Introduction
Genotypic variations generally affect phenotypes, but the presence of chaperones makes some phenotypes more robust to genetic and environmental changes than expected (Young et al., Reference Young, Moarefi and Hartl2001; Wagner, Reference Wagner2005). Phenotypes may be indeed regulated by canalization that is the result of intrinsic developmental buffering ensuring phenotypic robustness under genetic variation and environmental perturbation. As a consequence, animal phenotypes are remarkably consistent within a species under a wide range of conditions (Young et al., Reference Young, Moarefi and Hartl2001; Miklejohn & Hartl, Reference Miklejohn and Hartl2002; Wagner, Reference Wagner2005).
The presence of a mechanism able to buffer mutations could be particularly relevant for genes, whose expression is limited to specific development stage or to specific generations. An example in aphids is related to the reproduction mode since the typical annual life cycle of aphids is based on a cyclical parthenogenesis in which several apomictic parthenogenetic generations in spring and summer are followed by a single sexual generation (occurring in autumn) producing overwintering eggs (Moran, Reference Moran1992). Sexual reproduction (in term of females and males mating) occurs therefore in aphids only once per year in autumn and it is induced by decreasing day length and temperature (Moran, Reference Moran1992).
As a consequence of the fast life cycle (based not only on parthenogenesis, but also on viviparity), the generation time of aphids is on average 8 days long so that aphids may have more than 30 parthenogenetic generations before the single sexual generation. This means that apomictic parthenogenetic generations may accumulate mutations (that are not phenotypically visible) in genes involved in the determination of the sexual generation so they can lose the ability to produce sexuales with detrimental effects on their survival during winter (Moran, Reference Moran1992).
As frequently reported in the literature (for a review see Simon et al., Reference Simon, Rispe and Sunnucks2002), some aphid species/populations have lost their sexual phase becoming obligate parthenogens (frequently referred as asexual lines). Compared with cyclically parthenogenetic lines (sexual lines) that produce cold-resistant diapausing eggs after each annual sexual phase, asexual lines do not produce eggs, so that they are susceptible to freezing conditions (Rispe & Pierre, Reference Rispe and Pierre1998; Rispe et al., Reference Rispe, Pierre, Simon and Gouyon1998; Simon et al., Reference Simon, Rispe and Sunnucks2002). Furthermore, the origin of asexual lines has also its own importance since it shapes the genetic architecture of aphid populations by reducing the genetic recombination typically occurring in the amphygonic generation. As a consequence, the occurrence of mechanisms able to buffer mutations affecting the presence of the sexual generation could have a relevant adaptive value for aphids.
Molecular chaperones, also known as heat-shock proteins (hsps), are evolutionarily conserved molecules able to disaggregate and refold proteins destabilized by a variety of environmental stresses (Estruch, Reference Estruch2000; Gasch et al., Reference Gasch, Spellman, Kao, Carmel-Harel, Eisen, Storz, Botstein and Brown2000). In particular, hsps constitute a large family of proteins classified on the basis of their molecular weight (hsp10, hsp40, hsp60, hsp70, hsp90,…) that play crucial roles in folding/unfolding of proteins, assembly of multiprotein complexes, transporting/sorting of proteins into correct subcellular compartments, controlling cell-cycle and signalling and protecting cells against stress/apoptosis/genome instability (Morimoto et al., Reference Morimoto, Kline, Bimston and Cotto1997; Li & Srivastava, Reference Li and Srivastava2004; Richter et al., Reference Richter, Haslbeck and Buchner2010).
Among them, HSP90 proteins are present from bacteria to man, where they mediate many fundamental cellular processes, including cell cycle control, cell survival, hormone signalling and response to cellular stress (Zhao et al., Reference Zhao, Davey, Hsu, Kaplanek, Tong, Parsons, Krogan, Cagney, Mai, Greenblatt, Boone, Emili and Houry2005; Wandinger et al., Reference Wandinger, Richter and Buchner2008; Taipale & Jarosz, Reference Taipale and Jarosz2010). Furthermore, eukaryotic hsp90 promotes the formation of the correct conformation and activation of more than 200 proteins referred as hsp90 clients (Picard, Reference Picard2002; Zhao et al., Reference Zhao, Davey, Hsu, Kaplanek, Tong, Parsons, Krogan, Cagney, Mai, Greenblatt, Boone, Emili and Houry2005), differently from its prokaryotic homologue that seems to act on its own (Bardwell & Craig, Reference Bardwell and Craig1988).
