Introduction
Anorexia nervosa (AN) is a serious disorder that occurs predominantly among young women and carries a high risk of early death (Harris & Barraclough, Reference Harris and Barraclough1998; Millar et al. Reference Millar, Wardell, Vyvyan, Naji, Prescott and Eagles2005; Papadopoulos et al. Reference Papadopoulos, Ekbom, Brandt and Ekselius2009). The incidence of diagnosed AN in western societies rose during the 20th century (Lucas et al. Reference Lucas, Beard, O'Fallon and Kurland1991; Eagles et al. Reference Eagles, Johnston, Hunter, Lobban and Millar1995; Hoek, Reference Hoek2006) but seems to have stabilized with a point prevalence of around 0.3% among young females (Hoek, Reference Hoek2006). It is often a clandestine disorder that does not come to medical attention and actual lifetime prevalence rates in women are probably between 2% and 3% (Keski-Rahkonen et al. Reference Keski-Rahkonen, Hoek, Susser, Linna, Sihvola, Raevuori, Bulik, Kaprio and Rissanen2007; Isomaa et al. Reference Isomaa, Isomaa, Marttunen, Kaltiala-Heino and Bjorkqvist2009). As this epidemiology suggests, pregnancies among women with past or present AN are relatively common occurrences.
Ward's (Reference Ward2008) review concluded that the evidence about the effects of eating disorders upon pregnancy and foetal outcomes was ‘limited and sometimes conflicting’. She commented on methodological shortcomings in previous studies that included small sample sizes (<50 pregnancies) (Stewart et al. Reference Stewart, Raskin, Garfinkel, MacDonald and Robinson1987; Bulik et al. Reference Bulik, Sullivan, Fear, Pickering, Dawn and McCullin1999, Reference Bulik, von Holle, Siega-Riz, Torgersen, Lie, Hamer, Berg, Sullivan and Reichborn-Kjennerud2009; Franko et al. Reference Franko, Blais, Becker, Delinsky, Greenwood, Flores, Ekeblad, Eddy and Herzog2001; Koubaa et al. Reference Koubaa, Hällström, Lindholm and Hirschberg2005; Wentz et al. Reference Wentz, Gillberg, Anckarsater, Gillberg and Rastam2009) and an absence of control or comparison subjects (Stewart et al. Reference Stewart, Raskin, Garfinkel, MacDonald and Robinson1987; Brinch et al. Reference Brinch, Isager and Tolstrup1988; Franko et al. Reference Franko, Blais, Becker, Delinsky, Greenwood, Flores, Ekeblad, Eddy and Herzog2001). Diagnosis has often been imprecise, using uncorroborated hospital or case-register data (Sollid et al. Reference Sollid, Wisborg, Hjort and Secher2004; Ekéus et al. Reference Ekéus, Lindberg, Lindblad and Hjern2006; Bansil et al. Reference Bansil, Kuklina, Whiteman, Kourtis, Posner, Johnson and Jamieson2008), maternal self-diagnosis (Micali et al. Reference Micali, Simonoff and Treasure2007a) or mixed samples with undifferentiated eating disorder diagnoses (Franko et al. Reference Franko, Blais, Becker, Delinsky, Greenwood, Flores, Ekeblad, Eddy and Herzog2001; Sollid et al. Reference Sollid, Wisborg, Hjort and Secher2004; Koubaa et al. Reference Koubaa, Hällström, Lindholm and Hirschberg2005; Bansil et al. Reference Bansil, Kuklina, Whiteman, Kourtis, Posner, Johnson and Jamieson2008). Some studies have been confined to hospital in-patients (Sollid et al. Reference Sollid, Wisborg, Hjort and Secher2004; Ekéus et al. Reference Ekéus, Lindberg, Lindblad and Hjern2006; Bansil et al. Reference Bansil, Kuklina, Whiteman, Kourtis, Posner, Johnson and Jamieson2008). Attempts to adjust for confounders have not been common; only four studies (Koubaa et al. Reference Koubaa, Hällström, Lindholm and Hirschberg2005; Ekéus et al. Reference Ekéus, Lindberg, Lindblad and Hjern2006; Micali et al. Reference Micali, Simonoff and Treasure2007a; Bulik et al. Reference Bulik, von Holle, Siega-Riz, Torgersen, Lie, Hamer, Berg, Sullivan and Reichborn-Kjennerud2009) took