbaum et al., Reference Buske-Kirschbaum, Jobst, Wustmans, Kirschbaum, Rauh and Hellhammer1997). The TSST-C is a common method used to elicit an HPA stress response in a laboratory context and includes a cognitive and social evaluative challenge (e.g., Dorn et al., Reference Dorn, Campo, Thato, Dahl, Lewin, Chandra and Di Lorenzo2003; Kudielka, Hellhammer, & Kirschbaum, Reference Kudielka, Hellhammer, Kirchbaum, Harmon-Jones and Winkielman2007). It consists of a story completion task with two confederate judges present and an age-graded serial subtraction, mental arithmetic task. The participants are asked to prepare a 5-min speech regarding the ending of a story, the beginning of which is read to the participants. They were also told that the story would be judged by two judges who would evaluate the story in comparison to stories told by others of the same age. The participants also were told that they would have 5 min to complete the story. The second part of the TSST-C is a mental arithmetic task, whereby participants serially subtract a number from a previous number. The judges sat expressionless holding a stopwatch during the TSST-C. The participants were debriefed about the TSST-C procedure at the end of each session. When asked at the end of the study if they remembered the debriefing, most children did not remember. Anecdotal evidence is that some children stated that they were worried about coming to the lab at Times 2 and 3 because of the mental arithmetic test, ostensibly the most traumatic aspect of the TSST-C procedure.
Young adolescents salivated into a 5-mL tube for 5 min per sample. Five cortisol saliva samples were collected: immediately after the consent/assent (Sample 1, T = 20 min); prior to the TSST-C (Sample 2, T = 5 min) and three samples post-TSST-C (Sample 3, T = 0 min post-TSST-C; Sample 4, T = 10 min post-TSST-C; Sample 5, T = 20 min post-TSST-C). All samples were assayed for cortisol using an enzyme linked immunoassay (Salimetrics, State College, PA). The test has a range of sensitivity from 0.003 to 1.8 μg/dl. Average intra- and interassay coefficients of variance were 5.34% and 9.86%, respectively. Samples were assayed in duplicate and the average used in analyses.
Diurnal testosterone
Parents and young adolescents were instructed on saliva collection procedures at home prior to leaving the lab. Saliva for diurnal testosterone assays was collected at home upon awakening, 40 min after awakening, at noon, at 4:00 p.m., and at bedtime. The instructions were to collect saliva upon awakening prior to brushing teeth, drinking, or eating. Samples were assayed for testosterone using an enzyme linked immunoassay kit developed for saliva (Salimetrics, State College, PA). The test has a range of sensitivity from 6.1 to 600 ng/ml. Average intra- and interassay coefficients of variation are 6.7% and 14.05%, respectively. Samples were assayed in duplicate and the average used in analyses.
Stage of puberty
Stage of puberty was assessed by a pediatric research nurse using Tanner criteria of genital and pubic hair stage for boys and breast and pubic hair stage for girls (Marshall & Tanner, Reference Marshall and Tanner1969, Reference Marshall and Tanner1970). The nurse first explained the five stages of puberty and showed the young adolescent and parent pictures of the stages of sexual maturity. If the adolescent did not consent to the physical exam (n = 8), the adolescents’ self-rating of his/her stage of pubertal development was substituted for the nurse rating. The correlation between nurse and adolescent rating of breast or genital stage was r = .76 (p < .01 for both boys and girls). Sixteen percent (N = 8 of the participants self-rated their pubertal development. Eighty-four percent (N = 127) had nurse ratings of pubertal stage. Eight participants (n = 2 boys, n = 6 girls) changed stages across all three time points (Time 1 to Time 2 to Time 3).
Child Behavior Checklist (CBCL)
The CBCL is a well-standardized, norm-referenced rating scale completed by parents (Achenbach, Reference Achenbach2001). Parents rate the adolescent's behavior on a 3-point scale on 113 behavioral and emotional problems. Four raw externalizing problem subscale scores were used in the current report for four subscales: rule breaking (α = 0.98, κ = 15), aggressive (α = 0.98, κ = 18), attention (α = 0.80, κ = 8), and social problems (α = 0.98, κ = 11). The results were identical when T scores were used.
Diagnostic Interview Schedule for Children (DISC-IV)
The DISC-IV is a structured, computer-administered interview that assesses symptoms of psychiatric disorders in children (DSM-IV criteria; Shaffer, Fisher, Lucas, Dulcan, & Schwa-Stone, Reference Shaffer, Fisher, Lucas, Dulcan and Schwab-Stone2000). Parent reports of symptoms, primarily mother reports, were used in the analysis given the higher reported correlation of parent scores across time (Piacentini et al., Reference Piacentini, Roper, Jensen, Lucas, Fisher, Bird and Dulcan1999). Psychiatric diagnoses are infrequently reported in a community sample; therefore, symptom counts of oppositional defiant disorder (ODD) and conduct disorder (CD) were used in the analysis (αs, ODD = 0.81, CD = 0.64). Mothers completed the DISC-IV in 90% of the cases.
