Introduction
Non-fragmented old-growth red pine and white pine forests are rare ecosystems in Ontario, Canada (Perera & Baldwin Reference Perera and Baldwin1993; Gillmore & Palik Reference Gillmore and Palik2006). These forests have unique environmental and structural characteristics compared to younger forests, including high volumes of coarse woody debris (CWD), high biomass, and high herbaceous and cryptogam richness (Carleton & Arnup Reference Carleton and Arnup1993; Carleton Reference Carleton2003). Many lichen species are unique to old-growth forests (Lesica et al. Reference Lesica, McCune, Cooper and Hong1991; McMullin et al. Reference McMullin, Duinker, Cameron, Richardson and Brodo2008). The functions of lichens in forest ecosystems include decomposition and nitrogen fixation (Pike Reference Pike1978; Knops et al. Reference Knops, Nash, Boucher and Schlesinger1991; Nash Reference Nash2008), and providing food and nesting materials for mammals (Hayward & Rosentreter Reference Hayward and Rosentreter1994; Terry et al. Reference Terry, McLellan and Watts2000). The high levels of CWD within old-growth forests (Harmon et al. Reference Harmon, Franklin, Swanson, Sollins, Gregory, Lattin, Anderson, Cline, Aumen and Sedell1986; Spies et al. Reference Spies, Franklin and Thomas1988; Siitonen et al. Reference Siitonen, Martikainen, Punttila and Rauh2000) represent important habitat for lichen communities (Harmon et al. Reference Harmon, Franklin, Swanson, Sollins, Gregory, Lattin, Anderson, Cline, Aumen and Sedell1986; Samuelsson et al. Reference Samuelsson, Gustafsson and Ingelög1994; Kruys et al. Reference Kruys, Fries, Jonsson, Lämås and Ståhl1999).
Due to temporal and spatial variability in individual tree mortality, old-growth forests generally have a relatively even distribution of CWD decay stages, and diameter sizes (Jonsson Reference Jonsson2000). However, characteristics of CWD, such as abundance, physical structure (Spies et al. Reference Spies, Franklin and Thomas1988; Sippola et al. Reference Sippola, Siitonen and Kallilo1998) and bark structure (McAlister Reference McAlister1997; Siitonen Reference Siitonen2001), differ between forest types, particularly those dominated by different tree species. Different types of CWD (e.g., log, stump, or snag), along with a variation in diameter sizes and decay stages create a variety of habitats, especially for lichen communities (Söderström Reference Söderström1988; Humphrey et al. Reference Humphrey, Davey, Peace, Ferris and Harding2002; Botting & DeLong Reference Botting and DeLong2009). Therefore, different types of old-growth forests are likely to harbour lichen communities of varying composition.
To better understand the lichen communities in different old-growth forests, we examined the species on CWD and trees in two old-growth forests, one red pine (Pinus resinosa) dominated (RPF) and the other white pine (Pinus strobus) dominated (WPF). We expect lichen diversity and community composition amongst different substratum types and CWD decay stages to differ within each of the forests, as many species require different substrata (McMullin et al. Reference McMullin, Duinker, Cameron, Richardson and Brodo2008; Smith et al. Reference Smith, Aptroot, Coppins, Fletcher, Gilbert, James and Wolseley2009). We predict that the intermediate decay stages will show the highest lichen diversity because they can harbour both epiphytic species and epigeic species, as they have characteristics of live trees (presence of bark) and decayed wood (Kruys et al. Reference Kruys, Fries, Jonsson, Lämås and Ståhl1999; Humphrey et al. Reference Humphrey, Davey, Peace, Ferris and Harding2002).
Our study contributes to a better understanding of the lichen biota in two old-growth pine forests. It provides information on lichen diversity and communities present on CWD and trees within these old-forest ecosystems, creates baseline data to monitor changes, and can act as a control when comparing lichen diversity in old and young forests.
