INTRODUCTION
Even after serious efforts of various national and international agencies, the deadline to control Leishmania donovani (Ld)-induced visceral leishmaniasis (VL) has been rescheduled again and again in the Indian subcontinent. This is due to the increasing rate of resistance to anti-leishmanial drugs, limited therapeutic options, lack of sterile cure, appearance of post-kala-azar dermal leishmaniasis, the existence of asymptomatic infection and HIV-VL co-infection, etc. Presently, there is no vaccine in use. Though few vaccine candidates are under clinical trial (Chakravarty et al. Reference Chakravarty, Kumar and Trivedi2011; Coler et al. Reference Coler, Duthie, Hofmeyer, Guderian, Jayashankar, Vergara, Rolf, Misquith, Laurance, Raman, Bailor, Cauwelaert, Reed, Vallur, Favila, Orr, Ashman, Ghosh, Mondal and Reed2015), above situation warrants the introduction of potent immunomodulators, which can be used in future vaccine modules. Most of the earlier proposed vaccines failed to ascertain the high degree of antigenicity and persistency, which are essential for a suitable vaccine candidate. Major hurdles on the way of anti-leishmania vaccine are variation in immunogenicity due to human lymphocyte antigen expression, gene variation as well as parasite-induced pathogenic pressure over vaccine candidate (Kumar and Engwerda, Reference Kumar and Engwerda2014). Many of the earlier subunits or protein vaccines (Skeiky et al. Reference Skeiky, Kennedy, Kaufman, Borges, Guderian and Scholler1998; Stager et al. Reference Stager, Smith and Kaye2000; Agallou et al. Reference Agallou, Margaroni and Karagouni2011; Ghosh et al. Reference Ghosh, Zhang and Matlashewski2001; Rafati et al. Reference Rafati, Zahedifard and Nazgouee2006; Goto et al. Reference Goto, Bogatzki, Bertholet, Coler and Reed2007; Carrillo et al. Reference Carrillo, Crusat, Nieto, Chicharro, Thomas and Martinez2008) failed due to the fact that they were either tested in murine model or with cell lines, which provided good data inputs but may not mimic human cells. The present study has been carried out on peripheral blood of various healthy individuals from different endemic and non-endemic zones and treated cases of VL to decipher above problem. In this study, the presence of T-cell epitopes restricted to CD4+ or CD8+ cells has been also examined on phosphoglycerate mutase (PGAM) of Ld.
PGAM is a cytosolic enzyme, involved in glycolysis as well as gluconeogenesis pathway. It catalyses the reversible conversion of 3-phosphoglyceric acid (3-PGA) to 2-phosphoglyceric acid (2-PGA). Unlike co-factor-dependent PGAM (dPGAM) of human subjects, Leishmania contains co-factor-independent PGAM (iPGAM). iPGAM exhibits no sequence similarity with human dPGAM (Chevalier et al. Reference Chevalier, Rigden, van Roy, Opperdoes and Michels2000; Nowicki et al. Reference Nowicki, Kuaprasert, McNae, Morgan, Harding, Michels, Fothergill-Gilmore and Walkinshaw2009), which suggest its vaccine potential as well as the drug target. Basic Local Alignment Search Tool protein (BLASTP) analysis of the Ld-iPGAM and whole human protein shows only 30% identity and 9% query cover particularly with neural cell adhesion molecule L1 isoform 3 precursors. iPGAM of L. donovani exist in both the promastigotes and amastigotes forms, which is also an additional advantage regarding its vaccine potential. It becomes critical enzyme in the amastigotes form for its existence in human host because gluconeogenesis but not glycolysis is an essential pathway for its survival during amastigote form (Naederer et al. Reference Naederer, Ellis, Sernee, De Souza, Curtis, Handman and McConville2006). Recombinant iPGAM of Brugia malayi was also explored for its vaccine utility (Singh et al. Reference Singh, Kushwaha, Rana and Bhattacharya2014) and have been suggested as a possible target for drug designing. In the present study, recombinant iPGAM of Ld (rLd-iPGAM) has been reported with its potential to upregulate host-protective immune response augmenting antigen-presenting cell (APC) function, activation of effector cells and required anti-leishmanial activity. In silico study has suggested that Ld-iPGAM bears major histocompatibility complex (MHC) class I and II restricted epitopes (6 HLA A0201 and 5 DRB104010). Bearing these epitopes, rLd-iPGAM induced upregulation of interleukin-1β (IL-1β) on APCs as well as IL-1β receptor and activation marker CD69 on CD4+ or CD8+ cells. This early activation was resulted in the dominance of host-protective cytokines, viz. IL-2, interferon-γ (IFN-γ), IL-17, IL-12 and tumour necrosis factor-α (TNF-α). Besides, it upregulated the expression of reactive oxygen species (ROS) and inducible nitric oxide synthase (iNOS) in target cells in the presence of meager suppressor of mother against decapentaplegic protein-4 (SMAD-4) expression and upregulated nuclear factor-κ light-chain enhancer of activated B cells p50 (NF-κB p50). Present study ensures rLd-iPGAM as a good immunoprophylactic agent, which can be explored in future vaccine designing.
MATERIALS AND METHODS
Leishmania donovani culture and isolation of genomic DNA
A reference strain of Ld (MHOM/IN/83/AG83) was cultured in Roswell Park Memorial Institute (RPMI) medium supplemented with 10% fetal bovine serum (FBS). Promastigotes culture was maintained as per protocol being used elsewhere (Singh et al. Reference Singh, Bimal, Narayan, Jee, Bimal, Das and Bimal2011). Late log phase parasite was harvested by centrifugation at 900 g at 4 °C for 20 min (Hermle, Germany). The pellets (108 promastigotes) were washed thrice with phosphate-buffered saline (PBS) and spun at 900 g for 20 min at 4 °C. The washed pellet was used to isolate DNA using DNA Purification Kit (Qiagen, Germany). Isolated DNA was electrophoresed on 0·8% agarose gel, and DNA concentration was evaluated and stored for further use.
Amplification and cloning of Ld-iPGAM
Ld-iPGAM gene-specific primers were designed manually and checked with NEB Cutter and Oligocaclc tools. Primers were as following: forward 5′ TTT TGG ATC CAT GTC GAA TCT CCT CTT GAC 3′ (BamHI restriction site is underlined) and reverse 5′ TTT TAA GTC TTC AGT TCT CGA CAT AGA TC 3′ (Hind III restriction site is underlined). These primers were synthesized commercially (IDT, Gurgaon, India). The Ld-iPGAM gene (NCBI Accession no. CBZ39131·1) was amplified using above primers in Thermal cycler (Bio-Rad, USA). The PCR condition was set as the first cycle at 95 °C for 2 min and 30 cycles at 94 °C for 1 min, 58·1 °C for 1 min, 72 °C for 1 min and last cycle at 72 °C for 10 min. The PCR product was electrophoresed on 1% agarose gel along with 1 kb ladder (BR Biochem. Life Sciences, India). The amplified product was eluted using gel extraction kit (Qiagen, Germany). Vector (pET-28a) was isolated from the fresh culture using plasmid isolation kit (Qiagen, Germany). Isolated pET-28a and eluted amplicon were digested separately with BamHI and Hind III (Promega, USA) and ligated using T4 DNA ligase (Fermentas, Thermo Fisher Scientific, USA). Competent Escherichia coli DH5-α cell was transfected with the recombinant plasmid. Next day, the transformation was confirmed by colony PCR as well as by restriction double digestion using BamHI and Hind III.
Expression and purification of iPGAM
The pET-28a-Ld-iPGAM construct was isolated from DH5-α and re-transformed in competent E. coli BL-21 (DE3, an expression host for recombinant construct). This transformation was again confirmed by colony PCR and restriction double digestion. A 50 µL fresh inoculum of transformed BL-21 was inoculated in 5 mL Luria broth supplemented 1 µg mL−1 kanamycin. The culture was incubated at 37 °C in a shaker incubator at 220 rpm till the O.D600 reached to 0·5–0·6. Further, 0·8 mm isopropyl β-D-1-thiogalactopyranoside was added to the culture and incubated at 25 °C for overnight. A 1 mL of the recombinant cells culture was pelleted and lysed with 2× sample loading buffer [100 mm Tris base (pH 6·8), 4% sodium dodecyl sulphate (SDS), 20% glycerol, 0·2% bromophenol blue, 200 mm β-mercaptoethanol] and electrophoresed in SDS-12% polyacrylamide gel electrophoresis (PAGE) to confirm the protein expression. The uninduced culture was run in parallel as a control. The result was analysed in comparison to protein marker (Puregene).
For the expression of recombinant protein, transformed BL-21 (with pET28a-rLd-iPGAM) was inoculated in 200 mL LB medium supplemented with 1 µg mL−1 kanamycin. The culture was extended as described earlier in this manuscript. The culture was harvested at 6000 g for 15 min and resuspended in 5 mL lysis buffer (50 mm Tris base pH 8, 300 mm NaCl, 0·1% Triton X-100) containing 50 µL of 1 mm phenylmethylsulfonyl fluoride, 50 µL of 10 mg mL−1 lysozyme and incubated for 2 h at 25 °C. The suspension was sonicated for 8 × 60 s (with 60 s intervals between each pulse) at 70 amplitude on the ice. Sonicated cells were centrifuged at 14 000 g for 15 min and the supernatant containing protein of interest was mixed with 600 µL Ni2+ nitrilotriacetic acid bead (Qiagen, Germany) in a column and incubated at 4 °C for 1 h in a shaker incubator. The column content was washed with buffer (50 mm NaH2PO4, 20 mm imidazole, 300 mm NaCl, pH 8) for 7–8 times and rLd-iPGAM was eluted using elution buffer (50 mm NaH2PO4, 250 mm imidazole, 300 mm NaCl, pH 8). The purity of eluted rLd-iPGAM was analysed by SDS-12% PAGE (Laemmli, Reference Laemmli1970). The concentration of purified recombinant protein was estimated by Bradford method using bovine serum albumin (BSA) as standard.