Composite score
A composite score of the four CBCL subscales scores and ODD and CD symptom scores was calculated to estimate total antisocial behavior. Cronbach α for the total antisocial behavior score was 0.74 for boys, and 0.78 for girls. The subscale scores each were used in separate analyses.
Data analysis
The data were analyzed for outliers and normalcy of the distributions. An outlier was considered to be any raw data point greater than ±2 SD from the mean. The percent of data points within each variable considered to be outliers (±2 SD from the mean) ranged from 0% to 7%. Data points identified as outliers were replaced with a value of ±2 SD from the mean, and all subsequent analyses were performed using the adjusted for outliers data.
Cortisol reactivity was calculated with respect to increase in area under the curve (AUCi; Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, Reference Pruessner, Kirschbaum, Meinlschmid and Hellhammer2003) using samples 2 (T = 5 min immediately pre-TSST-C), 3 (T = 0 min immediately post-TSST-C), and 4 (T =10 min post-TSST-C) of salivary cortisol. Diurnal testosterone was calculated as AUC with respect to ground (AUCg; Pruessner et al., Reference Pruessner, Kirschbaum, Meinlschmid and Hellhammer2003) and utilized testosterone Samples 1 (awakening) and 2 (40 min postawakening), 3 (noon), 4 (4:00 p.m.), and 5 (bedtime).
Tests of the hypotheses
The changes in cortisol AUCi and testosterone AUCg were estimated in two ways. First, a 3 (age) × 3 (time) analysis of variance was done assessing mean changes in cortisol AUCi and testosterone AUCg from Time 1 to Time 3 (12 months later), separately for boys and girls given: (a) within-sex associations between hormones and antisocial behavior and (b) sex differences in mean levels of hormones. Second, linear growth models were fit in SAS (Version 9.22) for each individual cortisol AUCi and testosterone AUCg, and intercepts and slopes were extracted and used to answer the question of whether there was a relation between change in cortisol AUCi and change in diurnal testosterone AUCg over time. Cortisol AUCi and testosterone AUCg were separately regressed on time for each individual (Cortisol ti = β0i + β1i [Time ti ] + e ti ; Testosterone ti = β0i + β1i [Time ti ] + e ti ) and intercepts (β0i ), slopes (β1i ), and error variance terms (e ti ) were estimated. A Pearson correlation analysis by sex was performed on the intercepts and slopes to test for interindividual differences in intraindividual longitudinal change in cortisol AUCi and testosterone AUCg. The covariates of Tanner stage, age, and multiple other covariates were not included as the theoretical interest was in cortisol reactivity and testosterone in the population.
Rule-breaking, aggressive, social, and attention behavior problems, and CD and ODD symptoms were analyzed separately based on previous studies showing differences in the association between cortisol and antisocial behavior across behaviors (e.g., Randazzo, Dockray, & Susman, Reference Randazzo, Dockray and Susman2008). A second rationale for considering separately each type of antisocial behavior across puberty was that different forms of aggressive and delinquent behavior are positively related to testosterone indicating that specific positive links are dependent on different social or physiological context in which relationships are assessed (van Bokhoven et al., Reference van Bokhoven, van Goozen, van Engeland, Schaal, Arseneault, Séguin and Tremblay2006). Further, genetic influences work differently for different types of antisocial behavior, for instance, delinquency and aggression (Loeber & Stouthamer-Loeber, Reference Loeber and Stouthamer-Loeber1998).
To test the second hypothesis, total antisocial behavior and the CBCL and DISC-IV subscales were separately regressed on adolescent age, cortisol AUCi, testosterone AUCg, and the Cortisol AUCi × Testosterone AUCg interaction, controlling for Time 1 antisocial behavior by sex. Multiple regression analyses were performed on Time 1 variables predicting Time 2 antisocial behavior and Time 1 variables predicting Time 3 antisocial behavior controlling for Time 1 antisocial behavior. Time 1 to Time 2 and Time 1 to Time 3 (vs. Time 2 to Time 3) are the most important time points to examine as they represent the shortest and longest time spans in this longitudinal study. Tanner stage of puberty was controlled for in the initial regression analyses, but stage was not related to antisocial behavior; therefore. it was dropped from analyses presented. The analyses present below include age as a covariate in the regression analyses. In subsequent analyses, Tanner stage or change in stage is used as a covariate and is presented below.