Methods
Sites
The RPF examined is within the Wolf Lake Forest Reserve (46°51′36″N, 80°38′34·8″W) 50 km north-east of Greater Sudbury, Ontario, Canada. This site is within the Great Lakes-St. Lawrence forest, which is considered a transition zone between the temperate forest of southern Ontario and the boreal forests of northern Ontario (Carleton Reference Carleton2003). The last fire to have occurred in the Wolf Lake Forest Reserve was in 1978, where the forest fire regime frequency is 70 years (Iles Reference Iles1990). Average annual precipitation is 807 mm, with an average annual temperature of 4·9°C (Leithead et al. Reference Leithead, Anand and Silva2010). The red pine stands assessed range in age from 140 to 220 years (Ontario Ministry of Natural Resources 2010; Anand et al. Reference Anand, Leithead, Silva, Wagner, Ashiq, Cecile, Dorbyshev, Bergeron, Das and Bulger2013). The winds around the Wolf Lake Forest Reserve move in a south-westerly direction, thereby limiting the effect of industrial pollution from the City of Greater Sudbury on the vegetation present within the reserve (Winterhalder Reference Winterhalder1984; Joner et al. Reference Joner, Anand and Pillar2012). Information on lichen species present within the Wolf Lake Forest Reserve is limited; currently, there is only one study (Carleton & Gordon Reference Carleton and Gordon1992) that observed four common lichen species in the reserve [Cladonia chlorophaea (Flörke ex Sommerf.) Spreng., C. coniocrea (Flörke) Spreng., Hypogymnia physodes (L.) Nyl., and Vulpicida pinastri (Scop.) J.-E. Mattsson & M. J. Lai].
The WPF examined is in the northern part of the Chiniguichi Waterway Provincial Park (46°53′20·4″N, 80°40′19·2W″)(Ontario Ministry of Natural Resources 2003). It is located directly north-east of the Wolf Lake Forest Reserve boundary. The stands assessed in the WPF range from 180 to 220 years old (Ontario Ministry of Natural Resources 2010). The WPF is relatively anthropogenically undisturbed as there has not been any harvesting activity within the past 100 years, there are no hiking trails present within it, and it has a similar proximity to industrial emission sources as the Wolf Lake Forest Reserve (Tim Lehman, Area Forester, Ministry of Natural Resources, pers. comm.). There is no information on lichen species present within the Chiniguichi Waterway Provincial Park. In addition, there are no annual climate data available for this forest specifically, but it is in close proximity to the RPF (less than 10 km) so they likely share similar climates.
Field sampling
Five potential transects were established in a north-south orientation at least 50 m from forest edges, thus eliminating the impact of edge effects (Esseen & Renhorn Reference Esseen and Renhorn1998). The beginning of each transect was randomly established in each of the designated study areas by dividing aerial photographs into square quadrants (100×100 m), with each quadrant being assigned a number and using a random number generator to select quadrants. The beginning of each transect was situated in the middle of the south end of each quadrant. Transects were 20 m wide and ranged from 50 m to 200 m long. Transects ended within 50 m of the forest edge or where the forest composition (i.e., red pine or white pine dominance, depending on the stand) changed.
The four substratum types (trees, stumps, logs, and snags) were sampled along each transect. One piece of CWD was sampled at the site of a dead tree (i.e., if a log was sampled then the associated stump was not sampled). The minimum spacing between sampling units was 5 m in order to limit the amount of samples from similar microhabitats. Sampling for each substratum type was concluded once the quota (20) was reached. Due to irregular shapes of forest stands and the proximity of the starting point of each transect to forest edges or transition zones, 4 of the 5 potential transects were utilized in the WPF in order to meet the substratum sample quota (80), while 5 of the 5 potential transects in the RPF were utilized in order to meet the substratum sample quota (80). The total lengths of transects utilized in each forest were approximately equal (600 m). Within each of the two forests, only the CWD and trees of the dominant tree species were sampled in order to better distinguish differences between the two forest types.
Snags were at least 10 cm in diam. and 1·5 m tall (Svensson et al. Reference Svensson, Johansson and Thor2005). A 10×10 cm clear plastic grid (plot) separated into 1×1 cm squares was used to examine the lichen species richness and abundance on the snags. Plots were aligned along each trunk on either the north (10 snags per forest type) or south (10 snags per forest type) side of the snag. Plot centres were aligned down the trunk, with the first plot established at the ground level of each snag. Plots were separated by a distance of 40 cm between adjacent top and bottom edges for a total of five plots (0–10 cm, 40–50 cm, 90–100 cm, 140–150 cm, and 190–200 cm or apex plot). If the snag was less than 2·5 m tall, the fifth plot was set on its apex (apex plot) (McMullin et al. Reference McMullin, Lacey and Newmaster2011). The sampling method for trees was the same as that for snags except there was no apex plot included.
