INTRODUCTION
Coastal waters receive metals originating from industrial waste runoff, atmospheric deposition and shipyard activity. Bivalve molluscs can accumulate high concentrations of metals by their intense filtration activity for respiratory and nutritional purposes (Cheggour et al. 2001; Tran et al. 2001; Griscom et al. 2002). Resulting damage, as oxidative stress leading to lipid peroxidation, is modulated by antioxidant systems and metallothioneins (MT) (Géret et al. 2002; Ciocan and Rotchell, 2004; Funes et al. 2006). MT have been identified in approximately 50 aquatic invertebrate species, the majority of which are molluscs and crustaceans (Cotou et al. 2001; Tanguy et al. 2003; Liang et al. 2004). They are low-molecular weight, cysteine-rich proteins acting as metal-chelating agents for the excess of metals in the cells. MT are induced following metal exposure by a large number of metals, notably by cadmium (Cd) (Bordin et al. 1997; Baudrimont et al. 1997b, 1999), but are also influenced by a wide range of other factors like hormones, second messengers, physical stress, etc (Kägi, 1991; Baudrimont et al. 1997a; Cotou et al. 2001; Leiniö and Lehtonen, 2005; Amiard et al. 2006). They are then considered as stress proteins. Metals, however, often reduce animals' performance of crucial activity and increase bivalve mortality rates (Sokolowski et al. 1999; Barlow and Kingston, 2001; Neuberger-Cywiak et al. 2003).
Coastal ecosystems usually exhibit a rich and abundant fauna, including parasites (Kesting et al. 1996; Thomas et al. 1997; de Montaudouin et al. 2000; Mouritsen and Poulin, 2002; Lafferty et al. 2004). Bivalves are particularly infected by digenean trematodes (de Montaudouin et al. 2000), using their filtration activity as a major infection route (Wegeberg et al. 1999). These parasites use bivalves, such as the cockle Cerastoderma edule, as first or second intermediate host. In the first intermediate host, the parasite reproduces asexually in host tissues, particularly the gonad and digestive gland. The parasite eventually sheds larvae (cercariae) in the water that will infect another host species. This type of infection has a particularly detrimental effect on its host (Deltreil and His, 1970; Jonsson and André, 1992; Thieltges, 2006). As second intermediate host, the individual harbours cercariae in their latent form, i.e. the metacercariae. The stress inflicted by the metacercariae is less intense than in the former situation but cannot be neglected (Holmes and Bethel, 1972; Dobson, 1988; Poulin, 1994; Desclaux et al. 2002, 2006).
MT concentration alteration by parasites in cockles has been previously observed (Baudrimont et al. 2006). These results showed that parasitic infection enhances MT concentrations during the rest period. On the contrary, MT levels are reduced during the gametogenesis phase compared with healthy cockles, probably because of host castration by the parasite. Therefore, we can speculate whether, in the case of metal contamination, the bivalves are able to produce sufficient amounts of MT in order to counteract metal toxicity. Moreover, digenean infection in adult cockles can reach 100% in the second intermediate host and 50% in the first intermediate host (de Montaudouin et al. 2000; Desclaux et al. 2002). The objective of this study was thus to assess, through experimental contamination, the effect of parasite in the bioaccumulation of cadmium (Cd) by cockles, and to measure their MT concentrations. Experiments were performed in both infection patterns: cockle as first intermediate host with Labratrema minimus and as second intermediate host with Himasthla elongata. Results of both a previous paper (Baudrimont et al. 2006) and of the present work should elucidate some interactions occurring between parasites, MT synthesis and metal bioaccumulation in a contaminated environment.
MATERIALS AND METHODS
Biological models
The host was the edible cockle Cerastoderma edule, a common suspension-feeding bivalve living in sandy, shallow sediments along the European Atlantic coast (Tebble, 1966). The cockle is a potential host for several digeneans using this bivalve either as a first intermediate or as a second intermediate host.
Among parasites utilizing the cockle as first intermediate host, Labratrema minimus was chosen. It is a bucephalid, using goby Pomatoschistus minimus as the second intermediate host and sea bass Dicentrarchus labrax as final host. In the first intermediate host, the cockle, L. minimus reproduces asexually in a sporocyst form that invades the whole organism and particularly the digestive gland, the gonads and the gills (Maillard, 1976). Infection occurs in adults, generally with low prevalence (de Montaudouin et al. 2000; Desclaux et al. 2002).