account of maternal smoking (which may be more common in women with eating disorders) (Micali et al. Reference Micali, Simonoff and Treasure2007a; Bulik et al. Reference Bulik, von Holle, Siega-Riz, Torgersen, Lie, Hamer, Berg, Sullivan and Reichborn-Kjennerud2009); five considered maternal age (Bulik et al. Reference Bulik, Sullivan, Fear, Pickering, Dawn and McCullin1999, Reference Bulik, von Holle, Siega-Riz, Torgersen, Lie, Hamer, Berg, Sullivan and Reichborn-Kjennerud2009; Ekéus et al. Reference Ekéus, Lindberg, Lindblad and Hjern2006; Micali et al. Reference Micali, Simonoff and Treasure2007a; Bansil et al. Reference Bansil, Kuklina, Whiteman, Kourtis, Posner, Johnson and Jamieson2008) and only two adjusted for pre-pregnancy body mass index (BMI) (Micali et al. Reference Micali, Simonoff and Treasure2007a; Bulik et al. Reference Bulik, von Holle, Siega-Riz, Torgersen, Lie, Hamer, Berg, Sullivan and Reichborn-Kjennerud2009). Of the 11 studies identified of pregnancy outcomes in AN, all but two (Ekéus et al. Reference Ekéus, Lindberg, Lindblad and Hjern2006; Bulik et al. Reference Bulik, von Holle, Siega-Riz, Torgersen, Lie, Hamer, Berg, Sullivan and Reichborn-Kjennerud2009) concluded that women with past or present AN had babies of lower birthweight. However, neither of the studies that adjusted for maternal pre-pregnancy BMI (Micali et al. Reference Micali, Simonoff and Treasure2007a; Bulik et al. Reference Bulik, von Holle, Siega-Riz, Torgersen, Lie, Hamer, Berg, Sullivan and Reichborn-Kjennerud2009) found that babies were lighter than those in comparison pregnancies. Other findings have included higher rates of preterm deliveries (Brinch et al. Reference Brinch, Isager and Tolstrup1988; Sollid et al. Reference Sollid, Wisborg, Hjort and Secher2004), more infants who were small for their gestational age (Koubaa et al. Reference Koubaa, Hällström, Lindholm and Hirschberg2005), and increased rates of caesarean sections (Bulik et al. Reference Bulik, Sullivan, Fear, Pickering, Dawn and McCullin1999; Franko et al. Reference Franko, Blais, Becker, Delinsky, Greenwood, Flores, Ekeblad, Eddy and Herzog2001).
Given the frailty of the evidence base, the medical profession has perhaps erred on the side of caution (Franko et al. Reference Franko, Blais, Becker, Delinsky, Greenwood, Flores, Ekeblad, Eddy and Herzog2001; Mitchell & Bulik, Reference Mitchell and Bulik2006; Micali et al. Reference Micali, Simonoff and Treasure2007a). Only Ekéus et al. (Reference Ekéus, Lindberg, Lindblad and Hjern2006), following their case-register study in Sweden, reached a distinctly different conclusion: ‘Special obstetric monitoring of pregnant women with a history of anorexia nervosa does not seem to be warranted in a country with a satisfactory maternity surveillance’.
In summary, in the relatively common clinical circumstance of a woman with a history of AN becoming pregnant or contemplating a pregnancy, there is a dearth of good evidence underlying traditionally cautious advice. We sought to address some of the methodological deficiencies of previous studies and to add to the evidence on which such advice is based.
Method
Subjects
As described in previous studies (Eagles et al. Reference Eagles, Johnston, Hunter, Lobban and Millar1995, Reference Eagles, Easton, Nicoll, Johnston and Millar1999; Millar et al. Reference Millar, Wardell, Vyvyan, Naji, Prescott and Eagles2005), women who presented to psychiatric services in North East Scotland and were diagnosed with AN since 1965 have been identified. This cohort has been successively updated and enlarged, most recently up to the end of 1999. For the present study, it was enlarged further to include cases presenting between 1 January 2000 and 31 December 2007.