Stumps were at least 10 cm in diam. and less than 1·5 m tall (Nascimbene et al. Reference Nascimbene, Marini, Motta and Nimis2008). Five 10×10 cm plots were established on each stump, one on top of the stump, and four at each of the cardinal points (Nascimbene et al. Reference Nascimbene, Marini, Motta and Nimis2008). North and west plots were placed at ground level while south and east plots were placed halfway between ground level and the stump top.
Logs were defined as fallen wood at least 10 cm in diam. Five 10×10 cm plots were established along the top of the log, starting at the broadest point and separated by 40 cm from plot top to the next plot's base (Nascimbene et al. Reference Nascimbene, Marini, Motta and Nimis2008).
Lichen samples were taken when new species were encountered or when species could not confidently be identified in the field. Each piece of CWD had its diameter measured and stage of decay visually assessed on a scale of 1–5 (Table 1). The immediate canopy closure above each piece of CWD was assessed using a spherical densiometer and following methods outlined by Lemmon (Reference Lemmon1957).
Table 1. Characteristics used to identify decay classes for logs, snags and stumps. Log decay classes 1–5 (Maser et al. Reference Maser, Anderson, Cromack, Williams, Martin and Thomas1979; Sollins Reference Sollins1982; Nascimbene et al. Reference Nascimbene, Marini, Motta and Nimis2008). Snag decay classes 1–5 (Sollins Reference Sollins1982; Nascimbene et al. Reference Nascimbene, Marini, Motta and Nimis2008). Stump decay classes 1–5 (Motta et al. Reference Motta, Berretti, Lingua and Piussi2006).

Lichen identification
Lichen species were identified using stereo and compound microscopes with the help of chemical reactions from spot tests (Brodo et al. Reference Brodo, Sharnoff and Sharnoff2001). Species that could not be identified by morphology or chemical spots tests were further examined with thin-layer chromatography (Orange et al. Reference Orange, James and White2001). Immature specimens lacking fruiting bodies, spores or reliable chemistry were only identified to the genus level (Micarea spp., Lepraria sp., and Cladonia spp.).
Data analysis
Lichen species richness and diversity for each piece of CWD and tree was calculated using the Shannon Wiener diversity index (Shannon Reference Shannon1948). All statistical analyses were performed using SPSS version 20. In order to test the normality assumption of parametric statistical analyses, we used the Shapiro-Wilk test. In all cases, except for one, the data were not normally distributed (P<0·05) and as a result we utilized non-parametric statistical analyses to analyze the data. We utilized the Kruskal-Wallis tests to determine whether there were significant (P≤0·05) differences in (percent) canopy openness and diameter between substratum types in each forest and (ii) lichen diversity or species richness between substratum types and decay stages of each type of CWD. In the case of a significant result in Kruskal-Wallis tests, we used pairwise comparison in order to assess where the difference was.
Lichen community patterns were explored with multivariate analyses in PC-ORD ver. 6 (McCune & Mefford Reference McCune and Mefford1999). Nonmetric multidimensional scaling (NMS; Kruskal Reference Kruskal1964) was selected as the ordination method due to its effectiveness in handling ecological community data (e.g. it does not assume linear relationships among variables; McCune & Grace Reference McCune and Grace2002). Data was first square-root transformed to reduce skewness, and very rare species (those with only one occurrence across all samples) were removed from the dataset in order to reduce the noise in the data. NMS was first performed on the lichen abundance data averaged by CWD type within a stand to explore broad patterns. Next, data at the level of individual CWD pieces were ordinated with field-measured fine-scale environmental data (canopy openness, and CWD piece diameter and decay class) for each stand type separately, to investigate more precisely the relationships among lichen communities, CWD type, and environment. A multi-response permutation procedure (MRPP) was performed to test for differences among CWD types within each stand. MRPP calculates a P value and an agreement statistic, A, defining within-group homogeneity. A values close to zero indicate low within-group homogeneity and low levels of difference among groups. Conversely, A values closer to 1 indicate within-group homogeneity, and thus more meaningful differences among groups. MRPP analyses of ecological communities often report A values near or below 0·1, even when there are distinct differences between groups (McCune & Grace Reference McCune and Grace2002). A Bonferroni correction was applied to the multiple comparisons to reduce the type I error rate.