Preparation of soluble leishmanial antigen
For the preparation of soluble leishmania antigen (SLA), the late log phase parasites were centrifuged in 15 mL centrifuge tubes (Tarson, India) at 900 g for 20 min in a cooling centrifuge (Hermle, Germany). The pellet was washed twice with PBS by centrifuging at 900 g at 4 °C for 20 min. The washed Ld pellet was subjected to six freezes and thaw cycle. The lysate was centrifuged at 30 000 g for 30 min, and the supernatant was collected in aliquots and stored at −80 °C for further use.
Confirmation of purified protein by Western-blot and lipopolysaccharide (LPS) test
Western blotting was performed to confirm the presence of recombinant protein using anti-hexahistidine antibody (Towbin et al. Reference Towbin, Staehelin and Gordon1979). Briefly, the purified iPGAM was electrophoresed in SDS-12% PAGE and transferred to 0·22 µm nitrocellulose membrane (Sigma-Aldrich, USA) in the presence of transfer buffer (25 mm Tris base, 0·2 m glycine, 20% methanol, pH 8·2) using semi-dry blotter (Bio-rad, USA) at 15 V for 30 min. The transferred nitrocellulose paper was treated with blocking buffer (5% BSA, PBS) for overnight and washed with Tris buffer saline-tween 20 [TBS-T (Tris base, NaCl, 0·1% tween-20 and 0·2% BSA, pH 7·5)] for three times. The membrane was incubated with anti-hexahistidine antibodies (1 : 2500 for 1 h, Santa-Cruz Biotech, USA) followed by three washing with TBS-T. The membrane was further incubated with horseradish peroxidase conjugated anti-rabbit antibody (1 : 1000, MERCK Biosciences, Bangalore, India). The blotted membranes were introduced with 3-3′-diaminobenzidine (0·06% H2O2, Ameresco, Solon, USA) solution till the band appeared without background. The membrane was rinsed with plenty of distilled water to stop the reaction and analysed. The concentration of bacterial LPS contamination was determined in the rLd-iPGAM using Limulus amoebocyte lysate test (Thermo-Scientific, USA) as per manufacturer's instructions. A 0·12 µg mg−1 of LPS contamination was detected in the rLd-iPGAM. The contamination was removed using a polymyxin B-agarose column (Sigma, USA) according to the manufacturer's instructions. The purified rLd-iPGAM was then stored at −80 °C for further use.
Enzymatic activity of rLd-iPGAM
The enzymatic activity of rLd-iPGAM was carried out spectrophotometrically by measuring the decrease of UV absorbance at 340 nm due to oxidation of nicotinamide adenine dinucleotide hydrogen (NADH). As it is known that the amount of product produced in the reaction corresponds to the amount of NADH oxidized to nicotine adenine dinucleotide in the conversion of 3-PGA to 2-PGA. The assay was performed using 1 mL mixture containing 0·1 m HCl pH 7·6, 1 mm MgCl2, 1 mm adenosine diphosphate, 0·56 mm NADH, 0·1 mm CoCl2, 5 mm 3-PGA, 1 U mL−1 pyruvate kinase (Sigma), 1 U mL−1 enolase (Sigma), 1 U mL−1 pyruvate dehydrogenase (Sigma) and 1 µg mL−1 rLd-iPGAM at 25 °C.
Fluorescence staining of early activation marker CD69 on lymphocytes
Whole blood from the healthy subject (n = 13, observation of clinical symptoms and haematological changes expressed in a different study group has been attached in online Supplementary Table S1) was used to study the expression of surface markers lies on innate immune cells. A 100 µL whole blood was taken in culture plate (Nunc, Denmark), stimulated with or without rLd-iPGAM and incubated in the CO2 incubator at 37 °C for 24 h (for that blood was mixed with RPMI medium 1640+10% FBS in 1 : 1 ratio). Anti-CD69 fluorescein isothiocyanate (FITC) antibody (BD Biosciences) was added and further incubated for 20 min at room temperature in dark condition. A 1 mL fluorescence-activated cell sorting (FACS) lysis buffer™ (1×) was added, vortexed and incubated for 10 min. Cells were centrifuged at 200 g for 5 min. The pellet was washed with 2 mL PBS, and finally, samples were suspended in 500 µL PBS and acquired to study the fluorescence on FACS Caliber™ (BD). In order to explore the role of rLd-iPGAM on CD4+ and CD8+ cells, expression of CD69 was also evaluated on peripheral blood mononuclear cells (PMNCs). For this, PMNCs were isolated from peripheral blood of healthy subjects (n = 13) by density gradient centrifugation over Histopaque1077 (Sigma-Aldrich). The PMNCs were washed with PBS and counted on 0·1 mm Naubauer chamber (Fein Optia, JENA, Germany). Cells were suspended at 1 × 106 PMNCs mL−1 in RPMI-1640 complete media (RPMI + FBS). The cells were stimulated with or without rLd-iPGAM and incubated in the CO2 incubator at 37 °C for 24 h. The cultured cells were washed and stained with anti-CD4 Phycoerythrin Cy5 (PECy5) or anti-CD8 PECy5 and anti-CD69 FITC by following protocols discussed above.
Fluorescence staining of T cells and macrophages for qualitative evaluation of rLd-iPGAM-induced intracellular cytokines
PMNCs (1 × 106 cells mL−1) from healthy and treated VL subjects (n = 13, online Supplementary Table S1) were stimulated with or without Ld or rLd-iPGAM or phorbol 12-myristat 13-acetate (PMA) and ionomycin and incubated in the CO2 incubator at 37 °C for 96 h. The culture was blocked using GolgiPlug (1 µg mL−1, BD) before 4 h of harvesting. Non-adherent cells were collected from culture supernatant and harvested to study intracellular cytokine of innate and adaptive immune cell. Centrifuged supernatant was used for enzyme-linked immunosorbent assays (ELISA). Adherent cells (macrophages) were scraped out by keeping the plate on ice using ice-chilled PBS and used for onward macrophage study. Cells were washed with PBS and surface antibody, like anti-CD4 PECy5, anti-CD8 PECy5, anti-CD14 PerCP and anti-IL-R PE, was added in respective falcon tubes and incubated for 20 min at 4 °C. A cell sample was again washed with PBS. Cytofix (BD Biosciences) was added and incubated for 30 min at 4 °C. After incubation, cells were permeabilized by adding 1 mL permwash buffer (1×) (BD Biosciences) and kept at 4 °C for 5 min. Samples were washed and intracellular antibodies, like anti-IL-1β PE, anti-IFN-γ FITC, anti-IL-10 PE, anti-IL-2 FITC, anti-IL-4 FITC, anti-IL-12 FITC, anti-IL-17A PE, anti-transforming growth factor (TGF)-β FITC, anti-NF-κ β P50 PE, anti-SMAD PE, were added in respective tube followed by incubation for 30 min at 4 °C. Samples were gently mixed with 1 mL permwash buffer (1×) and washed thoroughly. Tubes were further centrifuged and washed with 2 mL stain solution (PBS with 0·09% NaN3 and 1% FBS). Finally, cell pellets were suspended in 500 µL stain buffer and acquired on FACS Caliber (BD).
Quantitative evaluation of rLd-iPGAM-induced cytokine expression by PMNCs
The cytokines, like IL-10, IFN-γ, TNF-α and IL-12, were evaluated in culture supernatants of PMNCs stimulated with or without rLd-iPGAM or Ld for 96 h. Phytohaemagglutinin (PHA) stimulation was used as positive control. Quantitative evaluations of cytokines were performed using kit contents and user manual of BD OptEIA kit. Absorbance was measured at 540 nm in ELISA reader (Bio-Rad).
Assessment of lymphocyte proliferation responses after rLd-iPGAM stimulation
PMNCs from healthy and treated VL subjects were cultured in 96-well flat-bottom tissue culture plates (Nunc, Denmark) with or without SLA or rLd-iPGAM or PHA (1 µg mL−1, Sigma) as positive control. The LTT (lymphocyte transformation test) assay was carried out following protocol by Garg et al. (Reference Garg, Gupta, Tripathi, Naik and Sundar2005) but using 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT, Roche diagnostics) instead of 3H thymidine. The culture was incubated at 37 °C in 5% CO2 for 3 days in case PHA and 5 days for unstimulated blank, SLA, and rLd-iPGAM. A 50 µL of XTT was added to 100 µL of the culture of each well. Absorbance was measured at 480 nm with 650 nm as reference wave length.
Evaluation of the effect of rLd-iPGAM on induction of ROS
PMNCs from the healthy subjects were taken in 12-well plate and incubated at 37 °C in CO2 incubator with 5% CO2 for overnight. Next days, adherent macrophages were collected from the cultured plate by following the same protocol as mentioned earlier. A 100 µL of cultured and washed macrophages from healthy subjects were taken into four different 12 × 75 mm2, Falcon™ tubes. First tube without any stimulation was taken as negative control, second tube was challenged with Ld. Similarly, third tube was stimulated with rLd-iPGAM and lastly, the LPS was added in a fourth tube as a positive control. All tubes were incubated in a water bath for 15 min. All tubes were taken out from water bath and added with 200 µ m dihydrorhodamine 123. A 4 µ m n-formylmethionyl-leucyl-phenylalanine was added in LPS as well as rLd-iPGAM containing tubes. Further tubes were incubated at 37 °C for 10 min. The samples were treated with 1 mL 1× FACS lysis buffer™ (BD) and centrifuged at 200 g for 5 min. The supernatant was discarded and resuspended in 500 µl stain buffer (PBS+1% FBS+0·09% NaN3). Samples were acquired on FACS Caliber™ using Cell Quest software (BD). The ROS produced by stimulated cells were measured based on mean fluorescence intensity (MFI) detected by the flow cytometry.
Evaluation of rLd-iPGAM-induced nitric oxide (NO) synthesis in PMNCs culture supernatant
PMNCs from healthy donor were cultured (1 × 106 cells mL−1) with or without rLd-iPGAM or Ld or PHA in 5% CO2 incubator at 37 °C for 96 h. Nitrate and nitrite were quantified to evaluate NO synthesis in the culture supernatant of PMNCs in the presence or absence of rLd-iPGAM, Ld and PHA. The assay was performed using the contents and kit manual of NO Assay Kit (Thermo Fisher). Absorbance was recorded at 540 nm wavelength.