All women presenting for the first time and acquiring a case-register or case-record diagnosis of AN were initially eligible for inclusion. The case records were then scrutinized and to satisfy continuing eligibility criteria it was necessary that patients had: (1) a confirmed case-record diagnosis of AN; (2) a significant recorded weight loss at presentation; (3) recorded amenorrhoea and/or evidence of characteristic psychopathology; and (4) no other diagnosis (that might be regarded as primary) of severe psychiatric illness such as schizophrenia or bipolar affective disorder. These criteria were adopted across the entire cohort and were those used in our previously published studies (Eagles et al. Reference Eagles, Johnston, Hunter, Lobban and Millar1995, Reference Eagles, Easton, Nicoll, Johnston and Millar1999; Millar et al. Reference Millar, Wardell, Vyvyan, Naji, Prescott and Eagles2005).
Database linkage
All women with a diagnosis of AN, identified as above, were linked by Community Health Index (CHI) numbers to the Aberdeen Maternity and Neonatal Databank (AMND). The AMND has been collecting data since 1950 on all pregnancy-related events occurring in women resident in Aberdeen and surrounding areas. It currently contains details of more than 150 000 women (www.abdn.ac.uk/amnd).
Women without AN
Each woman with a history of AN who was linked with the AMND was matched by age, year of delivery of first baby and parity (primigravida/multigravida) to five women without AN (‘non-AN’ women) occurring in the AMND. There were no other exclusion criteria for the matched non-AN women.
Pregnancy and perinatal outcomes
Data on pregnancy outcomes comprised the occurrence of miscarriage, ectopic pregnancy, therapeutic termination, threatened miscarriage, pregnancy-induced hypertension (gestational hypertension, pre-eclampsia or eclampsia), antepartum haemorrhage, induction of labour, malpresentation or position, and instrumental or caesarean delivery. Perinatal outcomes included stillbirth, neonatal death, preterm delivery (before 37 completed weeks of gestation) and low birthweight (LBW <2500 g). Standardized birthweight (SBW) scores (Campbell et al. Reference Campbell, Hall, Lemon, Carr-Hill, Pritchard and Samphier1993), which contain adjustments for maternal and pregnancy parameters and give an indication of intrauterine growth restriction (IUGR), were also compared. Because low maternal BMI in early pregnancy may relate to outcome of the pregnancy in women with a history of AN, we also compared women with a BMI of ⩽20 kg/m2 against mothers with a BMI >20 kg/m2.
Statistical analysis
To avoid non-independent observations, women with twin pregnancies were excluded from all analyses. The comparison of sociodemographic factors between women with a history of AN and non-AN women was based on univariate conditional logistic regression. Pregnancy outcomes were compared using multi-level logistic regression based on the generalized estimating equation (GEE) assuming an exchangeable correlation among pregnancies within a woman (Hardin & Hilde, Reference Hardin and Hilbe2003).
The logistic models were first adjusted for BMI, then also for smoking, social class and marital status, and for other pregnancy-related events that may have confounded the observed association (for example, induction of labour or pre-eclampsia for the outcome of preterm delivery). No adjusted model was necessary for the outcome of SBW score because this parameter is already adjusted for maternal height and parity, and also for the baby's gender and gestational age.
Results
Subjects
The cohort of women fulfilling the criteria for AN outlined above had numbered 487 up to the end of 1999. A further 178 female patients aged ⩽13 years had been seen for the first time by psychiatric services from 1 January 2000 to 31 December 2007 and had received a case-record diagnosis of AN. Of these patients, following scrutiny of their case records, 110 satisfied inclusion criteria for the study. This gave a total cohort of 597 patients presenting from 1965 to 2007 inclusive; these 597 patients constituted 65% of the total of 916 patients who had been given a case-register or case-record diagnosis of AN during this period.
Of these 597 women, 180 were matched through their CHI number as having at least one pregnancy recorded on the AMND. Four of these women were subsequently omitted due to a twin pregnancy. Of the remaining 176 women, the first pregnancy predated their diagnosis of AN in 42. The remaining 134 women (who delivered a total of 230 babies) were duly matched with 670 women who had delivered a total of 1144 babies. Figure 1 shows a flow diagram of the population selection for analysis.

Fig. 1. Flow diagram of population selection for analysis.
Baseline factors in matched groups
Table 1 shows baseline sociodemographic factors, height, smoking status and BMI in the women with a history of AN and the matched non-AN women at the time of their first pregnancies. As expected, BMIs were significantly lower in the AN women, but no other significant differences emerged.
Table 1. Baseline factors in women with a history of anorexia nervosa (AN) and the matched non-AN women

Values are given as % (n) or mean (standard deviation).