Results
Lichen biodiversity
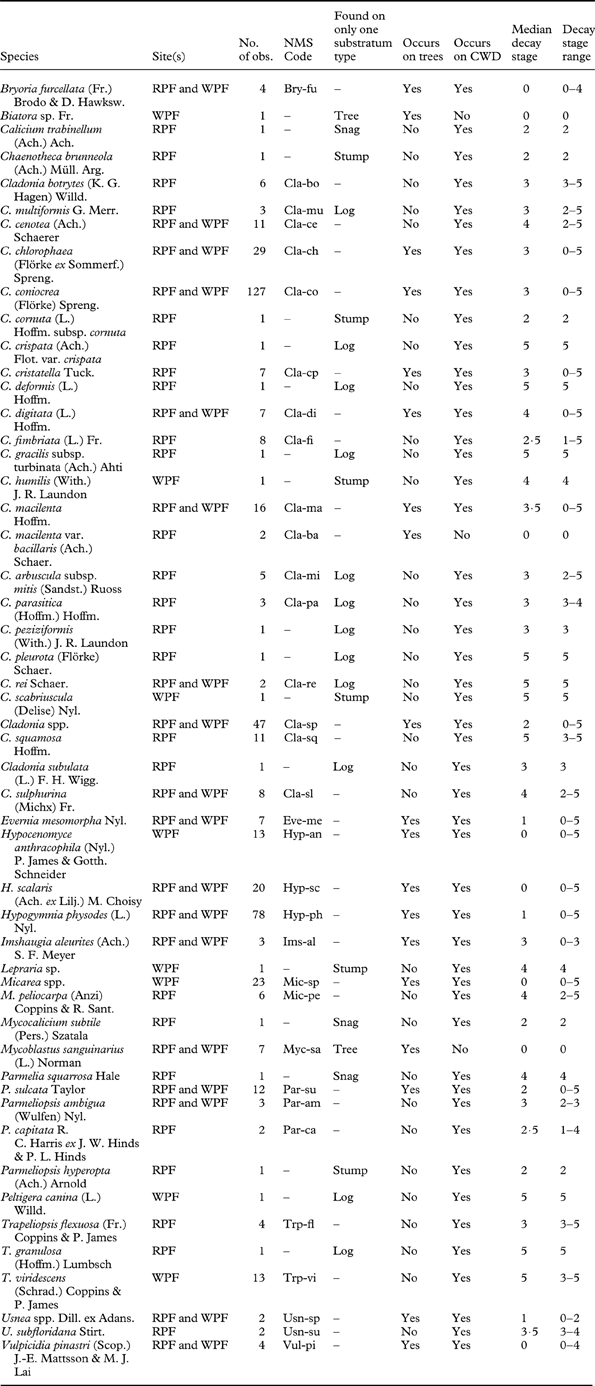
The RPF contained 43 lichen species, of which 25 (58·1%) were unique to that forest (Table 2). The WPF contained 26 species, of which 8 (30·8%) were unique to that forest (Table 2). In addition, in the RPF, 22 (51·2%) of the lichen species were substratum specific (found only on one substratum type; Mycoblastus sanguinarius was only found on trees; Chaenotheca brunneola, Bryoria furcellata, Cladonia cenotea, C. cornuta, and Parmeliopsis hyperopta were exclusively on stumps; Calicium trabinellum, Hypocenomyce scalaris, Mycocalicium subtile, and Parmelia squarrosa only occurred on snags; and Cladonia multiformis, C . crispata, C. deformis, C. digitata, C. gracilis subsp. turbinata, C. arbuscula subsp. mitis, C. parasitica, C. peziziformis, C. pleurota, C. rei, C. subulata, and Trapeliopsis granulosa were found only on logs), while in the WPF, 10 (38·5%) of the lichen species were found on one substratum type (Mycoblastus sanguinarius and Biatora sp. were exclusively on trees; Cladonia humilis, Lepraria sp. and Cladonia scabriuscula occurred only on stumps; Parmelia sulcata, Parmeliopsis ambigua, and Vulpicida pinastri were found only on snags; and C. rei and Peltigera canina were only on logs).