In aphids, hsps have been studied in few species using next-generation transcriptome sequencing and proteomic analyses (Nguyen et al., Reference Nguyen, Michaud and Cloutier2009; Li et al., Reference Li, Zhang, Luo, Wang and Lv2013) showing that hsps segregated into six families (hsp90, hsp70, hsp60, hsp40, shsp and hsp10). At a functional level, the involvement of hsps has been assessed in the response to heat stress in Aphis gossypii (Glover, 1877) and Macrosiphum euphorbiae (Thomas, 1878) and hsps have been described as essential for survival and detoxification of xenobiotics in Myzus persicae (Figueroa et al., Reference Figueroa, Prunier-Leterme, Rispe, Sepulveda, Fuentes-Contreras, Sabater-Munoz, Simon and Tagu2007; Ramsey et al., Reference Ramsey, Wilson, de Vos, Sun, Tamborindeguy, Winfield, Malloch, Smith, Fenton, Gray and Jander2007).
Interestingly, at present the role of hsps in mutation buffering has never been studied in aphids, despite its involvement in the maintenance of the phenotypic plasticity could allow aphids to occupy a wide variety of environments. For instance, M. persicae exhibits a dimorphism as alate (winged) and apterous forms (Braendle et al., Reference Braendle, Davis, Brisson and Stern2006). The switch to the alate morph seems to be triggered by several mechanisms in response to sub-optimal environmental conditions such as crowding, poor nutrition and presence of predators (Braendle et al., Reference Braendle, Davis, Brisson and Stern2006) so that winged adults are relevant for the aphid dispersal and survival (Müller et al., Reference Müller, Williams and Hardie2001). In this way, the ability to buffer potentially harmful mutations affecting the formation of wings results important for aphids. At the same time, it could be interesting to evaluate if mutations in the hsp90 gene are related to a pronounced genomic instability in aphids and, in particular, in the peach potato aphid M. persicae, where recurrent chromosomal rearrangements have been observed in some asexual lineages (Manicardi et al., Reference Manicardi, Nardelli and Mandrioli2015).
In the present paper, we amplified and sequenced the hsp90 gene of 17 lineages of the peach potato aphid M. persicae (Sulzer, 1776) with different reproductive modes (obligate vs. cyclical parthenogenesis), propensity to develop winged females and karyotype stability. As a whole, our results showed that mutations which altered HSP90 proteins were present in aphid lineages with obligate parthenogenesis only, suggesting that hsp90 could play a role in buffering hidden mutations that could disrupt cyclical parthenogenesis.
Materials and methods
Specimens of M. persicae were obtained from 17 aphid lineages maintained as a colony of parthenogenetic females on pea (Pisum sativum) plants at 19°C with a light-dark regime of 16 h light and 8 h darkness (Table 1). M. persicae clone 7 was kindly supplied by John Margaritopoulos (Greece); clones K10, K5, P type, D type and 229 were kindly supplied by Brian Fenton (Scotland), whereas clones 33H, 1, 19, 64, 4H1, SA1 and 48 were kindly supplied by Emanuele Mazzoni (Italy). M. persicae clones MO1, MO3, RE2a and RE2b were sampled in Italy.
Table 1. List of the studied M. persicae clones.

RNA extraction was performed with the ‘SV Total RNA Isolation System’ (Promega, Madison, WI, USA), accordingly to the supplier's suggestions. Amplification of an internal portion of hsp90 gene was performed by RT-PCR with the ‘Access RT-PCR System’ (Promega) with the primers F-hsp90Eso2 (5′-TCTGGTACCAAGGCCTTCAT) and R-hsp90Eso2 (5′-CTCGGAGGCTTC AACTTCAG) at an annealing temperature of 53°C for 1 min. Primers were designed according to the sequence XM_001943137, annotated as hsp83-like in the pea aphid Acyrthosiphon pisum (Boyer de Fonscolombe, 1841).