Evaluation of iNOS by semi-quantitative RT-PCR
Transcription of iNOS was evaluated by isolating the total RNA from PMNCs culture stimulated with or without rLd-iPGAM using Trizol. Reverse transcription was performed using an anchored oligo dT (H-dT11 M, where M represents adenine, cytosine, or guanine; GenHunter). The synthesized cDNAs were amplified by PCR for iNOS gene. The condition for RT-PCR included an initial denaturation at 94 °C for 5 min, 94 °C for 45 s, 56 °C (iNOS) or 58 °C glyceraldehyde 3-phosphate dehydrogenase (GAPDH) for 45 s, and 72 °C for 1 min, followed by a final extension at 72 °C for 5 min. RT-PCR was performed for 25 cycles, which was within the linear range of amplification of the corresponding mRNA species. The sequences of primers-iNOS: forward primer- 5′ AGCCAGAAGCGCTATCACGAAGAT-3′ reverse primer 5-AATG CAGAGCTGGCTCCATCCTTA-30 GAPDH Primer Fwd 5′TCAT CATCTCTGCCCCCTCTG3′, Rev 50-CGCCTGCTTCACCACCTTCTT-3. The RT-PCR end product was also run on 1·5% agarose gel, stained with ethidium bromide, and finally documented and quantified using the Bio-Rad gel documentation system and associated Quantity One software. PCR products were compared with respect to the GAPDH.
Evaluation of NO, TNF-α, IL-12 and IL-10 by rLd-iPGAM stimulated THP-1 cells
The human monocytic cell line, THP-1, was maintained in RPMI-1640 medium supplemented with 10% FBS at 37 °C in a humidified atmospheric incubator with 5% CO2. The 1 × 106 THP-1 cells mL−1 were cultured with 5 nm phorbol 12-myristate 13-acetate in 24-well plate for 48 h in order to get differentiation and maturation into macrophages. After incubation, cells were washed and cultured in fresh medium in the presence or absence of rLd-iPGAM or LPS. The culture supernatant was collected for evaluation of NO production using a kit (Thermoscientific) and cytokine ELISA for TNF-α, IL-12 and IL-10, using the BD OptEIA kit.
MHC class I and II epitopes prediction
For the screening of promising MHC class I and II epitopes, we mainly relied on HLA-A*0201 and HLA DRB1 0401 population. The amino acid sequence of 2,3-bisphosphoglycerate-independent PGAM (ACC No: CBZ39131·1) was retrieved from NCBI (http://www.ncbi.nlm.nih.gov/). The retrieved sequence was screened for 9 mer HLA-A*0201 restricted epitope using SYFPEITHI (which predicts the antigen-specific cytotoxic T-cell epitopes using matrix-based algorithm; Schuler et al. Reference Schuler, Nastke and Stevanović2007), RANKPEP (Reche et al. Reference Reche, Glutting, Zhang and Reinherz2004) and IEDB (http://tools.iedb.org) according to our previous methodology with certain alteration (Dikhit et al. Reference Dikhit, Kumar, Sahoo, Mansuri, Amit and Ansari2015; Amit et al. Reference Amit, Dikhit, Mahantesh, Chaudhary, Singh, Singh, Singh, Das, Pandey, Ali, Narayan, Sahoo, Das and Bimal2016). As it is known that RANKPEP is a position-specific scoring matrices-based bioinformatics tool, which is used to predict peptide binders to MHC-I molecules from protein sequences or sequences alignment. Besides, it also predicts the MHC-I ligands having C-terminal end, which is likely to be resultant of proteasomal cleavage. IEDB is the library of an experimentally measured immune epitopes. The database includes the tools that predict the MHC class I and II binding epitopes.
For 15 mer HLA DRB1 0401 restricted epitopes, SYFPEITHI, IEDB and NETMHC II (Nielsen and Lund, Reference Nielsen and Lund2009) servers were used. The consensus epitopes were selected based on Troat et al. theory as described previously (Dikhit et al. Reference Dikhit, Kumar, Sahoo, Mansuri, Amit and Ansari2015; Amit et al. Reference Amit, Dikhit, Mahantesh, Chaudhary, Singh, Singh, Singh, Das, Pandey, Ali, Narayan, Sahoo, Das and Bimal2016). BLASTP search against Homo sapiens was performed (Altschul et al. Reference Altschul, Madden, Schäffer, Zhang, Zhang and Miller1997; Kar et al. Reference Kar, Ansari, Suryadevara, Sahoo, Sahoo and Dikhit2013), and the peptides with 100% identity were excluded from the study.
Statistical analysis
Statistical analysis was performed using Graph Pad Prism 6. One-way analysis of variance was performed to evaluate the statistical significance of obtained data.
RESULTS
Ld-iPGAM was successfully cloned, expressed, purified and confirmed
Ld-iPGAM gene was amplified by PCR using genomic DNA of a reference Ld strain MHOM/IN/83/AG83. The amplified product was cloned, expressed and examined by SDS-PAGE. Overexpressed ~61 kDa band of Ld-iPGAM was recognized in comparison to SLA and uninduced BL-21 (Fig. 1A). The purified recombinant protein, as shown in Fig. 1B, was confirmed by Western blotting as an anti-hexahistidine monoclonal antibody reactive 61 kDa band (Fig. 1C). The specific activity of rLd-iPGAM measured after performing enzymatic assays was 32 U mg−1.
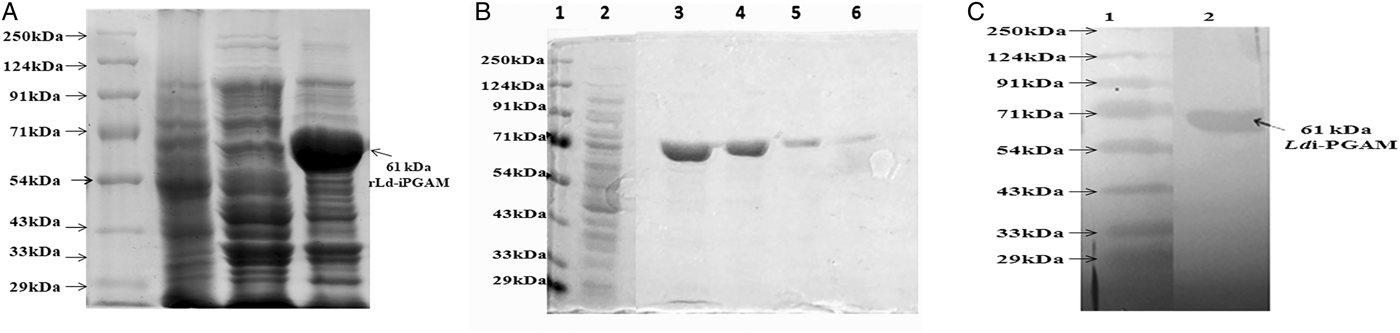
Fig. 1. The expression and purification of rLd-iPGAM. (A) SDS-12% PAGE of expressed protein (rLd-iPGAM) stained with coomassie brilliant blue R 250 (CBB R 250). Lane 1: protein molecular weight marker, lane 2: SLA, lane 3: uninduced transformed BL-21 and lane 4: expressed ~61 kDa rLd-iPGAM. (B) SDS-12% PAGE of purified rLd-iPGAM. Lane 1: protein molecular weight marker, lane 2: SLA and lanes 3, 4, 5 and 6: first, second, third and fourth elutions of purified iPGAM. (C) Western blotting of rLd-iPGAM protein showing reactivity with anti-hexahistidine antibody. Lane 1: protein marker, lane 2: purified rLd-iPGAM stained with anti-hexahistidine antibody.
rLd-iPGAM-induced dose-dependent expression of CD69 on lymphocytes
rLd-iPGAM-induced expression of CD69 was examined on human lymphocytes to evaluate the antigenicity as well as the optimal concentration of the antigen. Flow-cytometry data showed maximum (>10%) upregulation of CD69 on lymphocytes at 5 µg mL−1 rLd-iPGAM. The expression of CD69 was dose-dependent with further dilutions of rLd-iPGAM (Fig. 2A). However, some diminishing effect was also noticed on higher concentration of rLd-iPGAM. This experiment was also carried out on CD4+ and CD8+ cells (Fig. 2B and C). There was 2·3- and 2·06-fold (P⩽0·001) higher expression of CD69 on CD4+ and CD8+ cells, respectively, in response to 5 µg mL−1 rLd-iPGAM (Fig. 2D and E). PMA and ionomycin induded 9·8- and 7·5-fold higher expression of CD69 on CD4+ and CD8+ cells, respectively. Therefore, the 5 µg mL−1 concentration of rLd-iPGAM was taken into account for further experiments.