Post hoc analyses were performed on the significant regression models to delineate the relation between high, moderate, and low levels of cortisol AUCi and testosterone AUCg relative to the sample, by sex (Jaccard & Turrisi, Reference Jaccard and Turrisi2003). Moderate (mean centered), high (mean centered + 1 SD), and low (mean centered – 1 SD) variables were calculated for cortisol AUCi and testosterone AUCg. The significant antisocial behaviors were regressed on all possible combinations of two for high, moderate, and low cortisol AUCi and testosterone AUCg, and the respective interaction term. In some biomedical fields, there are cutoffs for identifying individuals with clinically elevated and attenuated concentrations of cortisol and testosterone; however, currently there are no set points for what is considered to be low or high cortisol or testosterone in a nonclinical population. In this post hoc analysis, by creating three arbitrary groups for cortisol AUCi and testosterone AUCg based on the distribution of the sample, we are able to describe categories of meaningful relations as opposed to cutoffs based on clinical norms.
Results
Descriptive statistics
Means and standard deviations are provided in Table 1. To assess longitudinal changes in cortisol reactivity AUCi and diurnal testosterone AUCg, a 3 (age; between subject) × 3 (Time 1, 2, and 3; within subject) generalized linear model repeated-measures analysis of variance was done. A Bonferroni adjustment was used for multiple comparisons. The mean longitudinal changes for cortisol reactivity (AUCi) and diurnal testosterone (AUCg) appear in Figure 1.
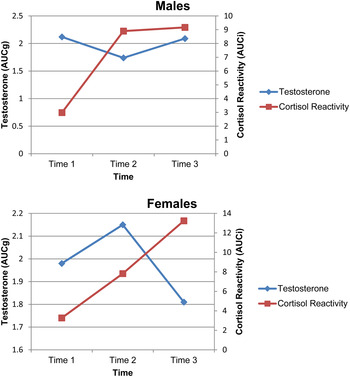
Figure 1. (Color online) Mean cortisol reactivity and diurnal testosterone for males and females across 1 year at 6-month intervals.
Table 1. Means and standard deviations for age, Tanner stage, cortisol AUCi, testosterone AUCg, and antisocial behaviors at T1, T2, and T3
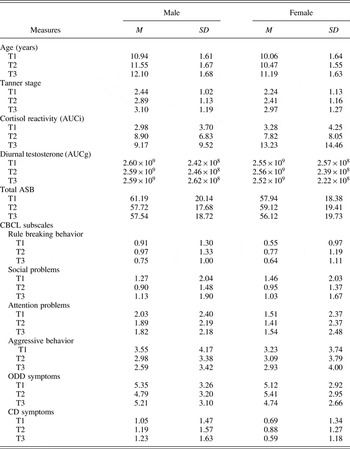
Note: T1–T3, Times 1–3; Tanner stage, genital stage in males, breast stage in females; AUCi, area under the curve increase; AUCg, AUC with respect to ground; CBCL, Child Behavior Checklist; ASB, antisocial behavior; ODD, oppositional defiant disorder symptoms; CD, conduct disorder symptoms.
Cortisol
For boys there was a main effect for age, F (2, 52) = 3.48, p = .038, with cortisol AUCi being higher at age 13 than at age 9. There also was a within-subjects effect for time, F (1.80, 93.73) = 18.57, p < .001, with cortisol AUCi being higher at Time 2 and Time 3 than at Time 1. The Time × Age interaction was not significant. For girls, there was a within-subject effect for Time, F (1.62, 94.03) = 18.79, p < .001, with cortisol AUCi being higher at Times 2 and 3 than at Time 1, and Time 3 being higher than at Time 2.
Testosterone
For boys and girls, there were no significant changes in diurnal testosterone AUCg across age or time. To further examine the lack of differences, the morning awakening response in testosterone was considered. Testosterone has a diurnal variation characterized by the highest concentrations upon wakening (Mitamura et al., Reference Mitamura, Yano, Suzuki, Ito, Makita and Okuno1999). Given this diurnal variation, we then analyzed the mean versus diurnal testosterone AUCg for the awakening and 40 min postawakening samples using a 3 (age) × 2 (time; at awakening and 40 min postawakening) analysis of variance. For awakening testosterone level, there was a significant effect for age, F (2, 32) = 23.06, p < .001, with awakening testosterone level increasing with age for boys. For 40-min postawakening testosterone level, there was a significant main effect for time, F (2, 68) = 5.42, p = .007, with higher 40-min postawakening testosterone level at Time 3 than at Time 1. There was also a significant main effect for age, F (2, 34) = 15.28, p < .001, with higher postawakening mean testosterone level at ages 11 and 13 than at age 9, and higher postawakening testosterone level at age 13 higher than at age 11. In girls, there were no significant age or time changes for awakening or 40-min mean testosterone levels.
Correlations
The correlations between all variables appear in Table 2. The significant correlations were primarily between the antisocial behaviors. In addition, chronological age was positively related to cortisol AUCi in boys and girls, and testosterone AUCg in boys and Tanner stage was related to diurnal testosterone for both boys and girls.