Comparisons with all women on the AMND
The women with a history of AN were compared to their matched non-AN women and also to all non-AN women registered on the AMND from 1965 to 2007 in terms of parity and age at first delivery. The mean age at first delivery was 2 years older in the AN group than in the non-AN AMND women. Perhaps related to this finding, the AN group had slightly fewer children than all non-AN women on the AMND.
Early pregnancy loss
Table 2 also shows that, compared to their matched non-AN counterparts, women with AN had a very similar incidence of miscarriage, termination and ectopic pregnancy. For comparative purposes, rates of these events are also shown for all pregnancies recorded on non-AN AMND women between 1965 and 2007. Rates of miscarriage were lower among these women (9.5% v. 15.7%).
Table 2. Number of children, mean age at first delivery and early pregnancy loss in women with a history of anorexia nervosa (AN), their matched non-AN women and the non-AN women from the entire AMND

AMND, Aberdeen Maternal and Neonatal Databank.
Values are given as % (n) or mean (standard deviation).
Pregnancy and perinatal outcomes
The 230 completed pregnancies of the AN women and the 1144 pregnancies in their matched non-AN women are compared in Table 3. The unadjusted comparison shows that the risk of LBW was significantly greater in the babies of mothers with a history of AN. Following adjustment for BMI, the risk of LBW was no longer significantly related to AN status. Babies of AN mothers were more likely to have SBWs below the median and had lower mean SBWs than babies of non-AN mothers. The adjusted relative risk (RR) for antepartum haemorrhage was significantly higher among women with a history of AN [RR 1.70, 95% confidence interval (CI) 1.09–2.65].
Table 3. Relative risk (RR) of pregnancy and perinatal outcomes among women with a history of anorexia nervosa (AN) compared to their matched controls
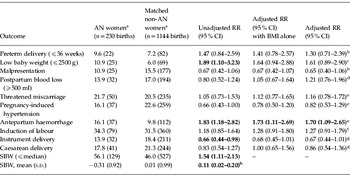
SBW, Standardized birthweight; s.d., standard deviation; CI, confidence interval; BNI, body mass index.
Statistically significant RRs are shown in bold.
a Values are given as % (n) unless stated otherwise.
b Adjusted for BMI, smoking status, social class, marital status, pre-eclampsia, antepartum haemorrhage and induction of labour.
c Adjusted for BMI, smoking status, social class, marital status, pre-eclampsia, antepartum haemorrhage, induction of labour and baby gender.
d Adjusted for BMI, smoking status, social class, marital status and mode of delivery.
e Adjusted for BMI, smoking status, social class and marital status.
f Adjusted for BMI, smoking status, social class, marital status, pre-eclampsia and antepartum haemorrhage.
g Adjusted for BMI, smoking status, social class, marital status, pre-eclampsia, antepartum haemorrhage and induction of labour.
h Regression coefficient (95% CI).
Low-weight AN mothers
For only 20% (43/215) of the AN mothers with a BMI recorded in early pregnancy was the BMI <20 kg/m2 (Table 4). The AN mothers with BMI <20 kg/m2 had babies whose SBW was more likely to be below the median value than it was for babies of AN mothers with a higher BMI (RR 2.15, 95% CI 1.05–4.42). Mean SBWs did not differ significantly between these groups and nor did any other outcome analysed.
Table 4. Relative risk (RR) of selected pregnancy and perinatal outcomes among women with a history of anorexia nervosa (AN) stratified by BMI ⩽20 kg/m2 and BMI >20 kg/m2
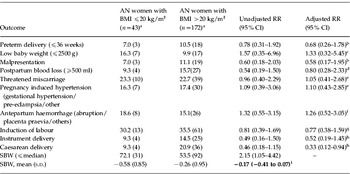
BMI, Body mass index; SBW, standardized birthweight; s.d., standard deviation; CI, confidence interval.
Statistically significant RRs are shown in bold.
a Values are given as % (n) unless stated otherwise.
b Adjusted for smoking status, social class, marital status, pre-eclampsia, antepartum haemorrhage and induction of labour.
c Adjusted for smoking status, social class, marital status, pre-eclampsia, antepartum haemorrhage, induction of labour and baby gender.
d Adjusted for smoking status, social class, marital status and mode of delivery.
e Adjusted for smoking status, social class and marital status.
f Adjusted for social class and marital status.
g Adjusted for smoking status, social class, marital status, pre-eclampsia and antepartum haemorrhage.
h Adjusted for smoking status, social class, marital status, pre-eclampsia, antepartum haemorrhage and induction of labour.
i Regression coefficient (95% CI).