Parasites using the cockle as second intermediate host are much more prevalent (Lauckner, 1983; de Montaudouin et al. 2000). Here the aim was to experimentally infect cockles. Consequently, the parasite model had to be rare in the field to distinguish control and parasitized individuals. In our area (Arcachon Bay, Atlantic coast of France), Himasthla elongata is rare (de Montaudouin et al. 2000) although it is a common species in northern countries (Lauckner, 1984; Lebour, 1911). This echinostomatid parasitizes the periwinkle Littorina littorea (gastropod) as first intermediate host and a gull as final host. In the second intermediate host, the cockle, this parasite mostly infects the foot.
The effect of infection on metal exposure was experimentally assessed in both situations (first and second intermediate host).
Cadmium exposure of cockles utilized as first intermediate host
Cockles were collected in February 2002 at Ile aux Oiseaux (Arcachon Bay) where Labratrema minimus prevalence is relatively high (≅20%). Identification of infected and non-infected cockles by L. minimus was performed by cercariae emission. Each cockle was isolated in a dish with filtered seawater at about 20 °C for 16 h. Each dish was verified under the stereomicroscope to detect L. minimus cercariae before experimentation. The cockles were fed with a concentrated diatom culture, Skeletonema spp., during a 1-week acclimatization period and during exposure periods in experimental units (E.U.). Each E.U. consisted of a glass aquarium (12×12 cm in surface, 30 cm in height) lined with an alimentary plastic bag and filled with synthetic sea water (Instant Ocean, salinity=30). The water was aerated by a diffuser and the temperature was fixed at 15 °C (±1 °C). pH was not controlled but remained stable (8·36, standard deviation=0·05). Pure sand (98% silica – grain size: 800–1400 μm) was introduced to obtain a 3-cm deep layer. Five 25-mm long cockles per E.U. were put in. The experiment followed a two-way ANOVA design, with cockle condition (four levels: (1) no parasite infection, no cadmium exposure, (2) no infection, with Cd exposure, (3) with infection, no Cd exposure, and (4) with infection and Cd exposure) and time (two levels: t0 and t7d, from exposure) as fixed factors. Each treatment was triplicated. Therefore, a total of 4×2×3=24 E.U. were utilized. After 7 days exposure, progressive cockle mortality occurred.
A single Cd level of contamination was selected, i.e. 15 μgCd L−1. This value is ca. 300 times higher than that measured in the unpolluted site of Arguin (Arcachon Bay) (Baudrimont et al. 2006) but of the same magnitude as in polluted areas (Andrès et al. 1999). After the first contamination (t0) and in order to maintain constant contamination throughout the exposure period, a daily addition of aqueous Cd solution was performed, adjusted according to the decrease in Cd concentration in each E.U. after quantification by Atomic Absorption Spectrophotometry (AAS) using a graphite tube atomizer (Varian AA 400). The measured concentration (mean±S.D.) was 15·7±2·9 μgCd L−1. When a cockle died, it was replaced by a new marked individual which was not considered for analysis at the end of the experiment but facilitated the maintenance of a constant biomass in aquaria.
At the end of each treatment, MT and cadmium concentrations in cockles were measured in the whole soft body without the foot, after freezing at −80 °C under nitrogen in polyethylene bags in order to minimize metallothionein oxidation. The a posteriori verification of the presence/absence of L. mimimus, the natural abundance of Himasthla quissetensis and the reproductive stage of cockles (Gimazane and Lubet, 1972; Baudrimont et al. 2006) were assessed in the foot. In addition, metal concentrations (Cd, Pb–Zn, Cu–Hg) in 3 cockles from the origin site were measured by Atomic Absorption Spectrophotometry (AAS).
A side-experiment was concomitantly performed with 3 parasitized cockles maintained for 1 week in a Cd-contaminated EU (15 μgCd L−1). At completion, cockles were opened and L. minimus sporocysts carefully removed under a stereomicroscope. Parasites and cockle flesh were weighed and Cd concentration measured in parasites as previously described.
Cadmium exposure of cockles utilized as second intermediate host
In this experiment, cockles were experimentally infected by Himasthla elongata. H. elongata cercariae were collected from infected Littorina littorea. Infected snails were kept at 20 °C, and fed with Ulva lactuca (green algae). To induce cercariae emission, each periwinkle was isolated in a dish with seawater at 15 °C and a cockle, initially free of H. elongata, was introduced. Cockles came from Banc d'Arguin, Arcachon Bay (June 2001), where the parasite H. elongata is absent from the cockle population (Desclaux et al. 2002). The same protocol was followed with healthy cockles (without H. elongata), except that cockles were introduced with periwinkles free of parasites. After 6 h in contact (or not) with cercariae, cockles were transferred in running seawater for 24 h, in order to insure correct encystment on newly parasitized cockles. Then, the Cd exposure treatment (t0) started. The methodology was exactly the same as in the previous experiment except that the Cd exposure lasted 14 days and that the exposure concentration was only 12·3 μgCd L−1, instead of 15 μgCd L−1 during the whole experiment, due to initial dosage difficulties. ANOVAs were performed at 7 days and 14 days.