Table 2. Correlations between age, Tanner stage, cortisol reactivity (AUCi), testosterone (AUCg), and antisocial behavior for boys and girls at the first occasion of measurement (Time 1)

Note: Values for boys are above the diagonal and for girls are below the diagonal. AUCi, Area under the curve increase; AUCg, AUC with respect to ground; ASB, total antisocial behavior; RBB, rule breaking behavior; CDS, conduct disorder symptoms; ODDS, oppositional defiant disorder symptoms
*p < .05. **p < .01.
Tests of hypotheses
Hypothesis 1: Parallel changes in cortisol and testosterone
Interindividual differences in intraindividual change were assessed by multiple regression, regressing cortisol AUCi and testosterone AUCg on time (1, 2, and 3) for each individual, and performing a correlation on cortisol AUCi and diurnal testosterone AUCg slopes and intercepts. For boys, the cortisol AUCi slopes and intercepts (R = –.83, p ≤ .0001) and the testosterone AUCg slopes and intercepts (R = –.797, p ≤ .0001) were negatively correlated. For girls, the cortisol AUCi slopes and intercepts (R = –.92, p ≤ .0001) and the testosterone AUCg slopes and intercepts (R = –.94, p ≤ .0001) also were negatively correlated. The lower the intercept, the faster the rate of increase in cortisol AUCi and testosterone AUCg in boys and girls, typical of normative developmental changes. There were no significant correlations between testosterone AUCg and cortisol AUCi intercepts and slopes, suggesting two independent growth trajectories across 1 year.
Hypothesis 2: Time 1 predicting Time 2 antisocial behavior (6 months later)
Regression analysis was used to test the hypotheses regarding the interaction between cortisol reactivity AUCi, a vulnerability, and diurnal testosterone AUCg and antisocial behavior. Time 1 total antisocial behavior, social problems, attention problems, rule breaking, aggressive behavior, CD, or ODD symptoms were entered first into the regression models in predicting the same variable at Time 2 or Time 3. The regression statistics appear in Table 3. The post hoc tests appear in Figure 2 by antisocial behavior and time and sex.
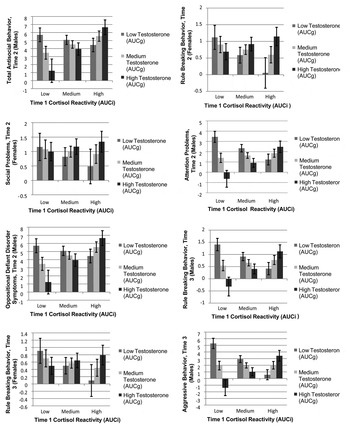
Figure 2. Mean cortisol reactivity and diurnal testosterone for girls and boys across 1 year at 6-month intervals by Time 2 and Time 3 for males and females.
Table 3. Regression of antisocial behaviors (total and subscales) on age, antisocial behavior (Time 1), cortisol reactivity, diurnal testosterone, and the interaction of cortisol reactivity and diurnal testosterone.

Note: ASB, Antisocial behavior; CDS, conduct disorder symptoms; ODD, oppositional defiant disorder symptoms.
a Control refers to the value of antisocial behavior at Time 1 predicting the same Time 2 or Time 3 antisocial behavior (e.g., rule breaking behavior at Time 1 predicting rule breaking behavior at Time 3).
†p < .10. *p < .05. **p < .01.
Total antisocial behavior
For boys, the two-way interaction of Time 1 cortisol AUCi and diurnal testosterone AUCg predicted unique variance in total antisocial behavior at Time 2. We plotted the interaction between cortisol AUCi and diurnal testosterone AUCg for low, moderate, and high cortisol AUCi and testosterone AUCg. When testosterone AUCg was low, boys with low cortisol AUCi were reported to have more antisocial behavior than when testosterone AUCg was high. When testosterone AUCg was high, boys with high cortisol AUCi were reported to have more antisocial behavior problems than when testosterone AUCg was low. There was no significant interaction for girls.
Rule breaking
There was no significant interaction for boys. For girls, the interaction between cortisol AUCi and testosterone AUCg was significant. When testosterone AUCg was low, there were no significant differences between girls with low, moderate, or high testosterone AUCg when cortisol AUCi was low. When testosterone AUCg was high, girls with high cortisol were reported to have more rule-breaking behavior than when testosterone AUCg was moderate or low.
Social problems
For girls, the interaction between cortisol AUCi and testosterone AUCg was significant. However, none of the post hoc tests were significant.
Attention problems
The findings were similar to those for total antisocial behavior. For boys there was a significant interaction of cortisol AUCi and testosterone AUCg. The post hoc analysis showed that when testosterone AUCg was low, boys with low cortisol AUCi were reported to have more attention problems than boys with high or moderate cortisol AUCi. When testosterone AUCg was high, boys with high or moderate cortisol AUCi were reported to have more attention problems than boys with low testosterone AUCg. There were no significant interactions for girls.
Aggressive behaviors
Unanticipated was that there were no significant findings for boys or girls for aggressive behaviors.