Discussion
Main findings
In this study there were some differences in pregnancy and perinatal outcomes between women with a history of AN and their matched comparison group. Unadjusted RR was higher among the AN women for LBW, but this difference did not persist following adjustment for maternal BMI. SBWs were lower for babies of mothers with a history of AN. The adjusted RR among AN women was higher for antepartum haemorrhage. The magnitudes of these risks were relatively small and their clinical relevance for psychiatric and obstetric management of pregnancies in women with a history of AN is discussed below.
Strengths and limitations
Our cohort of females with AN derives from a defined geographical area of North East Scotland with a population of some 550 000. From 1965 to 1999, cases were identified through the Aberdeen Psychiatric Case Register (APCR). The APCR ceased recording new data in 1999, and since 2000 case-record diagnoses have been collected from psychiatric medical record departments in Grampian. We believe that this has given rise to inclusion of all patients diagnosed with AN for the from 1965 to 2007, but it is possible that a few may have been missed, particularly in the years since the APCR ceased to function. The diagnostic criteria we deployed were designed to strike a balance between stringency and the practicalities of obtaining detailed information from case records. In that one-third of those patients who received a case-register or case-record diagnosis of AN did not fulfil our inclusion criteria, we consider that this balance was broadly appropriate. The inclusion of only 65% of originally diagnosed patients does highlight the problems inherent in studies that have included, without further scrutiny, all case-register or hospital diagnoses of eating disorders (Sollid et al. Reference Sollid, Wisborg, Hjort and Secher2004; Ekéus et al. Reference Ekéus, Lindberg, Lindblad and Hjern2006; Bansil et al. Reference Bansil, Kuklina, Whiteman, Kourtis, Posner, Johnson and Jamieson2008). Although we envisage that our cohort is representative of all patients with AN who presented to psychiatric services (including in-patients, out-patients and day patients), it is possible that those matching the AMND are not entirely typical. The region is characterized by low geographical mobility, but patients who were diagnosed in North East Scotland with AN and who moved out of the area before becoming pregnant would not appear on the AMND. These more geographically mobile women may differ, for example by occupation or social class, from those who do not move out of the area.
The comprehensive data available through the AMND constitute a major strength of the study. This permitted accurate matching of patients with non-AN women for maternal age, year of first delivery and parity. It also facilitated statistical adjustments for potentially important confounding variables such as maternal BMI, smoking status and social class. Although nearly all pregnancy-related variables were available, weight gain during pregnancy is not routinely collected on the AMND. This would have been of potential relevance, especially because women with eating disorders have been found to have higher gestational weight gains, which may mitigate against poor pregnancy outcomes in AN (Bulik et al. Reference Bulik, von Holle, Siega-Riz, Torgersen, Lie, Hamer, Berg, Sullivan and Reichborn-Kjennerud2009).
The design of our study precluded our having knowledge of subjects' symptoms of AN at the time of their pregnancies. Such data would have permitted scrutiny of possible associations between pregnancy outcomes and enduring symptoms of AN. As a broad outcome measure of AN, we compared women with BMIs below and above 20 kg/m2, although weight restoration does not always predict hormonal or nutritional health (Loucks, Reference Loucks2007).
With a total of 230 pregnancies in women with a history of AN, our study is not the largest of its type. Sollid et al. (Reference Sollid, Wisborg, Hjort and Secher2004) investigated 504 pregnancies but the mothers, all of whom had been psychiatric in-patients, had mixed eating disorder diagnoses recorded on a national case register. Micali et al. (Reference Micali, Simonoff and Treasure2007a) had a sample size only slightly smaller than our own (171 pregnancies), but their diagnoses were based on maternal self-report. Bansil et al. (Reference Bansil, Kuklina, Whiteman, Kourtis, Posner, Johnson and Jamieson2008) studied 1668 women in the USA who had been admitted to hospital to deliver babies and had contemporaneously been diagnosed with an active eating disorder. Women with AN were not separately diagnosed, and there were high rates of co-morbidity of alcohol/drug misuse with eating disorders. Ekéus et al. (Reference Ekéus, Lindberg, Lindblad and Hjern2006) studied 1000 pregnancies of women previously diagnosed as in-patients with AN on the Swedish National Case Register. Of these larger studies, only Micali et al. (Reference Micali, Simonoff and Treasure2007a) were able to adjust for maternal BMI.