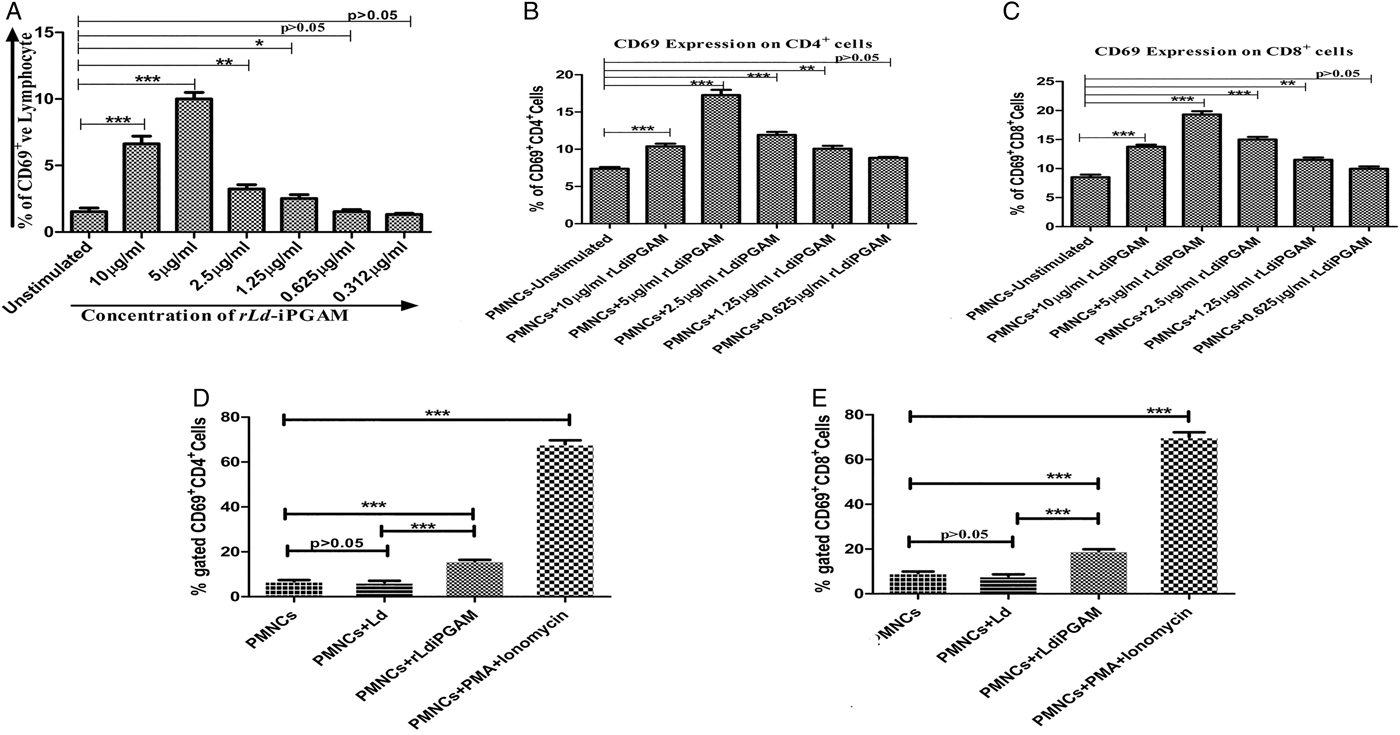
Fig. 2. The expression of CD69 on whole lymphocytes, CD4+ as well as CD8+ cells. (A) Expression of CD69 on whole lymphocytes (gated population of whole blood cells) upon stimulation with or without rLd-iPGAM in a series of 2-fold serial dilution. (B) Expression of CD69 on CD4+ cells (gated population from PMNCs) upon stimulation with or without rLd-iPGAM in a series of 2-fold serial dilution. (C) Expression of CD69 on CD8+ cells (gated population from PMNCs) upon stimulation with or without rLd-iPGAM in a series of 2-fold serial dilution. (D) Expression of CD69 on CD4+ T cells (gated population from PMNCs) after stimulation with or without Leshmania donovani (Ld) or rLd-iPGAM or PMA and ionomycin. (E) Expression of CD69 on CD8+ T cells (gated population from PMNCs) after stimulation with or without Ld or rLd-iPGAM or PMA and ionomycin. Bar represents the mean ± s.d. of five different experiments. Asterisk (*) is significant of (P < 0·05), double asterisk (**) is significant of (P < 0·01) and triple asterisk (***) is significant of (P < 0·001).
rLd-iPGAM stimulation induced higher expression of IL-1β and 1L-1R
After finalizing the concentration of rLd-iPGAM for in vitro studies, its effect on phagosome was evaluated. For this, effect of rLd-iPGAM on a leucocyte endogenous mediator and mononuclear cell factor was evaluated. rLd-iPGAM stimulation induced 1·90-fold higher IL-1β expression in macrophages in comparison to unstimulated control (Fig. 3A). rLd-iPGAM stimulation also induced higher expression of IL-β receptor (IL-1R) on CD4+ and CD8+ cells in comparison to unstimulated control (Fig. 3B and C).

Fig. 3. The secretion of IL-1β by macrophages and expression of its receptor IL-1R on CD4+ and CD8+ cells after stimulation with or without Leshmania donovani (Ld) or rLd-iPGAM or PMA and ionomycin. (A) Secretion of IL-1β by CD14+ macrophages. (B) Expression of IL-1R on CD4+ cells. (C) Expression of IL-1R on CD8+ cells. Bar represents the mean ± s.d. of three different experiments. Asterisk (*) is significant of (P < 0·05), double asterisk (**) is significant of (P < 0·01) and triple asterisk (***) is significant of (P < 0·001).
rLd-iPGAM-induced host-protective immune activation in healthy as well as in treated VL subject
Early cytokine IL-2 released from T lymphocytes for its activation towards effector function was also evaluated in response to rLd-iPGAM. Percentage of IL-2+CD4+ cell was increased by 1·90- and 2·16-fold in healthy and treated VL subjects, respectively, after stimulation with rLd-iPGAM in comparison to unstimulated control (Fig. 4A). Depriving and generous effect was also noticed after Ld or PMA and ionomycin stimulation, respectively.

Fig. 4. The secretion of IL-2 and IL-4 by CD4+ cells after stimulation with or without Leshamnia donovani (Ld) or rLd-iPGAM or PMA and ionomycin. (A) IL-2 secreted by CD4+ cells. (B) IL-4 secreted by CD4+ cells. Bar represents the mean ± s.d. of two different experiments. Asterisk (*) is significant of (P < 0·05), double asterisk (**) is significant of (P < 0·01) and triple asterisk (***) is significant of (P < 0·001).
As it is well known that cellular activation either proceed towards helper cell type 1 (Th1) or type 2 (Th2). Evaluation of a signature cytokine IL-4 for Th2 was essential to decipher the pathogenic response of rLd-iPGAM. Upon stimulation with the recombinant protein, expression of the IL-4+CD4 cell was reduced by 0·27- and 0·40-fold in healthy and treated VL subjects, respectively (Fig. 4B). However, an increasing trend of IL-4+CD4 cells were observed in samples stimulated with Ld or PMA and ionomycin.
On the other hand, a prompt effect of rLd-iPGAM was observed on IFN-γ, which is pre-requisite for protection against leishmaniasis. rLd-iPGAM stimulation induced 1·72-fold (P < 0·05) and 1·35-fold (P < 0·05) higher expression of IFN-γ in CD4+ cells from healthy and treated VL donor, respectively (Fig. 5A). As expected, in samples of treated VL donor, but not in healthy donor, IFN-γ expression was higher after Ld stimulation. PMA and ionomycin treatment, as positive control increased IFN-γ by 3·22- and 1·56-fold in healthy and treated subjects, respectively. At this point, evaluation of a signature regulatory cytokine IL-10 was crucial for rLd-iPGAM. rLd-iPGAM stimulation reduced the expression of IL-10 by 0·33- and 0·48-fold in both healthy as well as treated VL donors (Fig. 5C). Stimulation with Ld as well as PMA and ionomycin increased IL-10 expression in healthy samples. However, PMA and ionomycin but not Ld increased IL-10 expression in treated subjects.
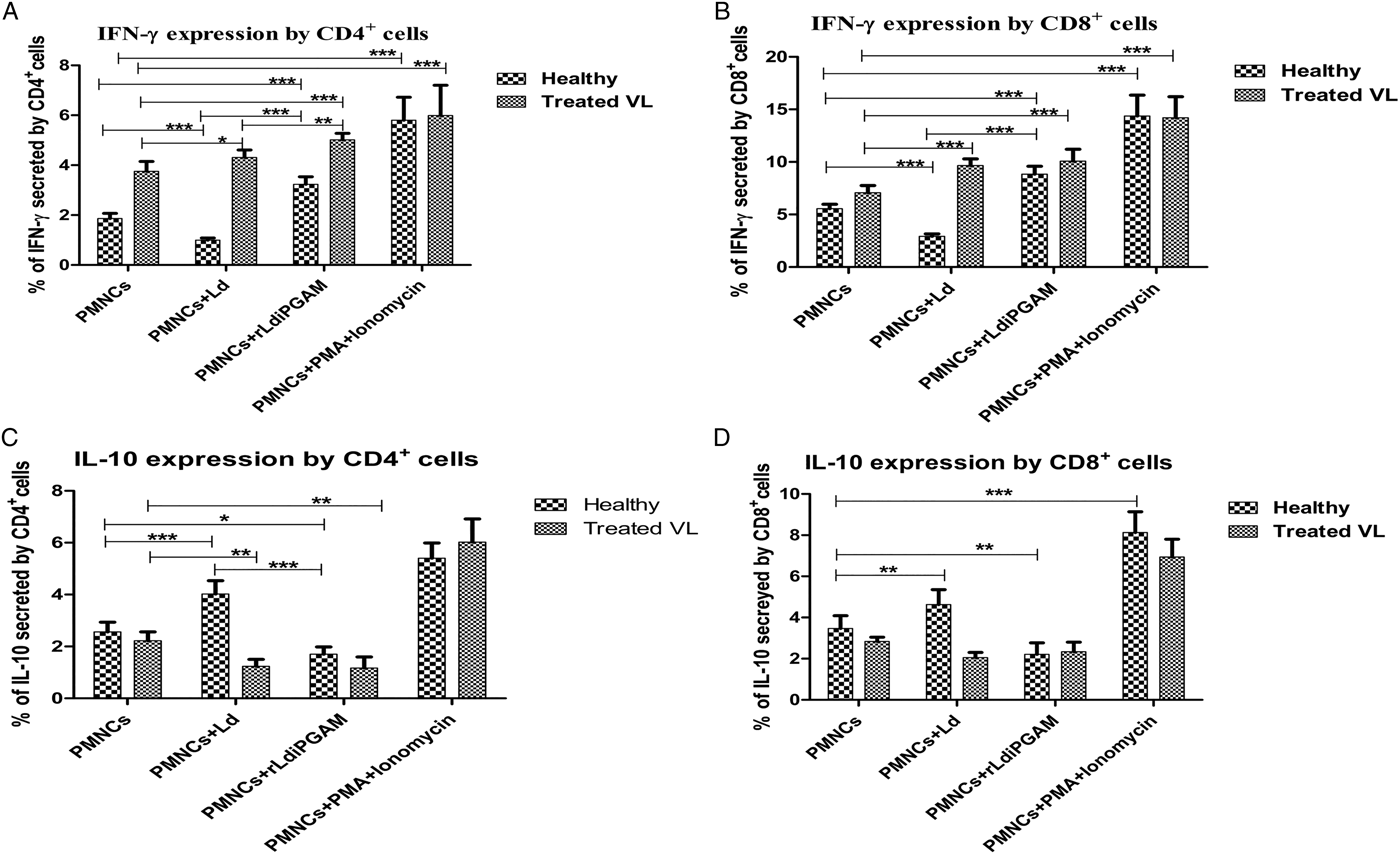
Fig. 5. The secretion of intracellular cytokines IFN-γ and IL-10 by CD4+ and CD8+ cells after stimulation with or without Leshmania donovani (Ld) or rLd-iPGAM or PMA and ionomycin. (A) IFN-γ secreted by CD4+ cells. (B) IFN-γ secreted by CD8+ cells. (C) IL-10 secreted by CD4+ cells. (D) IL-10 secreted by CD8+ cells. Bar represents the mean ± s.d. of four different experiments. Asterisk (*) is significant of (P < 0·05), double asterisk (**) is significant of (P < 0·01) and triple asterisk (***) is significant of (P < 0·001).