DISC-R symptoms: ODD symptoms
For boys, the findings for ODD symptoms were similar to those for CBCL behavior problems even though the methodology was different as the ODD symptoms were derived from a parent psychiatric interview of the adolescent's symptoms versus a problem checklist. There was a significant interaction between cortisol AUGi and testosterone AUCg. When testosterone AUCg was low, boys with low cortisol AUCi were reported to have more ODD symptoms than when testosterone was moderate or high. When testosterone AUCg was high, boys with high AUCi cortisol were reported to have more ODD symptoms than when testosterone AUCg was low. For girls, there were no significant findings.
CD symptoms
The interactions were not significant.
Time 1 predicting Time 3 antisocial behavior (12 months later)
Rule breaking
For the 12-month longitudinal time period, the findings had similarities with the 6-month time point, yet some differences were also noted. For boys, there was a significant interaction between cortisol AUCi and testosterone AUCg and rule-breaking behavior problems 12 months later. The post hoc analysis showed that when testosterone AUCg is low, boys with low cortisol AUCi were reported to have more rule-breaking problems than boys who were reported to have high or moderate testosterone AUCg. When testosterone AUCg is high, boys with high cortisol AUCi were reported to have more rule-breaking behavior than boys with low testosterone AUCg.
For girls, there was also a significant interaction between AUCi and AUCg, but none of the post hoc tests showed significant differences.
Aggressive behaviors
For boys, there was a significant interaction between cortisol AUCi and testosterone AUCg and aggressive behavior problems. Post hoc tests showed that when testosterone AUCg was low, boys with low or moderate cortisol AUCi were reported to have more aggressive behavior problems than boys with high testosterone AUCg. When testosterone AUCg was high, boys with high cortisol AUCi were reported to have more aggressive behavior problems than boys with moderate or low testosterone AUCg. There were no significant findings for girls.
Given that Tanner stage can be related to cortisol reactivity, diurnal testosterone, and antisocial behavior, Tanner stage was a covariate in additional analyses. The findings when including pubertal stage at Time 1 (instead of age at Time 1) were as follows: there were fewer significant two-way interactions between cortisol reactivity and diurnal testosterone when pubertal stage at Time 1 was included in the model in place of age, for both males and females. In males, there was a significant interaction between cortisol reactivity and diurnal testosterone when predicting aggressive behavior at Time 2 (F = 13.58, p = .000, β = 3.64, p = .016) and Time 3 (F = 18.09, p = .000, β = 3.96, p = .005). In the models controlling for age, only the Time 3 finding for aggressive behavior was significant. The results from a post hoc analysis show that when testosterone AUCg is low, boys with low cortisol reactivity have more aggressive behavior problems at Time 2 than boys with high testosterone AUCg. In females, there is a significant two-way interaction between cortisol reactivity and testosterone AUCg when predicting Time 2 social problems (F = 18.45, p = .000, β = 2.53, p = .013) and rule-breaking behavior at Time 2 (F = 8.17, p = .000, β = 3.15, p = .014).
The next analysis addressed the question of whether changes in Tanner stage influence antisocial behavior. Given the high correlation between Tanner stage at Time 1 and Time 3 (r = .81, p < .001) both Tanner Stages 1 and 2 cannot be included in the same analyses. Thus, we created a difference score between Time 1 and Time 2 Tanner stage, and Time 1 and Time 3 Tanner stage. The two difference scores are not correlated with Time 1 Tanner stage (p > .05) and represent the magnitude of change in Tanner stage across the study. Then an additional analysis was done again controlling for Time 1 Tanner stage and the applicable difference score (Time 2–Time 1 for Time 2 outcomes; Time 3–Time 1 for Time 3 outcomes). For males, the results are consistent with the findings when we only controlled for Time 1 Tanner stage. There are significant two-way interactions between cortisol reactivity and testosterone AUCg when predicting Time 2 aggressive behavior (F = 10.78, p = .000, β = 3.494, p = .025) and Time 3 aggressive behavior (F = 14.62, p = .000, β = 3.902, p = .008). The post hoc tests were in the same direction as when we controlled for only Time 1 Tanner stage.
For females, there is a significant two-way interaction between cortisol reactivity and testosterone AUCg when predicting Time 2 social problems (F = 16.12, p = .000, β = 2.537, p = .013) and Time 2 rule-breaking behavior (F = 6.85, p = .000, β = 2.292, p = .027). These findings are consistent with the findings when only controlling for Time 1 Tanner stage. One new finding emerged when controlling for the change in Tanner stage; the Cortisol Reactivity × Testosterone AUCg interaction was significant when predicting Time 3 social problems (F = 24.84, p = .000, β = 2.414, p = .019). This finding was not present in the original model (controlling for Time 1 age or in the model controlling for Time 1 Tanner stage).