Maternal weight, birthweight and infant health
For many years, LBW has been associated with poorer health outcomes, not only in infancy and childhood but also into adult life (Wilcox, Reference Wilcox2001; Stein et al. Reference Stein, Zybert, van der Pal-de Bruin and Lumey2006; Micali & Treasure, Reference Micali and Treasure2009). However, LBW is a complex phenomenon and is an inexact predictor of health because LBW infants comprise a mixture of preterm, IUGR and constitutionally small babies (Wilcox, Reference Wilcox2001; Goedhart et al. Reference Goedhart, van Eijsden, van der Wal and Bonsel2008). Although famine conditions during pregnancy result in LBW babies (Rush, Reference Rush2001), maternal nutritional deprivation affects foetal growth only below a threshold (Stein et al. Reference Stein, Ravelli and Lumey1995), presumably equivalent to a significant and active eating disorder. In addition, although higher maternal BMI at conception correlates with higher birthweights and with lower rates of IUGR, it is also associated with increased foetal death rates (Naeye, Reference Naeye1990; Rush, Reference Rush2001). Concomitantly, mothers with low BMIs before pregnancy are more likely to have light babies than mothers with higher BMIs (Sebire et al. Reference Sebire, Jolly, Harris, Regan and Robinson2001; Bhattacharya et al. Reference Bhattacharya, Campbell, Liston and Bhattacharya2007) and the babies have lower perinatal death rates (Naeye, Reference Naeye1990).
Against this backdrop, it would be simplistic to be concerned about all LBW babies born to mothers with a history of AN, especially if no account has been taken of maternal BMI. In the present study, LBW was only significantly related to maternal AN status prior to adjustment for BMI. However, as opposed to LBW per se, SBW (which corrects for maternal BMI, gestational age, parity and baby gender) is a clearer indication of possible IUGR. Mean SBWs were also lower in babies of AN mothers than in those of non-AN mothers.
It is important to attempt to clarify the possible clinical significance of this finding. Babies with IUGR are at increased risk from a range of adverse early and later outcomes (Botero & Lifshitz, Reference Botero and Lifshitz1999; Cooke, Reference Cooke2007), and outcomes can be ameliorated with early detection and prompt management (Brodsky & Christou, Reference Brodsky and Christou2004; Breeze & Lees, Reference Breeze and Lees2007). It could thus be argued that vigilance for IUGR is important in the pregnancies of mothers with a history of AN, especially when these women have a low pre-pregnancy BMI. Fewer babies of AN mothers are abnormally large, and it would seem alarmist to be routinely concerned that these babies are at risk of IUGR, although obstetric services may want to factor a history of maternal AN into a holistic assessment of risk.
Early pregnancy loss
In comparison with our matched mothers, the AN women in our study did not differ significantly with regard to the frequency of either previous miscarriage or medical terminations of pregnancy. In comparison with all 54 100 women recorded in the AMND, the AN women again did not differ significantly in rate of pregnancy terminations, but their rates of miscarriage were higher. We thought it likely that this difference arose because the AN women were on average 2 years older (Table 2) at the time of their first delivery, and miscarriages tend to become more common with increasing maternal age (Nybo Andersen et al. Reference Nybo Andersen, Wohlfahrt, Christens, Olsen and Melbye2000). Bulik et al. (Reference Bulik, Sullivan, Fear, Pickering, Dawn and McCullin1999) also found higher rates of miscarriage in women with AN, but in their study the women were significantly younger than the control women. In the Norwegian Mother and Child Cohort Study, Bulik et al. (Reference Bulik, Hoffman, von Holle, Torgersen, Stoltenberg and Reichborn-Kjennerud2010) found that women with AN (who were again younger than comparison women) had more unplanned pregnancies and more terminations of pregnancy than did women without eating disorders. A similar finding of an increase in the proportion of unplanned pregnancies in the Avon Longitudinal Study (Easter et al. Reference Easter, Treasure and Micali2011) lends support to this possibility.