Metals and metallothionein analyses
Tissues (except the foot) of 2 pooled cockles per replicate were dried in absorbing paper, weighed and digested in high purity nitric acid at 95 °C during 3 h before metal analysis. Samples were diluted in ultra-pure water and analysed by Atomic Absorption Spectrophotometry (AAS) using air-acetylene flame (Varian AA 220 FS) for Zn and Cu, by AAS using a pyrolitic graphite furnace (Varian AA 400) for Cd and Pb, and by cold vapour atomic spectophotometry (Varian CETAC M-6000 Mercury Analyser) for Hg. Detection limits were 5, 20, 0·1, 2 and 0·01 μg L−1 for Zn, Cu, Cd, Pb and Hg respectively. As a check on the accuracy of the methods, certified biological reference samples were systematically analysed (Tort-2: Lobster hepatopancreas; Dolt-2: Dogfisher liver; Mess-3: Marine sediment, Reference Material for Trace Metals, NRC-CNRC, Ottawa, Canada).
MT concentrations were measured in the tissues without the foot in 3 other pools of 2 cockles by cold inorganic mercury (HgII) saturation assay coupled with cold vapour atomic spectophotometry as described by Baudrimont et al. (1999). This technique is based on the quantification of Hg bound to the saturated-MTs. The denaturation of non-MT proteins was performed with trichloroacetic acid and the scavenging of excess Hg not bound to the MTs removed with lyophilized pig haemoglobin (Sigma) at 0·2 g Hb ml−1 in 30 mM Tris-HCl buffer (pH 8·2 at 20 °C). The final supernatant was then quantitatively recovered and used for Hg determination by cold vapour atomic absorption spectrometry (Varian Cetac M-6000 Mercury Analyser). The detection limit was estimated at 1 ng Hg. MT concentrations in tissues were expressed in nmol of Hg-binding sites per gram (fresh weight). Owing to the fact that the exact quantity of Hg binding sites per MT molecule is unknown for this species, MT concentrations cannot be expressed in nmol MT g−1 (fresh weight), but in nmol sites Hg g−1 (fw).
RESULTS
Cadmium exposure of cockles utilized as first intermediate host
At the beginning of the experiment, cockles had similar shell length (25·2 mm±ES=0·4 mm), flesh fresh weight without foot (0·38±0·02 g fw) and Himasthla quissetensis abundance (12·5±2·2 cysts cockle−1). Mean metal concentrations in cockles (without foot) were 27, 807, 33, 11842 and 1220 ng g−1 (fw) for Cd, Pb, Hg, Zn and Cu respectively. Only the reproductive stage differed: stage b (intermediate gonadic development) for L. minimus-infected cockles and stage c (complete maturity) for cockles without this parasite.
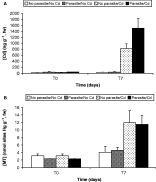
After 7 days exposure, Cd concentration in cockles was significantly different in relation to time (F-ratio=160·44; P<0·001), L. minimus presence (F-ratio=10·76; P<0·01) and Cd exposure (F-ratio=160·84; P<0·001) (Fig. 1A, Table 1). Cd concentrations remained logically low in treatments without Cd exposure. However, cockles with L. minimus infection were naturally more contaminated (42 ng g−1) than cockles without (27 ng g−1). When exposed for 7 days in Cd contaminated water (15 μgCd L−1), Cd concentration in cockle tissues increased by 30 in uninfected cockles and by 36 in L. minimus infected individuals, leading to a 2-fold higher bioaccumulation of this metal in parasitized individuals.

Fig. 1. Evolution of cadmium (A) and metallothionein (B) concentrations (±standard error) after 7 days of cadmium exposure (15 μgCd L−1) in cockles infected or not infected by Labratrema minimus.
Table 1. Results of three-way ANOVAs comparing the effect of time, Labratrema minimus presence and cadmium exposure (fixed factors) on cadmium and metallothionein concentrations in cockles after 7 days in Cd-contaminated water (P-values significant at P<0·05.)