In summary, the consistent pattern of findings showed that when testosterone was low, boys with low cortisol reactivity were reported to have more antisocial behavior problems compared to when cortisol reactivity or diurnal testosterone was moderate or high. When testosterone was high, boys with high cortisol reactivity were reported to have more antisocial behavior than boys with moderate or low diurnal testosterone. The pattern of findings was similar across 6 and 12 months, with fewer findings for girls than for boys. The findings were similar when controlling for age and Tanner stage or change in Tanner stage.
Discussion
Not until the last three decades did theories and biomarker methodologies become available for testing hypotheses regarding the vexing question of the interaction of indices of stress reactivity, cortisol, puberty-related changes in testosterone, and antisocial behavior during adolescence. These advances made it possible to explore a dual, HPA–HPG axis understanding of physiological responses during a challenging situation and an endogenous marker of puberty, testosterone, in young adolescents. To advance understanding of the growth and interaction between cortisol reactivity, diurnal testosterone, and antisocial behavior, this report hypothesized, first, that there are simultaneous, longitudinal trajectories of cortisol reactivity and diurnal testosterone during early adolescence. The findings show that cortisol reactivity and awakening and postawakening testosterone did change across 1 year. However, cortisol reactivity and diurnal testosterone did not change in synchrony given the lack of relationships between the two growth trajectories across 6 and 12 months. This finding suggests that increases in cortisol reactivity and testosterone are independent components of the developmental physiology of the endocrine system. The findings are novel in that the interactions between cortisol reactivity and diurnal testosterone and antisocial behavior were independent of chronological age. Parenthetically controlling for Tanner pubertal stage versus age is similar to controlling for age given the high correlation between age and stage (Dorn, Susman, Nottelman, Inoff-Germain, & Chrousos, Reference Dorn, Susman, Nottelmann, Inoff-Germain and Chrousos1990). Nonetheless, we went on to do additional analyses to assure that important findings regarding Tanner stage were not missed. As presented in the Results section, the findings were less frequent when stage versus age was used as a covariate in the models predicting antisocial behavior. For the significant models, the findings were similar to the findings when age was the covariate and the post hoc tests were in the same direction; the interaction showed that low cortisol reactivity and low testosterone and high reactivity and high testosterone predicted antisocial behavior. These similarities reflect the joint increasing age and stage during puberty.
A second hypothesis was that cortisol reactivity interacts with diurnal testosterone to predict antisocial behavior. The take-home message is that the interaction of cortisol reactivity and testosterone predicted antisocial behavior problems across 6 and 12 months in young adolescents. Understanding that HPA and HPG axes interactions foretell of future antisocial behavior is important for conceptualizing how two endocrine systems interact to influence the development of externalizing psychopathology.
With regard to our first hypothesis, there were no significant associations between cortisol reactivity or diurnal testosterone intercepts and slopes in spite of decades of speculation regarding increases in reactivity to stressors and rising testosterone levels during adolescence. The lack of association likely reflects the different mechanisms responsible for the secretion of cortisol and testosterone during puberty. Cortisol reactivity is influenced by genes, psychological issues, rearing experiences, and environmental influences secondary to novelty, challenging, and threatening circumstances (Chrousos & Gold, Reference Chrousos and Gold1992) as well as normal reproductive maturation (Legro, Lin, Demers, & Lloyd, Reference Legro, Lin, Demers and Lloyd2003). In contrast, diurnal testosterone changes are governed by the reactivation of the HPG axis during puberty that results in stimulation of gonadotropin releasing hormone, gonadotropins, and testosterone secretion, all of which are essential for mammalian reproduction (Grumbach & Styne, Reference Grumbach, Styne, Larsen, Kronenberg, Melmed and Polonsky1998). The different indices of cortisol (reactivity) and testosterone (diurnal) may also have contributed to the lack of correspondence between the two trajectories. Studies might profit from using the same endocrine index of change in future studies.