Antepartum haemorrhage
Following adjustment for BMI, our mothers with a history of AN were at significantly greater risk for antepartum haemorrhage (RR 1.73, 95% CI 1.11–2.19) than matched non-AN mothers. This is a new finding. Women with AN are reported to have increased likelihood of a bleeding diathesis (Mitchell & Crow, Reference Mitchell and Crow2006). Increased haemorrhagic tendency among our AN mothers may be more likely to reflect mild vitamin K deficiency. Females with AN have significantly reduced intake of dietary fats, not only when the disease is active but also at follow-up (Nova et al. Reference Nova, Varela, Lopez-Vidriero, Toro, Cenal, Casas and Marcos2001; Affenito et al. Reference Affenito, Dohm, Crawford, Daniels and Stiegel-Moore2002; Misra et al. Reference Misra, Tsai, Anderson, Hubbard, Gallagher, Soyka, Miller, Herzog and Klibanski2006), and vitamin K is fat soluble. The increased rates of antepartum haemorrhage could also be the result of reporting bias, women with AN being more likely to be anxious about their pregnancy and seek health care at a lower threshold of bleeding. It is important to note that specific types of antepartum haemorrhage (placenta praevia, placental abruption) did not differ significantly in the two groups, but only the more innocuous, and more common non-specific antepartum haemorrhage, was increased. In addition, if AN women had a bleeding diathesis, the rates of postpartum haemorrhage should also be increased.
Advice about pregnancy and AN
There is adequate evidence that active, significant AN during pregnancy is harmful to babies in utero (Treasure & Russell, Reference Treasure and Russell1988; Conti et al. Reference Conti, Abraham and Taylor1998; Bansil et al. Reference Bansil, Kuklina, Whiteman, Kourtis, Posner, Johnson and Jamieson2008). Thus, when women with significant AN become pregnant, advice advocating closely coordinated multidisciplinary care is entirely appropriate. Indeed, good cases have been made (partly in view of the clandestine nature of eating disorders) to screen pregnant women (Micali et al. Reference Micali, Treasure and Simonoff2007b; Soares et al. Reference Soares, Nunes, Schmidt, Giacomello, Manzolli, Camey, Buss, Drehmer, Melere, Hoffman, Ozcariz, Manenti, Pinheiro and Duncan2009) and to improve obstetric services in identifying women with active eating disorders (Leddy et al. Reference Leddy, Jones, Morgan and Schulkin2009).
The situation is less straightforward when women have remitted AN or continuing mild symptoms. The natural history of AN shows that the prognosis is not always good and that recovery is often slow. Steinhausen (Reference Steinhausen2002) reviewed more than 100 studies covering more than 5000 patients with AN and reported that just less than half had fully recovered at follow-up. It follows that many women will be contemplating a pregnancy while they are, at least to some degree, still symptomatic. Such women may often remain underweight; one study found that at a 12-year follow-up of women with AN, their mean BMI was 20.1 kg/m2 (Sullivan et al. Reference Sullivan, Bulik, Fear and Pickering1998). Furthermore, when women with eating disorders become pregnant, mptoms to improve during pregnancy (Bulik et al. Reference Bulik, von Holle, Hamer, Berg, Torgersen, Magnus, Stoltenberg, Siega-Riz, Sullivan and Reichborn-Kjennerud2007; Micali et al. Reference Micali, Treasure and Simonoff2007b; Crow et al. Reference Crow, Agras, Crosby, Halmi and Mitchell2008; von Soest & Wichstrom, Reference von Soest and Wichstrom2008) and pregnancy is sometimes a useful goal to promote motivation for recovery and engagement in therapy.
Against this backdrop, and in view of the level of increased risk of adverse outcomes detected in the present study, we suggest that previous advice – to delay pregnancies until complete remission and for routine close monitoring of pregnancies – may have been unduly cautious. As with any risk factor in medicine, it is important to consider the magnitude of that risk rather than to view it as an all-or-nothing phenomenon. Women with a history of AN, from the results of the present study, are at slightly, but statistically significantly, increased risk of having a baby with IUGR and of experiencing antepartum haemorrhage, but, from many angles, it is vital to view such risks in a holistic perspective.
Acknowledgements
We thank L. Murdoch for matching and extracting data from the AMND, and L. Hadden for secretarial work for the paper. The study was funded by the Chief Scientist's Office in Scotland. The North of Scotland Ethics Service declared that formal ethics committee approval was not required because all data were anonymized.
Declaration of Interest
None.