Table 2. Lichen species found in one or both of the study stands (RPF=red pine forest; WPF=white pine forest). Nomenclature is from Esslinger (Reference Esslinger2011). Species codes that each species were assigned in the NMS are noted. Substratum on which each species was found is noted along with median decay stage and decay stage ranges.
Canopy closure and substratum diameter
Within the RPF, there was no significant difference in surrounding percent canopy openness (χ2(df=3, n=80)=2·566, P=0·463) or substratum diameters (χ2(df=3, n=80)=6·783, P=0·079) between the substratum types. However, in the WPF, there was a significant difference in percent canopy openness among the different substratum types (χ2(df=3, n=80)=12·403, P=0·006), with trees having a significantly lower percent canopy openness than all three types of CWD (Fig. 1A). Substratum diameters in the WPF also showed a difference among the different substratum types (χ2(df=3, n=80)=12·403, P=0·006), but the pairwise comparison did not show evidence of a statistically significant difference which is likely due to the Bonferroni-corrected pairwise comparisons increasing the chance of a type II error occurring (Fig. 1B).

Fig. 1. A, the results of the pairwise comparisons assessing the difference in mean percent canopy openness amongst different substratum types within the WPF; B, the results of the pairwise comparisons assessing the difference in mean substratum diameter (cm) amongst the different types of substrata within the WPF; C, the results of the pairwise comparisons assessing the difference in mean Shannon-Wiener diversity amongst different substratum types within the WPF; D, the results of the pairwise comparisons assessing the difference in mean species richness amongst the different types of substrata within the WPF. It should be noted that the raw data are displayed in (A), (B), (C) and (D) rather than the ranked data, since the raw data is more meaningful for comparisons. In each plot, mean values with the same lower case letter are not significantly different at the P<0·05 level.
Substratum types
There was no difference in lichen diversity (χ2(df=3, n=80)=1·985, P=0·575) or species richness (χ2(df=3, n=80)=5·531, P=0·137) among the different substratum types within the RPF. While in the WPF, there was a statistically significant difference in lichen diversity (χ2(df=3, n=80)=23·652, P=0·0001) among the different substratum types; logs had lower lichen diversity than stumps and trees, and snags also had lower diversity than trees (Fig. 1C & D). In addition, there was a significant difference in species richness (χ2(df=3, n=80)=25·706, P=0·0001) among the different substratum types, with logs having lower species richness than trees, stumps and snags (Fig. 1C & D).
CWD decay stages
Lichen diversity is not significantly different between the decay stages of logs (χ 2(df=4, n=20)=2·496, P=0·645), snags (χ2(df=4, n=20)=4·806, P=0·308), or stumps (χ2(df=4, n=20)=1·564, P=0·815) within the RPF. There was also no significant difference in lichen species richness among the different decay stages of logs (χ2(df=4, n=20)=2·420, P=0·659), snags (χ2(df=4, n=20)=3·961, P=0·411), or stumps (χ2(df=4, n=20)=3·475, P=0·482).
WPF decay stages
Within the WPF, there was no significant difference in lichen diversity among the different decay stages of logs (χ2(df=2, n=20)=3·508, P=0·173), snags (χ2(df=3, n=20)=1·570, P=0·666) or stumps (χ2(df=3, n=20)=6·294, P=0·098). Also, there was no significant difference in lichen species richness among the different decay stages of logs (χ2(df=2, n=20)=5·389, P=0·068), snags (χ2(df=3, n=20)=1·085, P=0·781) or stumps (χ2(df=3, n=20)=3·180, P=0·365).
Community composition: multivariate analyses
NMS ordination of averaged lichen species abundance values for all CWD types in both forest stands resulted in a two-dimensional model of CWD-lichen community relationships that accounted for 86% of the total variation in the data. Final stress and instability for this optimal solution were low (5·7 and <10–5, respectively). The ordination diagram illustrates differences in lichen community composition among CWD types (Fig. 2). Within a forest type, trees are widely separated from logs in ordination space. Lichen communities on trees in the WPF included fruticose species (such as B. furcellata and Usnea spp.) and crustose species (such as H. anthracophila and Micarea spp.) that were absent from logs. Trees in the RPF hosted several species that were not found at all on logs in the same stand, including M. sanguinarius, C. macilenta var. bacillaris, V. pinastri, and H. scalaris. Within a forest type, snags and stumps are relatively close together in ordination space, indicating similar community composition.