Our second hypothesis predicted that cortisol reactivity and the moderating effects of diurnal testosterone would predict antisocial behavior. These fascinating interactions are consistent with earlier findings showing a similar interaction predicting antisocial behavior in adults (Dabbs, Jurkovic, & Frady, Reference Dabbs, Jurkovic and Frady1991; Mehta & Josephs, Reference Mehta and Joseph2010; Popma et al., Reference Popma, Vermeiren, Geluk, Rinne, van den Brink, Knol and Doreleijers2007). Overall, when diurnal testosterone was low, boys with low cortisol reactivity have more behavior problems (e.g., ODD symptoms and attention problems) than when diurnal testosterone was high or moderate. In addition, when diurnal testosterone was high, boys with high or moderate cortisol reactivity were significantly higher on antisocial behavior, attention behavior problems, and ODD symptoms than when testosterone was low or moderate. For low cortisol reactivity and low diurnal testosterone, the findings are consistent with previous studies showing that low cortisol reactivity and aggressive behavior were moderated by timing of physical maturation via Tanner stage, an alternative index of testosterone development (Susman et al., Reference Susman, Inoff-Germain, Nottelmann, Loriaux, Cutler and Chrousos1987). In that case, Tanner moderated the effect of cortisol reactivity on morningness versus eveningness, a different dimension of behavior compared to antisocial behavior. An adolescent with both low cortisol reactivity and low diurnal testosterone may be inhibited and avoids situations with excess arousal potential or contexts that call for dominance or aggression yet exhibits poor self-regulation in other-initiated stressful situations. Alternatively, an adolescent with low cortisol and low testosterone may be a child who externalizes aggression but is later in pubertal development than same-age peers and has lower testosterone (Susman et al., Reference Susman, Inoff-Germain, Nottelmann, Loriaux, Cutler and Chrousos1987). In contrast, an adolescent with high cortisol and high testosterone (positive coupling) may be the one who externalizes aggression and physical aggression in aversive contexts (e.g., rule breaking and aggressive behavior problems in boys and rule breaking in girls). Stress-related adrenal activation can directly produce cortisol increases and also lead to testosterone elevations via the peripheral conversion of adrenal androgens, a precursor to cortisol, to testosterone (Roney, Lukaszewski, & Simmons, Reference Roney, Lukaszewski and Simmons2007). Children high on cortisol reactivity can be characterized by high HPA axis arousal but poor self-regulation of the HPG axis resulting in antisocial behavior.
The specific mechanisms whereby the brain (that is rich in steroid hormone receptors) coordinates cortisol reactivity, testosterone, and aggressive behavior are unknown. Changes at the receptor, neurotransmitter, and HPA axis system level have been proposed to explain the interaction (e.g., Montoya, Terburg, Peter, & van Honk, Reference Montoya, Terburg, Bos and van Honk2012; Platje et al., Reference Platje, Popma, Vermeiren, Doreleijers, Meeus, van Lier and Jansen2015). Theorized areas of the brain involved in the interaction include the amygdala (fear), hippocampus (defective glucocorticoid feedback), and mesocortico limbic dopamine system (novelty seeking; Charmandari, Kino, Souvatzoglou, & Chrousos, Reference Charmandari, Kino, Souvatzoglou and Chrousos2003). Josephs, Sellers, Newman, & Mehta, (Reference Josephs, Sellers, Newman and Mehta2006) reported convincing evidence that the serotonin transporter linked polymorphic region (5-HTTLPR) genotype (presence of short or long alleles) plays an important role in cortisol-testosterone hormonal reactivity in response to threat. In three studies with male and female college students, Josephs et al. showed that threats to status via social exclusion, cognitive/perceptual failure, and physical competence all produced elevated cortisol levels in short allele carriers with higher testosterone levels. An unexpected finding was that 5-HTTLPR long allele homozygotes with higher testosterone showed lower cortisol levels in response to threat pattern of response that parallels that reported in psychopathic individuals.
High cortisol reactivity and diurnal testosterone is not necessarily detrimental as this pattern may aid youth in responding adaptively to the novel challenges of early adolescence. Higher diurnal testosterone also may contribute to adaptive responding to challenges that require physical strength. Although adaptive for certain challenges in some situations, higher cortisol reactivity and testosterone may have yet unidentified long-term health consequences like cardiovascular problems. Finally, similar to the existing literature using basal cortisol concentrations, the significant findings show that both hypo- and hypercortisol reactivity predict antisocial behavior (Popma et al., Reference Popma, Vermeiren, Geluk, Rinne, van den Brink, Knol and Doreleijers2007) depending on the level of diurnal testosterone. Charmandari et al. (Reference Charmandari, Kino, Souvatzoglou and Chrousos2003) suggest that adaptation to a challenge can be characterized by hypo- and hyperphysiological responses depending on genetics, rearing environment, and other unknown mechanisms. Cortisol suppresses testosterone, so when testosterone is high, cortisol should be low as in long-term exposure to stressors (Chrousos & Gold, Reference Chrousos and Gold1992). When cortisol is high, however, testosterone can also be high in spite of experiencing an emotionally stressful situation (Almeida, Petenusci, Franci, Silva, & Carvalho, Reference Almeida, Petenusci, Franci, Silva and Carvalho2000). In brief, reactivity to a stressor was characterized by both hypo- and hypercortisol reactivity and diurnal testosterone. Overall, the findings herein of low cortisol and low testosterone and more aggressive behavior are not in agreement with the findings in adults suggesting that low cortisol reactivity and high diurnal testosterone are predictive of antisocial behavior. The lack of consistency may derive from longitudinal versus cross sectional findings, developmental differences in young adolescents versus adults, and the metric of cortisol reactivity and diurnal testosterone versus basal levels. In agreement with the strong recommendation of Carré and Mehta (Reference Carré and Mehta2011), we highly recommend further studies aimed at explaining the links between cortisol and testosterone and aggressive behavior.