Like percentage of CD4+ IFN-γ + cells, CD8+ IFN-γ + cells were also upregulated in samples from treated VL and healthy human subjects in response to rLd-iPGAM (Fig. 5B). However, it was reduced by 0·48-fold in healthy samples and increased by 1·36-fold in treated VL samples in response to Ld. On the other hand, rLd-iPGAM stimulation reduced the percentage of IL-10 expressing CD8+ cells in samples of treated VL and healthy subjects (Fig. 5D). However, Ld stimulation upregulated IL-10 expressing CD8+ cells in samples from healthy, but not treated VL subjects. The PMA and ionomycin treatment showed positive response as expected.
Effects of rLd-iPGAM was also evaluated in the expression of another pro-inflammatory cytokine IL-17, induced by IL-1 and other inflamosomes, expressed by a set of T-helper and works synergistically with other inflammatory cytokines to protect from infection. rLd-iPGAM induced upregulation of CD4+ IL-17 cells as well as CD8+IL-17 in samples from healthy and treated VL donors (Fig. 6A and B). In comparison, Ld downregulated IL-17-positive CD4+ or CD8+ cells in healthy donors, whereas upregulated in samples from treated VL. Upregulated expression of IL17+ cells was observed after PMA and ionomycin stimulation.
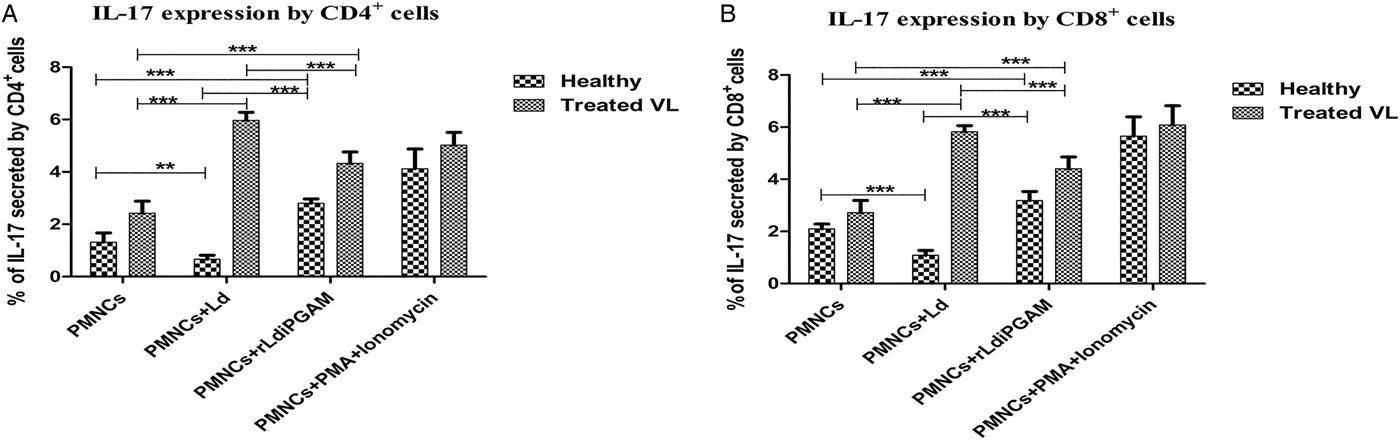
Fig. 6. The secretion of intracellular cytokines IL-17 by CD4+ and CD8+ cells after stimulation with or without Leshmania donovani (Ld) or rLd-iPGAM or PMA and ionomycin. (A) IL-17 secreted by CD4+ cells. (B) IL-17 secreted by CD8+ cells. Bar represents the mean ± s.d. of two different experiments. Double asterisk (**) is significant of (P < 0·01) and triple asterisk (***) is significant of (P < 0·001).
rLd-iPGAM charging boosts host-protective signalling of macrophages
Macrophage signalling plays a key role in providing immunity against the intracellular parasite Ld. Therefore, rLd-iPGAM-induced responses of macrophages were evaluated. IL-12 expressing CD14+ macrophages were increased significantly after stimulation with rLd-iPGAM or PMA and ionomycin in healthy and treated VL samples, respectively (Fig. 7A). Leishmania donovani stimulation, however, reduced IL-12 expressing macrophages in healthy donors, but increased in treated VL subjects.
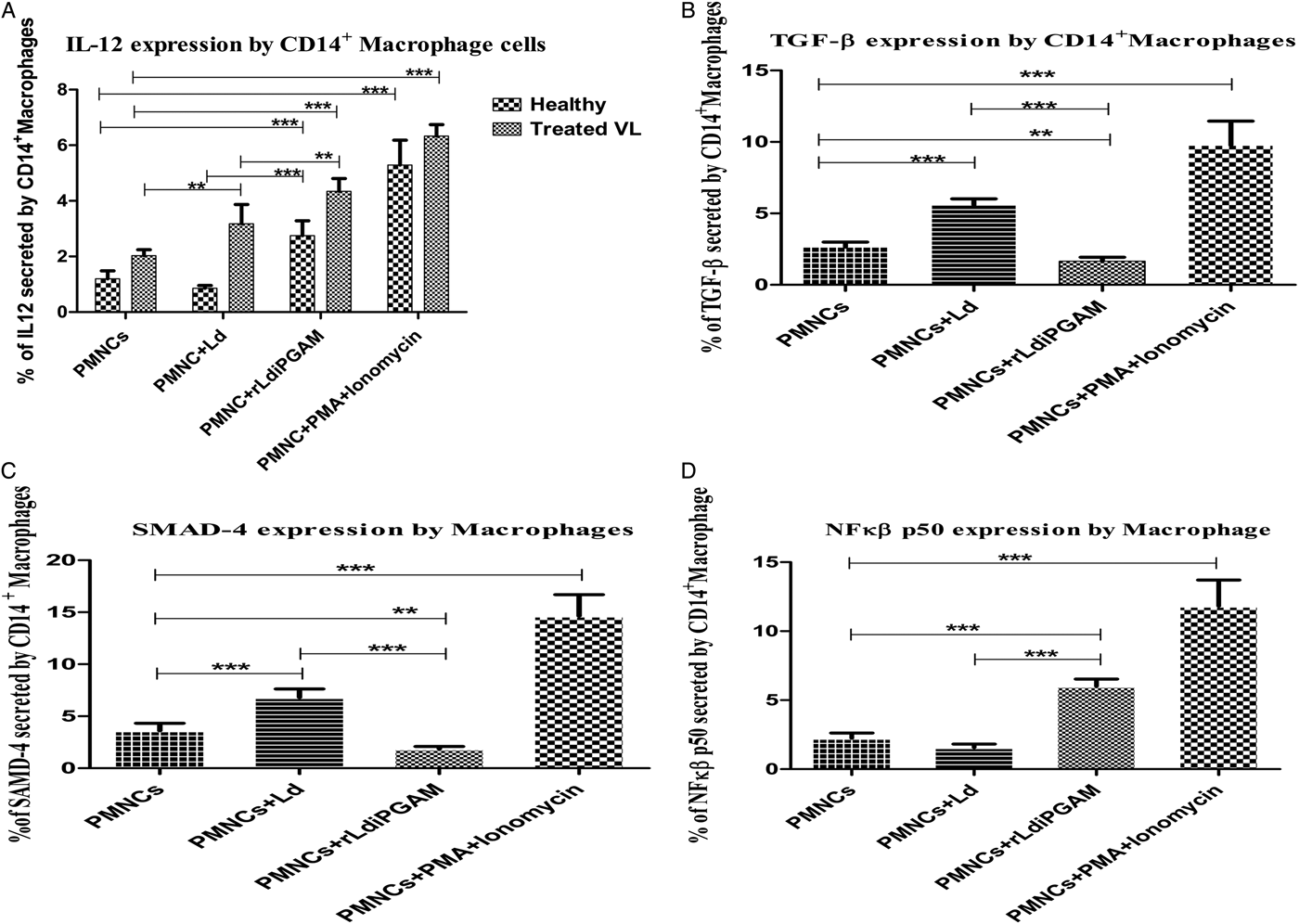
Fig. 7. The secretion of intracellular cytokines and transcription factors in macrophages, stimulated with or without Leshmania donovani (Ld) or rLd-iPGAM or PMA and ionomycin, as positive control. (A) IL-12 secreted by CD14+ macrophages. (B) TGF-β secreted by CD14+ macrophages. (C) SMAD-4 secreted by CD14+ macrophages. (D) NF-κβ p50 secreted by CD14+ macrophages. Bar represents the mean ± s.d. of four different experiments. Double asterisk (**) is significant of (P < 0·01) and triple asterisk (***) is significant of (P < 0·001).
Leishmania donovani is known to upregulate TGF-β, a negative regulator of immunity, under its survival strategy. Leishmania donovani as well as PMA and ionomycin were observed upregulating TGF-β expressing macrophages, which also resulted in higher expression of SMAD-4. But rLd-iPGAM stimulation, showing its immunogenic strength, reduced the expression of TGF-β +CD14+ macrophages (Fig. 7B) as well as SMAD-4 to half (Fig. 7C).
rLd-iPGAM-induced immune activation was expected to translate in higher expression of NF-κ β p50. There was 2·68-fold upregulation of NF-κ β p50 expression in macrophages after rLd-iPGAM stimulation (Fig. 7D). Whereas, pathogenic responses of Ld stimulation was resulted in reduced expression of NF-κ β p50. The PMA and ionomycin upregulated the NF-κ β p50.