Fig. 2. Nonmetric multidimensional scaling ordination of lichen community data on different types of CWD (and live trees) in two forest types. Axes one and two, respectively, represent 59·7% and 26·2% of the variation in the data. W=white pine forest, R=red pine forest, LT=live tree, ST=stump, S=snag, L= log. See Table 2 for species codes.
MRPP analyses confirmed lichen community differences among substratum types. Within each forest type, the lichen community on each CWD type differed significantly from that found on trees (e.g., WPF logs vs. trees; A=0·20, P=<0·0001), but did not differ from each other (e.g., RPF logs vs. snags; A=0·02, P=0·15).
The ordinations conducted at the level of individual CWD pieces and their associated environmental variables revealed that decay class was the only variable measured that correlated with lichen community variation among samples (correlations between ordination axis one and decay class: in WPF r 2=0·286, τ=0·352; in RPF r 2=0·432, τ=0·491).
Discussion
Diversity and richness: substratum types
There was no difference in lichen diversity or species richness amongst different substratum types within the RPF. This result could be attributed to the uniformity of canopy openness and habitat diameters amongst the sample sites, since higher light environments are often preferred over low light environments by many lichens (Muhle & LeBlanc Reference Muhle and LeBlanc1975; Chlebicki et al. Reference Chlebicki, Żarnowiec, Cieśliński, Klama, Bujakiewicz and Załuski1996), and differing diameters of CWD influence the number of lichen species present (Kruys et al. Reference Kruys, Fries, Jonsson, Lämås and Ståhl1999). Our prediction, that one substratum type will have higher lichen diversity and species richness compared to other types in a stand, was not verified in the RPF.
In the WPF, lichen diversity and species richness were influenced by substratum type, with logs being both less diverse and less species rich than stumps and trees. The result for the WPF supports the findings of Nascimbene et al. (Reference Nascimbene, Marini, Motta and Nimis2008) in the Italian Alps. They showed that lichen richness was affected by CWD type, with both snags and stumps being more species rich than logs, which were dominated by bryophytes. Humphrey et al. (Reference Humphrey, Davey, Peace, Ferris and Harding2002) found partly similar results in semi-natural planted forests in Great Britain. They showed that lichen richness differed with CWD type, with snags being richer than both stumps and logs; this was likely due to reduced competition with bryophytes. The trees in the WPF probably have higher species richness and diversity than snags, stumps, or logs due to the lack of competition with bryophytes and a longer period of time in which a stable habitat was available to colonize and develop. Our prediction, that some substratum types will have greater lichen diversity and species richness than others, is true in the WPF.
In both the RPF and WPF we found species that have been associated with old-growth forests in other studies. Mycoblastus sanguinarius and Usnea spp. were found to be more abundant in old-growth stands studied by Boudreault et al. (Reference Boudreault, Bergeron, Gauthier and Drapeau2002) in their assessment of lichen and bryophyte communities in mature to old-growth boreal black spruce forests in Quebec and Ontario, Canada. Crites & Dale (Reference Crites and Dale1998) examined the diversity of lichens, bryophytes, and fungi in aspen mixedwood boreal forests in Alberta, Canada. They found six lichen species exclusively in old-growth forests, two of which (Evernia mesomorpha and Calicium spp.) were found in this study. The epiphytic lichen species richness found in the RPF and WPF is comparable, given the differences in sampling, to those found in McMullin et al. (Reference McMullin, Duinker, Cameron, Richardson and Brodo2008), who examined the epiphytic lichen richness in young to old-growth coniferous forests in Nova Scotia, Canada. They found that epiphytic lichen richness in forests of similar ages to the WPF and RPF ranged from 16 to 41 species.
All of the 26 lichen species found in the WPF are new records for the Chiniguichi Waterway Provincial Park, and 39 of the 43 lichen species found in the RPF are new records for the Wolf Lake Forest Reserve. Our study provides a better understanding of lichen community composition in old-growth red and white pine forests and builds on the work of Carleton (Reference Carleton2003).