At the end of the experiment, MT concentration in cockles was significantly different in relation to time (F-ratio=29·27; P<0·001) and Cd exposure (F-ratio=9·07; P<0·05), but was not modified by L. minimus presence (F-ratio=0·19; P>0·05) (Fig. 1B, Table 1). MT concentrations in cockles were very low at the beginning of Cd exposure (t0), i.e. 2·7 nmol sites Hg g−1, fw. At completion (t7), mean MT concentrations of unexposed cockles had slightly increased (4·3 nmol sites Hg g−1, fw) when it was multiplied by 4·3 (11·7 nmol sites Hg g−1, fw) in the presence of Cd, in parasitized or healthy cockles.
Parasite mass represented 18 to 34% of the total parasite-host system. The Cd concentration in the parasite after 1 week contamination was 1450±121 ng g−1 (fw), i.e. similar to host concentration (1506±317 ng g−1).
Cadmium exposure of cockles utilized as second intermediate host
At the beginning of the experiment, cockles had similar shell length (27·9 mm±ES=0·2 mm), flesh fresh weight without foot (0·8±0·03 g fw) and Himasthla quissetensis abundance (1·5±0·2 cysts cockle−1). Mean metal concentrations in cockles (without foot) were 41, 236, 15, 11510 and 1223 ng g−1 (fw) for Cd, Pb, Hg, Zn and Cu respectively. Reproductive stage in Himasthla elongata infected cockles was similar to that in cockles without this parasite (stage c).
The success of experimental infection was moderate with a mean of 13·4 metacercariae of H. elongata recovered in cockle feet. The distribution of infection was unequal, ranging from 3 to 36 cysts per cockle (S.D.=7·2 cysts), but there was no significant difference (F-ratio=1·39; P>0·05) in infection success in relation with time and metal exposure.
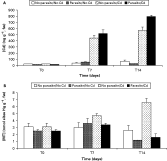
After 7 days exposure, Cd concentration in cockles significantly increased under contaminated conditions, from 29 to 482 ng g−1 (×16·6), with no effect of parasite infection (F-ratio=5·07; P=0·054) (Fig. 2A, Table 2). Simultaneously, MT concentration in cockles increased moderately, from 2·8 to 3·7 nmol sites Hg g−1 fw, with no effect of cadmium exposure (F-ratio=2·12; P>0·05) or parasite infection (F-ratio=0·42; P=0·054), although MT concentration was the highest, 4·7 nmol sites Hg g−1 fw, in the ‘no parasite/with cadmium’ treatment (Fig. 2B, Table 2).

Fig. 2. Evolution of cadmium (A) and metallothionein (B) concentrations (±standard error) after 7 and 14 days of cadmium exposure (12·3 μgCd L−1) in cockles infected or not infected by Himasthla elongata.
Table 2. Results of two-way ANOVAs comparing the effect of Himasthla elongata infection and cadmium exposure (fixed factors) on cadmium and metallothionein concentrations in cockles after 7 and 14 days in Cd contaminated water (P-values significant at P<0·05.)

After 14 days exposure, cadmium concentration in cockles had increased again under contaminated conditions, with a significantly higher value in parasitized cockles (795 ng g−1) than in uninfected individuals (569 ng g−1) (F-ratio=5·07; P<0·05) (Fig. 2A, Table 2). Simultaneously, MT concentrations in cockles had globally decreased (1·8 nmol sites Hg g−1, fw), except in unparasitized cockles exposed to Cd, the MT concentration of which significantly increased (6·6 nmol sites Hg g−1, fw; multiplied by 2·4 compared with t0) (Fig. 2B, Table 2).
DISCUSSION
Although both experiments involved the interaction of parasites and metal exposure on MT concentrations, fundamental differences exist. First, the interaction between the parasite and the host was dramatically different: as first intermediate host (first experiment), the cockle suffered much more than as second intermediate host (second experiment) (Desclaux et al. 2002). This difference will be discussed later for interpretation of the results. Secondly, the age of the infection process was not the same. In the first experiment, cockles were already infected by Labratrema minimus, from an unknown date. In the second experiment, the infection was controlled and the eventual perturbation in cockles can be due to both recent cercariae penetration through tissues, and metacercariae presence in tissues. Finally, the sampling period and site, and consequently the cockles' size and their ‘life-history’ were not similar in both experiments.