Qualitative data of rLd-iPGAM-induced cytokines was supported by quantitative assay by ELISA
Few of the key cytokines were also evaluated quantitatively to confirm flow data. For this, sandwich ELISA was performed with culture supernatants of PMNCs stimulated with or without rLd-iPGAM. Secretion of IFN-γ was increased by 2·46-fold, IL-10 was reduced by 0·32-fold, TNF-α and IL-12 were also significantly increased by 1·27- and 2·51-fold after stimulation with rLd-iPGAM in comparison to unstimulated control (Fig. 8A–D). In response to Ld stimulation, as predicted, IL-10 was raised by 1·2-fold. Unlike IL-10, the level of IFN-γ was decreased by 0·25-fold, TNF-α concentration was reduced to half, and the level of IL-12 decreased by 0·30-fold in comparison to unstimulated healthy control sample. Stimulation with PHA, as a positive control, increased the secretion of all these cytokines. All these cytokines except IL-10 and TNF-α were also increased in treated VL samples in response to rLd-iPGAM.
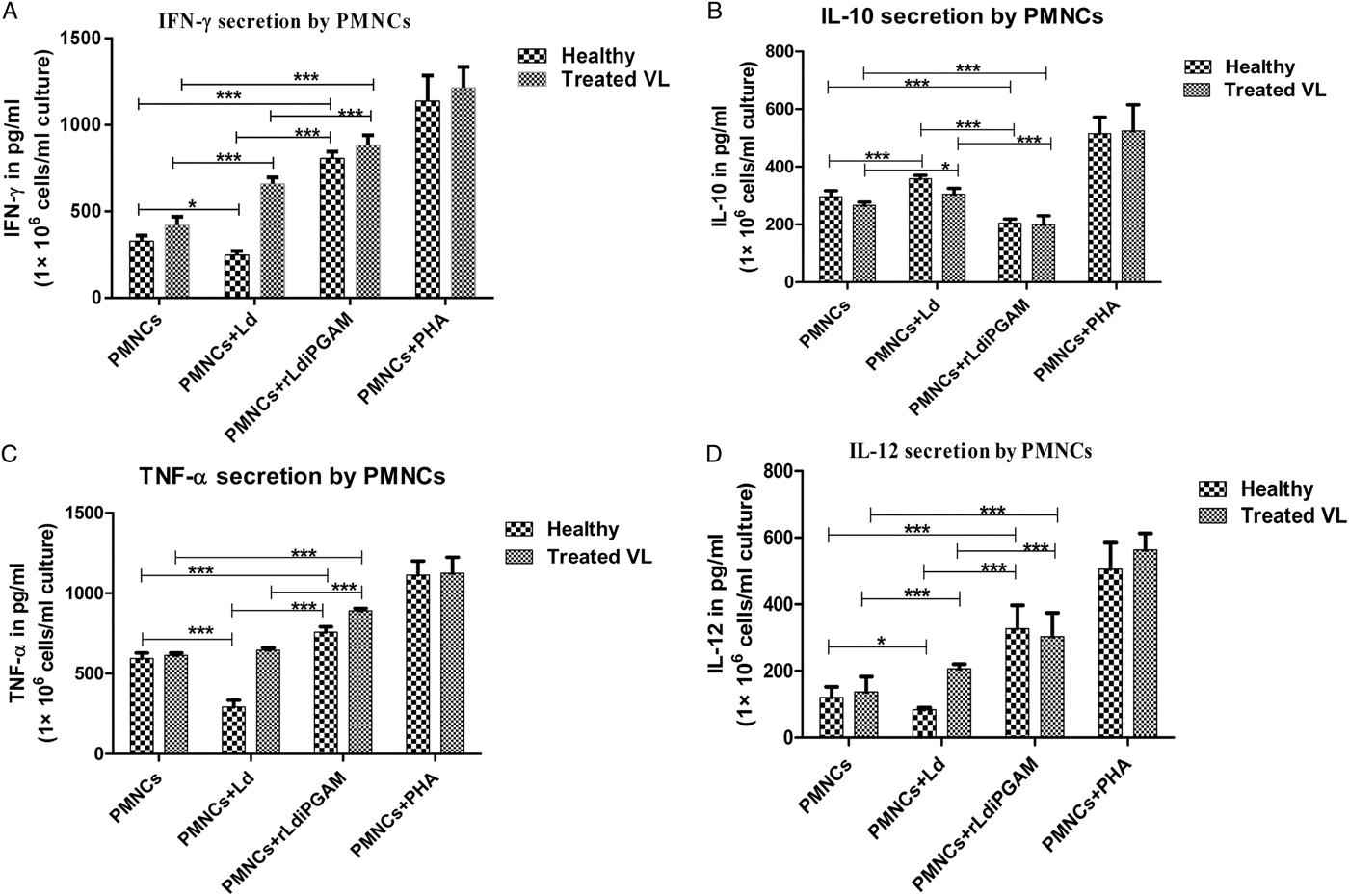
Fig. 8. The quantitative data of IFN-γ, IL-10, TNF-α and IL-12 evaluated by ELISA using culture supernatant of 1 × 106 PMNCs mL−1 incubated with or without Leshamania donovani (Ld) or rLd-iPGAM and PHA, as positive control. (A) IFN-γ in pg mL−1. (B) IL-10 in pg mL−1. (C) TNF-α in pg mL−1. (D) IL-12 in pg mL−1. Bar represents the mean ± s.d. of four different experiments. Asterisk (*) is significant of (P < 0·05), double asterisk (**) is significant of (P < 0·01) and triple asterisk (***) is significant of (P < 0·001).
rLd-iPGAM stimulation induced strong lymphoproliferative responses (LTT assay)
PMNCs cultured in the presence or absence of SLA, rLd-iPGAM and PHA were assessed by XTT. The results of the proliferative response of lymphocytes against rLd-iPGAM showed significantly higher stimulation in healthy and treated VL (mean OD 1·90 ± 0·186 and 2·386 ± 0·243) than SLA (mean OD 0·772 ± 0·105 and 1·146 ± 0·198) (Fig. 9A).
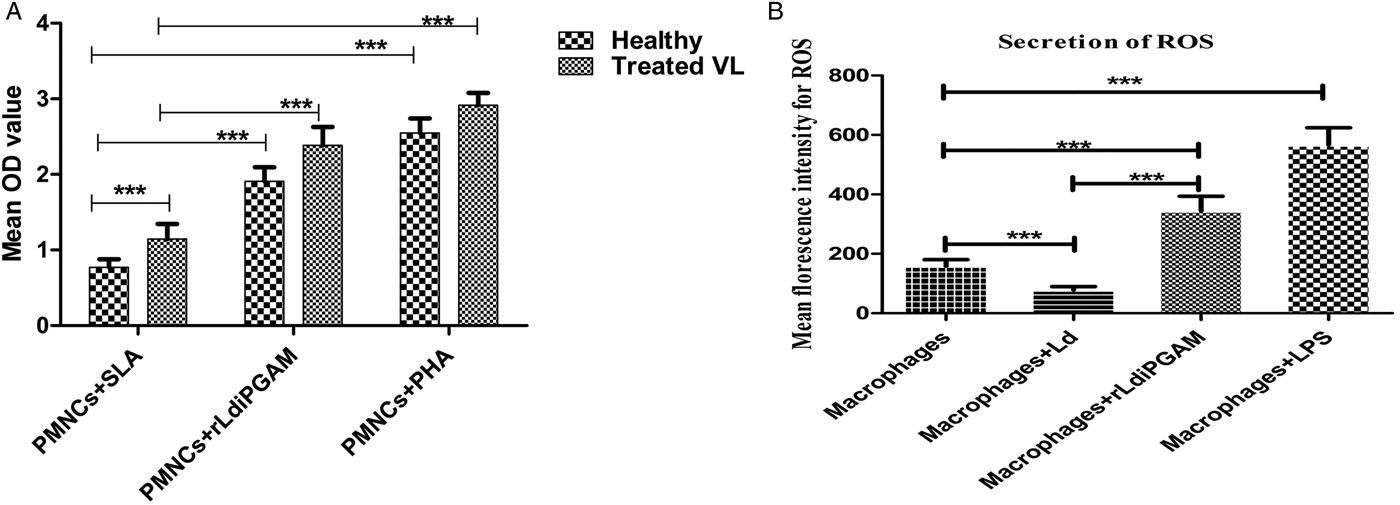
Fig. 9. Lymphocyte proliferation response shown by PMNCs and the ROS release by active macrophage. (A) LTT response of PMNCs from healthy donors against SLA, rLd-iPGAM and PHA. Proliferation was represented as the mean OD of stimulated culture−(minus) mean OD of unstimulated culture, as negative control. (B) The ROS produced by macrophages stimulated with or without Ld or rLd-iPGAM or LPS, as positive control. The data of ROS were measured via mean fluorescence intensity (MFI). Bar represents the mean ± s.d. of two different experiments. Triple asterisk (***) is significant of (P < 0·001).
rLd-iPGAM stimulation charged macrophages to enhance ROS
To ascertain whether the immunoprotective response of rLd-iPGAM translates into parasiticidal activities of macrophages, ROS activity was evaluated. Macrophages showed increased ROS production upon rLd-iPGAM stimulation in comparison to unstimulated control. The stimulated macrophages exhibited 2·1-fold higher ROS activity (MFI = 344·2) in comparison to unstimulated macrophage cells (MFI = 158·2). However, Ld challenge decreased the ROS activity (MFI = 78·2) by half and LPS stimulation increased it significantly (MFI = 566·6) (Fig. 9B).
rLd-iPGAM stimulation activated iNOS and induced NO synthesis
NO play a crucial role in the elimination of Leishmania amastigotes during the healing process. Leishmania is well known, and as also evidenced by the present result, for reducing NO synthesis; but rLd-iPGAM stimulation induced 1·8-fold higher production of NO (P < 0·05; Fig. 10A). The expression of iNOS transcripts also increased by 1·733-fold after rLd-iPGAM stimulation (Fig. 10B and C).