Comparison between RPF and WPF
The RPF had a higher alpha diversity than the WPF. Because we were only able to compare one stand of each species, it is not possible to attribute differences in lichen diversity to the dominant tree species. However, in the interests of increasing our understanding of lichen diversity and its variability among forest stands, we can suggest some of the factors that contribute to the patterns we observed. We observed that logs in the RPF had higher lichen diversity than logs in the WPF, which may be due to much of the CWD in the WPF being more decayed. This advanced decomposition could allow more time for colonization on logs by bryophytes, which may competitively exclude lichen (Botting & DeLong Reference Botting and DeLong2009). The larger number of species unique to the RPF compared to the WPF agrees with McAlister (Reference McAlister1997), who found that some cryptograms were only associated with the logs of certain tree species in a North Carolina forest. The larger number of species specific to a CWD type (only found on one type of CWD, snag, log or stump) found in the RPF suggests that a variety of CWD types, especially logs, is more important for increasing overall lichen diversity in the RPF compared to the WPF.
Diversity and richness: decay stages
In both the RPF and WPF, there was no difference in lichen diversity or species richness amongst the different decay stages of CWD. This may be due to the limited number of samples of CWD in some stages. Future studies should attempt to gather an even number of samples across all decay stages for more definitive results. Our second prediction, that some decay stages have higher lichen diversity and species richness than others, is not supported in the RPF or the WPF.
Most other studies did not support our findings as they showed that lichen diversity and species richness differed on different decay stages of CWD. Nascimbene et al. (Reference Nascimbene, Marini, Motta and Nimis2008) found that decay stage influenced lichen species richness on snags, stumps and logs. Botting & DeLong (Reference Botting and DeLong2009) analyzed the influence of CWD characteristics on bryophytes and macrolichen within sub-boreal spruce forests in British Columbia and found that decay stage influenced macrolichen species richness and abundance. This discrepancy may also be due to our limited availability of some decay stages. Our results were supported, however, by Lohmus & Lohmus (Reference Lohmus and Lohmus2001), who found that lichen diversity did not differ between snags of any decay stages in an old peatland forest in Estonia.
Community composition
The differences between lichen communities on trees and different types of CWD within each forest are due to changes in the physical structure and chemistry of the substratum (McMullin et al. Reference McMullin, Duinker, Richardson, Cameron, Hamilton and Newmaster2010), leading to changes from largely epiphytic species (e.g. E. mesomorpha, V. pinastri) to communities mostly comprised of epigeic (e.g., Cladonia spp.) species. Our results were supported by the findings of Söderström (Reference Söderström1988), who showed a shift in lichen communities from epiphytes (i.e. Bryoria, Usnea and Evernia) on trees to epixylics (i.e. Chaenotheca, and Calicium) or epigeics (i.e. Peltigera) on coniferous logs in a Swedish boreal forest.
Conclusion
Many lichen species rely on the habitat provided by old-growth forests (Lesica et al. Reference Lesica, McCune, Cooper and Hong1991; McMullin et al. Reference McMullin, Duinker, Cameron, Richardson and Brodo2008). We observed that there were differences in lichen biota diversity patterns between stands, which may be caused by the difference in the dominant tree species in each. Our study provides a unique inventory of an often-overlooked group in two rare and poorly understood ecosystems. Few old-growth forests remain in Ontario and the stands examined in this study at the Wolf Lake Forest Reserve are at risk of harvest due to mining leases that are still active in the area (Ontario Ministry of Natural Resources 1999; Anand et al. Reference Anand, Leithead, Silva, Wagner, Ashiq, Cecile, Dorbyshev, Bergeron, Das and Bulger2013). A greater understanding of the lichen communities in these forest types will assist with identifying stands of high conservation value, particularly those at risk.
We thank the Natural Sciences and Engineering Research Council of Canada for providing funding (Undergraduate Student Research Award to C. Wagner, Discovery Grant to M. Anand), Tim Lehman of the Ontario Ministry of Natural Resources, Cara Bulger and Arundhati Das for assistance with fieldwork, Jose Maloles for help identifying lichen specimens, Alice Cecile and Cara Bulger for assistance with statistical analyses, and Steven Newmaster for providing laboratory space and equipment for identifications.