Nevertheless, after 7 days, evolution of cadmium and MT concentrations was similar. There was an important bioaccumulation in cockles from the contaminated E.U. with higher concentrations in parasitized cockles. Parasites themselves could be involved in this accumulation. Indeed, most studies report metal uptake by endoparasites above environmental concentrations and to several thousand-fold higher levels than their hosts (Sures, 2004). These ratios, however, are based on host muscle metal concentrations which is not considered a target organ for Cd accumulation (Baudrimont et al. 1997b; Durrieu et al. 2005). Moreover, to our knowledge, very few studies have concerned digeneans (Sures et al. 1998). In the present study, high Cd concentrations were not due to the parasite itself. Indeed, for L. minimus, the ratio [Cd]parasite / [Cd]host was 0·96. For H. elongata, the volume of the maximum parasite load (36×190-μm diameter metacercariae) was negligible compared with 28-mm shell length cockles and was too small to provide enough material for Cd detection. Consequently, whatever the Cd concentration would be in the parasite, this will be insignificant compared with the total Cd bioaccumulation. Thus, high Cd concentrations measured in parasitized cockles can be explained by cell destruction, which facilitates the diffusion of cadmium in the tissues without utilizing membrane transporters. In contrast, membrane transporters may be saturated in healthy cockles. Moreover, parasitized cockles often remain at the sediment surface with gaping valves (Desclaux et al. 2002), facilitating a direct contamination through the mantle and the foot (Tran et al. 2001). Additionally, a preliminary experiment conducted with neutral red introduced in the water of E.U., as a marker of direct exposure, showed that the tissues of parasitized cockles were much more dyed than those of healthy bivalves, especially in the gills and mantle (data not shown). The difference of bioaccumulation between infected and non-infected cockles was even more obvious in cockles suffering from more severe infection, i.e. cockles infected by L. minimus (first experiment). In this case, cell lyses were more important. Nevertheless, absolute concentration values of cadmium in contaminated cockles were lower in the second experiment. This difference can be due to (1) a lower exposure concentration compared with the first experiment (see Materials and Methods section); (2) fresh weight of the whole soft body was twice as high for cockles in the second experiment, which has a diluting effect on cadmium concentration (Leung and Furness, 2001; Mourgaud et al. 2002); (3) a less deleterious effect of parasite infection by H. elongata (second experiment). If the difference of cadmium contamination between parasitized and unparasitized cockles was not apparent after 7 days (P=0·054), the difference became significant after 14 days (P<0·05). This result confirms that metacercariae may be deleterious (Holmes and Bethel, 1972; Lauckner, 1987; Fried et al. 1995) even though they often appear energetically inert and seem to evoke no immediate physiological response in the host (Coleman and Travis, 1998).
At the beginning of the experiment, MT concentration in cockles was very low in both experiments. After 7 days, MT concentration slightly increased in the first experiment, without parasite effect. In the second experiment, MT concentration remained constant the first week. But after 14 days, MT concentration decreased in H. elongata-infected cockles (contaminated or not). In contrast, MT concentration increased in contaminated uninfected cockles. Long delays (>20 days) between metal exposure and significant MT induction have already been observed (Géret et al. 2002), although a very short response (<4 days) can also occur (Chan et al. 2002). The decrease of MT concentration after 1 week suggests that MT turnover is short. Leung and Furness (1999) showed that the half-life of MT in mussels (25 days) was much shorter than that of residual cadmium (300 days). In all cases, the higher cadmium concentration observed in parasitized cockles compared with uninfected individuals was never compensated by an MT induction, MT concentration remaining stable or decreasing.
Metallothionein concentrations in aquatic organisms are commonly used as metal pollution biomarkers to assess water quality (Amiard et al. 2006). However, the use of the MT as biomarkers comes up against the problem of the complexity of mechanisms regulating their biosynthesis which is influenced by a wide range of other factors such as hormones, second messengers, cytotoxic agents and physical stress. A previous field monitoring conducted in a pristine environment (Baudrimont et al. 2006), demonstrated the complex interactions between parasite infection and host reproductive stage on MT concentration. In the present experimental study, the combined effect of contamination and infection on Cd bioaccumulation and MT concentration was also highlighted. Indeed, in the case of metal contamination, MT concentration was similar or lower in parasitized than in unparasitized cockles. Therefore, an alteration of the protective effect of metallothioneins in parasitized cockles after metal contamination was clearly evidenced, probably due to a depressed physiological response of the host, which could enhance its vulnerability. Consequently, these results highlight the limit of the use of MT as a biomarker of metal pollution in field monitoring if parasitism is not taken into account, which could lead to false-negative results.
We thank A. Palvadeau for her important work, C. Desclaux, V. Marie and V. Roques-Duflo for their assistance in the laboratory, and P. Lebleu and P. Marraco for their valuable help on the field. This study was partly financed by Programme National Environnement Côtier (PNEC).