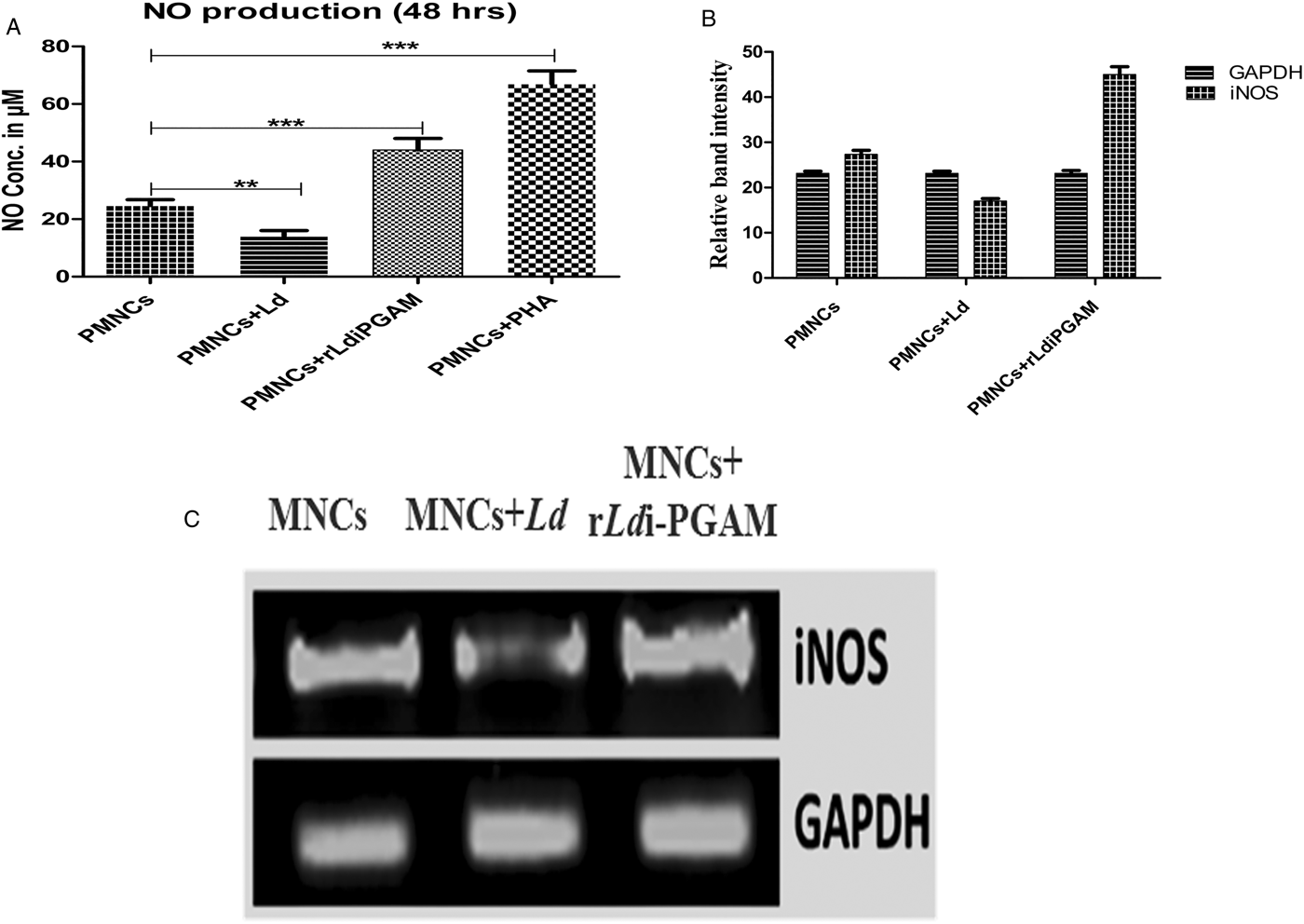
Fig. 10. Secretion of NO and activation of corresponding iNOS in PBMCs from healthy donor treated with or without Leshmania donovani (Ld) or rLd-iPGAM or PHA. (A) Amount of NO secreted. (B) Relative amplification of iNOS by real-time PCR (C) Amplification of iNOS in end product by RT-PCR in agarose gel. Bar represent the mean ± s.d. of single independent experiments. Double asterisk (**) is significant of (P < 0·01) and triple asterisk (***) is significant of (P < 0·001).
rLd-iPGAM charging directs the THP-1 cells towards host-protective responses
In support of the above experiments, immunomodulatory capacity of rLd-iPGAM was also evaluated on a monocyte cell line THP-1. NO generation increased by 2·16-fold in the THP-1 cells stimulated with rLd-iPGAM in comparison to unstimulated THP-1 cells (Fig. 11A). The NO production was 3-fold higher in response to LPS. Level of TNF-α and IL-12 was also increased by 2·28 and 2·69-fold (Fig. 11B and C). Unlike TNF-α and IL-12, secretion of IL-10 was decreased by more than half-fold as compared with unstimulated THP-1 cells (Fig. 11D). However, LPS stimulation significantly increased the level of TNF-α, IL-12 and IL-10.
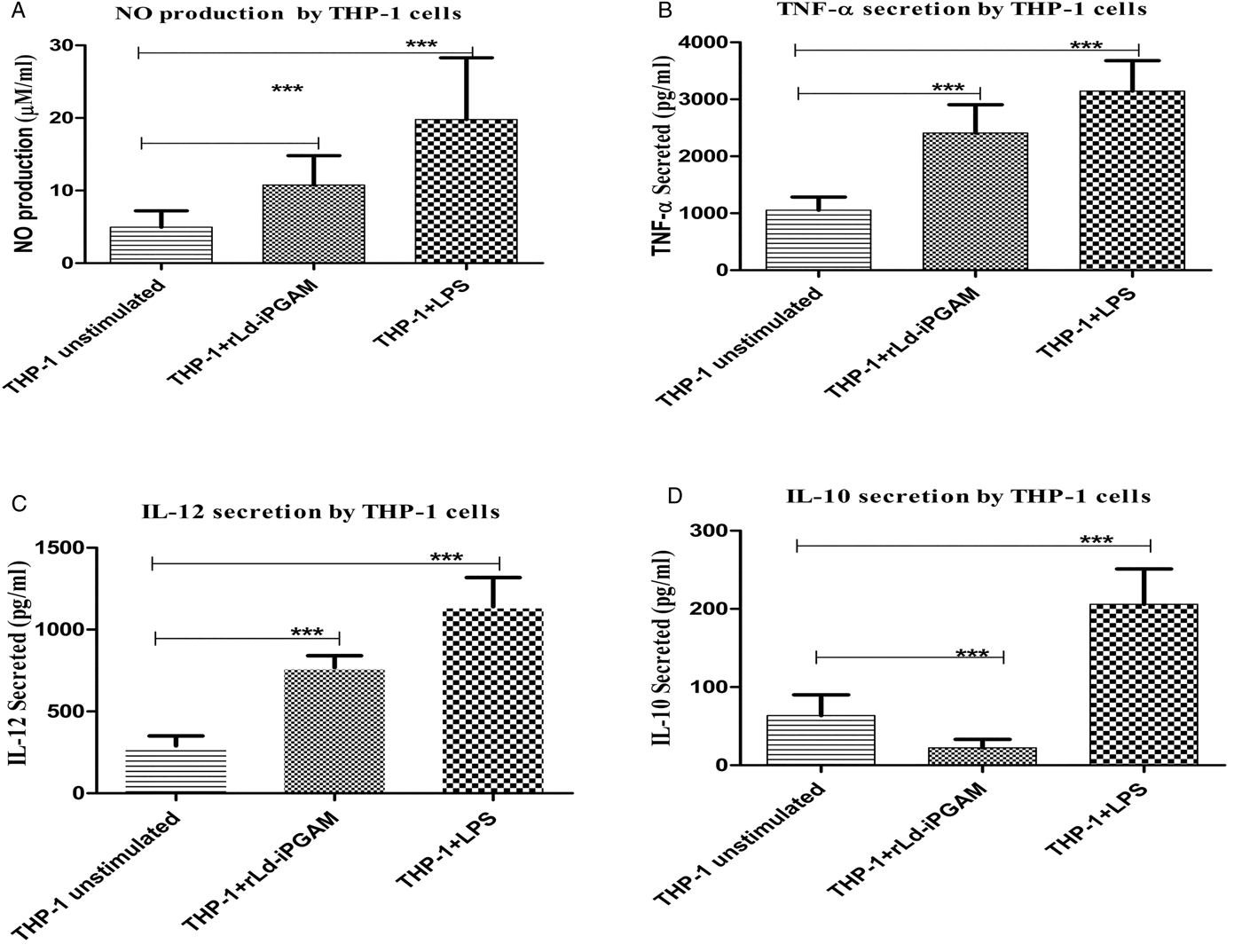
Fig. 11. The responses shown by THP-1 cell line stimulated with or without rLd-iPGAM or LPS. (A) NO production by THP-1 cell line. (B) TNF-α (in pg mL−1) secreted by THP-1 cell line. (C) IL-12 (in pg mL−1) secreted by THP-1 cell line. (D) IL-10 (in pg mL−1) secreted by THP-1 cell line. Bar represents the mean ± s.d. of four different experiments. Triple asterisk (***) is significant of (P < 0·001).
Immunoactivation potential of iPGAM was supported by in silico study
In the present study, possible MHC class I/II restricted epitopes were predicted based on Trost et al. theory by combining the predictions from different algorithms. The epitopes that are highly conserved will be tolerated in the host. BLASTP analysis revealed the peptide (SLAKLKDAV) with 100% query coverage and 88% identity against zinc finger homeobox 2, isoform CRA_d of H. sapiens and excluded from the study. The peptide ALKSGMYDV was found to be a consensus high binding affinity with MHC class I allele (online Supplementary Table S2) and shared only 67% identity with H. sapiens. Though, Syfpeithi score did not reflect the best binding affinity for the HLA DR1 restricted epitope (LGAFTKEGSTLHLIG), but IEDB and NETMHC II analysis revealed it as best possible epitope (online Supplementary Table S3).