The findings for girls were not above chance level, with the lack of findings consistent with previous literature (Granger et al., Reference Granger, Shirtcliff, Zahn-Waxler, Usher, Klimes-Dougan and Hastings2003). For girls, the interaction between cortisol reactivity and diurnal testosterone and antisocial behavior is unique. Few studies have examined cortisol and testosterone interaction in girls even though the hormone and deviant behavior changes during adolescence are typical for both boys and girls. Granger et al. (Reference Granger, Shirtcliff, Zahn-Waxler, Usher, Klimes-Dougan and Hastings2003) suggests reasons for why a direct comparison between testosterone and behavior relations is more difficult in girls than in boys. Testosterone is secreted from different sources in boys (testes) and girls (adrenals and ovaries), the levels in boys are higher and have more variability, the diurnal rhythm is more pronounced, and in boys, levels are more stable and show higher correlations with age and pubertal status (Granger et al., Reference Granger, Shirtcliff, Zahn-Waxler, Usher, Klimes-Dougan and Hastings2003). Thus, our fewer significant findings in girls is consistent with the sparse literature on testosterone and antisocial behavior in younger girls. For both boys and girls, the findings were consistent for the interaction between cortisol reactivity and diurnal testosterone and antisocial behaviors across 6 and 12 months.
One might question why chronological age versus Tanner stage was used to control for developmental differences in antisocial behavior. First, the correlation of age and Tanner is high and there is too much collinearity between the two to include both in a single statistical model. Second, one of the problems with using Tanner stage as a substitute for the endocrine milieu, that is, testosterone, is that Tanner stage does not map well onto hormone concentrations: for instance, there is wide variability in testosterone concentrations within each Tanner stage (see Huang et al., Reference Huang, Hillman, Ding, Biro, Dorn and Susman2012; Nottelmann et al., Reference Nottelmann, Susman, Dorn, Inoff-Germain, Loriaux, Cutler and Chrousos1987). Our focus is on testosterone concentrations as opposed to the external manifestation of puberty as we have been convinced by new literature that rises in testosterone do have an effect on brain development independent from Tanner, which in turn affects behavior (see Herting et al., Reference Herting, Gautam, Spielberg, Dahl and Sowell2014). In the interest of translating the current findings, age is likely earlier to interpret than pubertal stage for teachers, health care professionals, and others.
There are also limitations to the study. Given the small sample size, we had little power to detect the hypothesized interactions because interactions require more power than main effects. Nonetheless, given the novelty of the emphasis on the interactions between the stress and reproductive gonadal axes that undergo major organizational and maturational changes during puberty, it is prudent to balance the probability of Type I and II errors. An additional limitation is that saliva for cortisol and testosterone was collected on only 1 day at each time of measurement, and possible noncompliance with times of collection is a consideration. Replication of the findings needs to be done in a larger sample that includes youth who are ethnically diverse. In addition, testosterone reactivity needs to be assessed simultaneously with cortisol reactivity. Finally, we acknowledge the complexity of including many variables and possible interactions that must be considered in biobehavioral research. To control for these many variables (a) we used age as a covariate in the model, (b) pubertal stage was analyzed for interactions in a secondary analysis, (c) we justified the use of cortisol reactivity as a predictor (vs moderator) of antisocial behavior based on the current literature, and (d) we used statistical methods consistent with assessing developmental predictions over time. Other important strengths of the study are that the pattern of interactions was replicated across 6 and 12 months.
The findings suggest a platform on which to build future research. There is a need to examine estrogen as well as testosterone in relation to brain development and antisocial behavior as estrogen also changes dramatically at puberty, especially in girls. The simultaneous assessment of brain changes, via imaging of brain structure (or function) and stress and reproductive hormones and antisocial behavior is an important next step in understanding neuroendocrine mechanisms in antisocial behavior. In addition, future research should examine HPA activity and gonadal steroids for a longer time period given that the relation between steroids and antisocial behavior may be transformed as sexual maturity is reached. A future attempt to replicate these findings in a longer time frame using sophisticated growth curve statistical modeling could facilitate defining when to expect that diurnal rising testosterone is influential in moderating reactivity to contextual stressors as indexed by cortisol reactivity. Finally, an important question is whether both cortisol reactivity and testosterone concentrations can be changed to reduce the risk of antisocial behavior in future translational studies. Evidence suggests that reducing testosterone via multimethod treatment strategies leads to better treatment outcomes for disruptive behavior children (Shenk et al., Reference Shenk, Dorn, Kolko, Susman, Noll and Bukstein2012). Children with higher pretreatment testosterone levels were four times more likely to be in the low-response to treatment follow-up trajectory than children in a high-response trajectory. In brief, the study advances the field as the findings may signal the possibility of intervention effects on the stress response and testosterone physiology.