DISCUSSION
Immunity to Ld infection depends largely upon cell-mediated immunity, particularly the upregulation of Th1 cells (Howard and Liew, Reference Howard and Liew1984; Liew, Reference Liew1991a , Reference Liew b ). Besides CD8+ cells are also considered as a good contributor in the adaptive immune response during VL (Nateghi et al. Reference Nateghi, Keshavarz, Edalat, Sarrafnejad and Shahrestani2010). Therefore, T-cell stimulating antigens are being considered as a good vaccine target for intracellular pathogens (Kamhawi et al. Reference Kamhawi, Oliveira and Valenzuela2014).
rLd-iPGAM having immunogenic potential induced earlier inducible cell surface glycoprotein CD69 on lymphocytes. Though, the aim of this experiment was to find a suitable dose (5 µg mL−1) of rLd-iPGAM for further experiments, higher expression of CD69 on CD4+ and CD8+ cells was an early assurance of lymphocyte activation, phosphorylation and proliferation.
IL-1β is the member of IL-1 family and mediates the inflammatory responses. It is involved in cell proliferation, differentiation, apoptosis and facilitates host resistance to infection (Djalma et al. Reference Djalma, Diego, Vanessa, Larissa, Alexandre, Tiago, Fredy, Maria, Karina, Richard, Marcelo and Dario2013). rLd-iPGAM induced upregulation of IL-1β, which promote T-cell activation. Its counter-receptor on lymphocyte binds with a group of 11 cytokines and helps in inflammatory responses to infections. rLd-iPGAM stimulation was able to promote interaction of IL-1β with IL-1R to help in transendothelial migration of immunocompetent cells.
It is evidenced that IL-2 treatment induces antimicrobial activity by promoting IFN-y secretion (Christopher et al. Reference Christopher, El-Safi, Wynn, Maria, Satti, Kordofani, Hashim, Ali, Neva, Nutman and Sacks1993) and helps in resistance to infection (Murray et al. Reference Murray, Miralles, Mark and McDermott1993). CD8+ also supports Th1 response of CD4+ cells being prone to IL-2 expression (Coler et al. Reference Coler, Goto, Vanitha and Steven2007; Qila et al. Reference Qila, Woodward and Suzuki2013). rLd-iPGAM stimulation reverted the Ld-induced downregulation of IL-2 due to better expression through APCs and IL-1β interaction with IL-1β receptor upon CD4+ cells.
IL-4, a well-known Th2 cytokine in the Leishmania pathogenesis (Aseffa et al. Reference Aseffa, Gumy, Launois, MacDonald, Louis and Fabienne2002) was also regulated by rLd-iPGAM in both healthy as well as in treated samples. Though some of the authors do not recognize IL-4 as a pathogenic cytokine, its higher expression serves as a signature cytokine during VL (Swain et al. Reference Swain, Weinberg, English and Huston1990).
The immunoprophylactic effect of rLd-iPGAM was strengthened by the stimulatory response in upregulation of IFN-γ +CD4+ and IFN-γ +CD8+ cells and downregulation of IL-10+CD4+ and IL-10+CD8+ cells from healthy and treated VL subjects. Higher expression of IFN-γ +CD4+ and IFN-γ +CD8+ in rLd-iPGAM stimulated cells from a treated VL donor is probably due to the presence of memory cells against rLd-iPGAM. This possibility was also strengthened by the pathogenic effect of Ld stimulation on cells of the healthy donor, which was reversed by a higher Th1/Th2 ratio in the sample of treated VL cases; but we could not examine the rLd-iPGAM-induced dynamics of memory cell in the present study design.
IL-17 helps in protecting against human VL (Pitta et al. Reference Pitta, Romano, Cabantous, Henri, Hammad, Kouriba, Argiro, El Kheir, Bucheton, Mary, El-Safi and Dessein2009). It induces host-protective signalling against Ld (Nascimento et al. Reference Nascimento, Carregaro, Junior, Costa, Ryffel, Duthie, Jesus, Almedia and Silva2011; Huber et al. Reference Huber, Heink, Pagenstecher, Reinhard, Ritter, Visekruna, Guralnik, Bollig, Jeltsch, Heinemann, Wittmann, Buch, Prazeres da Costa., Brüstle, Brenner, Mak, Mittrücker, Tackenberg, Kamradt and Lohoff2013). Similar to IFN-γ, IL-17+CD4+ cells (Th-17) and IL-17+CD8+ cells (Tc-17) expression was raised upon stimulation with rLd-iPGAM in cells of treated VL. Cells of treated VL subject recognized Ld and responded well to express IL-17, which was also observed in the case of rLd-iPGAM stimulation. rLd-iPGAM induced higher expression of Th17 in the healthy sample as well, being more immunogenic in comparison to Ld parasite as a whole.
IL-12 interacts with T cells and helps the initiation and maintenance of Th1 responses (Ghalib et al. Reference Ghalib, Whittle, Kubin, Hashim, el-Hassan, Grabstein, Trinchieri and Reed1995; Murray et al. Reference Murray, Christine, Jianguo and Xiaojing2006). Thus, it mediates essential signalling between macrophages and T cells for the production of IFN-γ. Leishmania donovani downmodulate the IL-12 expression, which was reverted by rLd-iPGAM. Macrophages from treated VL responded both Ld and rLd-iPGAM producing IL-12. However, the response against rLd-iPGAM was better. Thus, rLd-iPGAM not only activated the T cells, but also modulated the macrophages for protective secondary signalling. IL-12 binding with IL-12 receptor induces expression of IFN-γ and IL-17. Leishmania donovani stimulation, as predicted, enhanced the secretion of TGF-β due to the presence of parasite-derived factor cathepsin-B. TGF-β enhances parasite survival in macrophages (Kira et al. Reference Kira, Schultz-Cherry, Rodriguez, Jeronimo, Nascimento, Goldman, Recker, Miller and Wilson2003) by downmodulating NO production (Rebecca et al. Reference Rebecca, Faleiro, Louise and Christian2014). rLd-iPGAM downmodulated TGF-β expression most probably due to lack of cathepsin-B or due to host immune pressure. This rLd-iPGAM-induced downregulation of TGF-β was resulted in decreased accumulation of SMAD-4 in the nucleus (Roelen et al. Reference Roelen, Cohen, Raychowdhury, Chadee, Zhang and Kyriakis2003). It is known that downregulated SMAD-4 fails to promote IL-1 receptor-associated kinase-M, which ultimately leads a concomitant upregulation of downstream NF-κβ p50 (Srivastav et al. Reference Srivastav, Saha, Barua, Ukil and Das2015).
The qualitative assay of cytokines evaluated by flow cytometry was supported by quantitative evaluation of few cytokines, like IFN-γ, IL-10, TNF-α and IL-12. IFN-γ is known to act in synergy with another macrophage-derived cytokine TNF-α. Therefore, along with IFN-γ, higher expression of TNF-α activates macrophages to express iNOS (Anselmo et al. Reference Anselmo, Giudice, Pereira, Guimaraes, De Jesus, Tatiana, Mary, Edgar and Roque2009). Therefore, upregulation of IFN-γ and TNF-α upon rLd-iPGAM stimulation increased the iNOS expression, which in turn enhanced the production of NO and promoting the clearance of Ld. Pro-inflammatory cytokines TNF-α and IL-12 play a critical role in the induction of NO and ROS. Thus, upregulation of TNF-α and IL-12 upon rLd-iPGAM stimulation boosting the leishmaniacidal activity of macrophages. Unlike IFN-γ, IL-10 was reduced in samples from healthy and treated VL subjects upon rLd-iPGAM stimulation. This extracellular cytokine data collected through ELISA finally confirmed the host-protective immune response established by recombinant protein.
Development of strong cell-mediated immunological response is the key of protective signalling against VL. The significant lymphoproliferative response shown by PMNCs against rLd-iPGAM stimulation ensures that recombinant protein having the potential to activate and develop strong T-cell-mediated immunity against pathogenesis.
ROS production through an oxidative burst is responsible for the leishmaniacidal activity (Murray, Reference Murray1981; Gannt et al. Reference Gannt, Goldman, McCormick, Miller and Jeronimo2001; Bisti et al. Reference Bisti, Konidou, Boelaert, Lebastard and Soteriadou2006), but it has been reported that Ld inhibits the production of O2 − inside the host, so the level of O2 − and H2O2 is significantly lower in monocytes from VL patient as compared with healthy subjects (Kumar et al. Reference Kumar, Pai and Sundar2001, Reference Kumar, Pai, Pandey and Sundar2002). Leishmania donovani survive inside the host by using the antioxidant system or by suppressing macrophages ROS production (Murray, Reference Murray1981). We observed that charging of rLd-iPGAM significantly increased the ROS generation by activating macrophages, which ensures that recombinant protein is able to defend the host from parasitic infection.
Binding of IFN-γ and TNF-α with macrophages activates iNOS, which in turn produces NO by breaking L-arginine to L-citrulline. NO plays a significant role in controlling parasite and overcome VL. It has direct parasiticidal activity in addition to its role in signalling to evoke innate macrophage responses (Pandya et al. Reference Pandya, Verma, Khare, Tiwari, Srinivasarao, Dube, Goyal and Mishra2016). This result was also supported by the confirmation of upregulation of iNOS in the presence of rLd-iPGAM.
Th1 stimulatory nature of rLd-iPGAM was validated and supported by directing the THP-1 cells through increasing the level of NO, TNF-α, IL-12 and simultaneously decreasing IL-10.
The efficacy of rLd-iPGAM was supported by in silico study, which suggested that this antigen has highly conserve MHC class I and II restricted epitopes that could trigger the desired immune response in the host. As previously reported of the 10% top scored peptides predicted by SYFPEITHI and BIMAS, 85% peptides have the ability to trigger a desired immune response (Rammensee et al. Reference Rammensee, Bachmann, Emmerich, Bachor and Stevanovic1999; Parker et al. Reference Parker, Bednarek and Coligan1994). Beside, highly conserved epitopes will be tolerated in the host. Therefore, this study recommends rLd-iPGAM as a potential immunomodulating agent, which can be used in future vaccine module.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182017001494.
ACKNOWLEDGEMENTS
Technical assistance rendered by Mr Sanjay Kumar Chaturvedi, T. A. and Mr B. N. Roy, Technician of the Microbiology Division, RMRIMS, Patna, India are acknowledged for their continuous support.
FINANCIAL SUPPORT
Financial support for chemical was provided by RMRIMS, ICMR, Patna. Student fellowship was supported by Department of Biotechnology, Govt. of India (grant number DBT/JRF/13/141/2087). Article communication number – RMRI 40/6/10/2016.