RACE amplification was done to complete the hsp90 sequence using the ‘5′/3′ RACE Kit’ (Roche), according to the supplier's datasheet.
Bioinformatic analyses of the obtained hsp90 sequences were performed by BLAST alignments in Genbank (http://blast.ncbi.nlm.nih.gov/Blast.cgi) both at DNA and protein level. A further search has been performed by BLAST alignments in aphid genomes at AphidBase (http://www.aphidbase.com). Sequence alignment has been performed using CLC Sequence Viewer (CLC Bio), whereas the search of conserved domain was done using the NCBI's conserved domain database according to Marchler-Bauer et al. (Reference Marchler-Bauer, Derbyshire, Gonzales, Lu, Chitsaz, Geer, Geer, He, Gwadz, Hurwitz, Lanczycki, Lu, Marchler, Song, Thanki, Wang, Yamashita, Zhang, Zheng and Bryant2015).
Functional assays have been performed using geldanamycin (Sigma Aldrich), an in vivo hsp90 inhibitor, at concentrations of 1.5 and 3 µg ml−1, according to functional analyses performed in Drosophila melanogaster (Crevel et al., Reference Crevel, Bates, Huikeshoven and Cotterill2001). Geldanamycin has been diluted in a sterile artificial diet solution consisting of 5% yeast extract in 30% sucrose in distilled water (pH adjusted to 7 with 5 M KOH) supplied in feeding chamber, according to Torres-Quintero et al. (Reference Torres-Quintero, Arenas-Sosa, Peña-Chora and Hernández-Velázquez2013). After 2 days of feeding in the artificial diet, ten M. persicae parthenogenetic females of the cyclical parthenogenetic lineages 64, 1, MO1, 48 and 4H1 have been moved to short photoperiods (8 h light:16 h dark) in order to induce the development of males according to Crema (Reference Crema1979). Males were daily counted for a total of 15 days making experiments in five replicas. Control experiments have been also performed in artificial diet with the same aphid lineages without geldanamycin. Male identification has been performed by the analysis of the karyotype obtained by aphid squashing, according to Mandrioli et al. (Reference Mandrioli, Manicardi, Bizzaro and Bianchi1999), and successive silver staining of chromosomes, as reported in Mandrioli et al. (Reference Mandrioli, Manicardi and Marec2003).
Statistical analyses were performed using the Student's t-test, whereas box-plot graphs were obtained using the BoxPlotR tool (freely available online at the address http://shiny.chemgrid.org/boxplotr/).
Results
Sequence analysis revealed that in M. persicae lineage 1 (used as a reference in view of its standard karyotype and reproductive mode based on cyclical parthenogenesis), the hsp90 cDNA consisted of 2148 bp and coded for a 715 amino acid (aa) long peptide, size that is in accordance to that reported for other insects in GenBank.
At a sequence level, the alignment of the M. persicae HSP90 with homologous proteins of the pea aphid A. pisum (XP_001943172), the fly D. melanogaster (NP_523899), the medfly Ceratitis capitata (Wiedemann, 1824) (CAJ28987) and the honey bee Apis mellifera (Linnaeus, 1758) (XP_006571335) revealed a similarity ranging from 75 to 96% (fig. 1a). In particular, A. pisum and M. persicae showed an 86% similarity in their coded HSP90 proteins. Furthermore, M. persicae HSP90 possessed histidine kinase-like ATPases (HATPase_c) and HSP domains typically observed in the hsp90 superfamily (fig. 1b).

Fig. 1. (a) Alignment of the HSP90 amino acidic sequence of M. persicae (MP) with homologues from A. pisum (AP), D. melanogaster (DM), C. capitata (CC) and A. mellifera (AM) revealed a widespread conservation of this protein in insects. (b) The M. persicae HSP90 protein presents the two conserved domains typically observed in proteins of the hsp90 family. HATPase_(c) Histidine kinase-like ATPases. HSP90: hsp90 domain.
The amplification, sequencing and assembly of the Hsp90 cDNA have been successively performed in other 16 lineages and the obtained sequences have been aligned in order to identify the presence of mutations and their effects on the coded protein (Table 2, fig. 2).
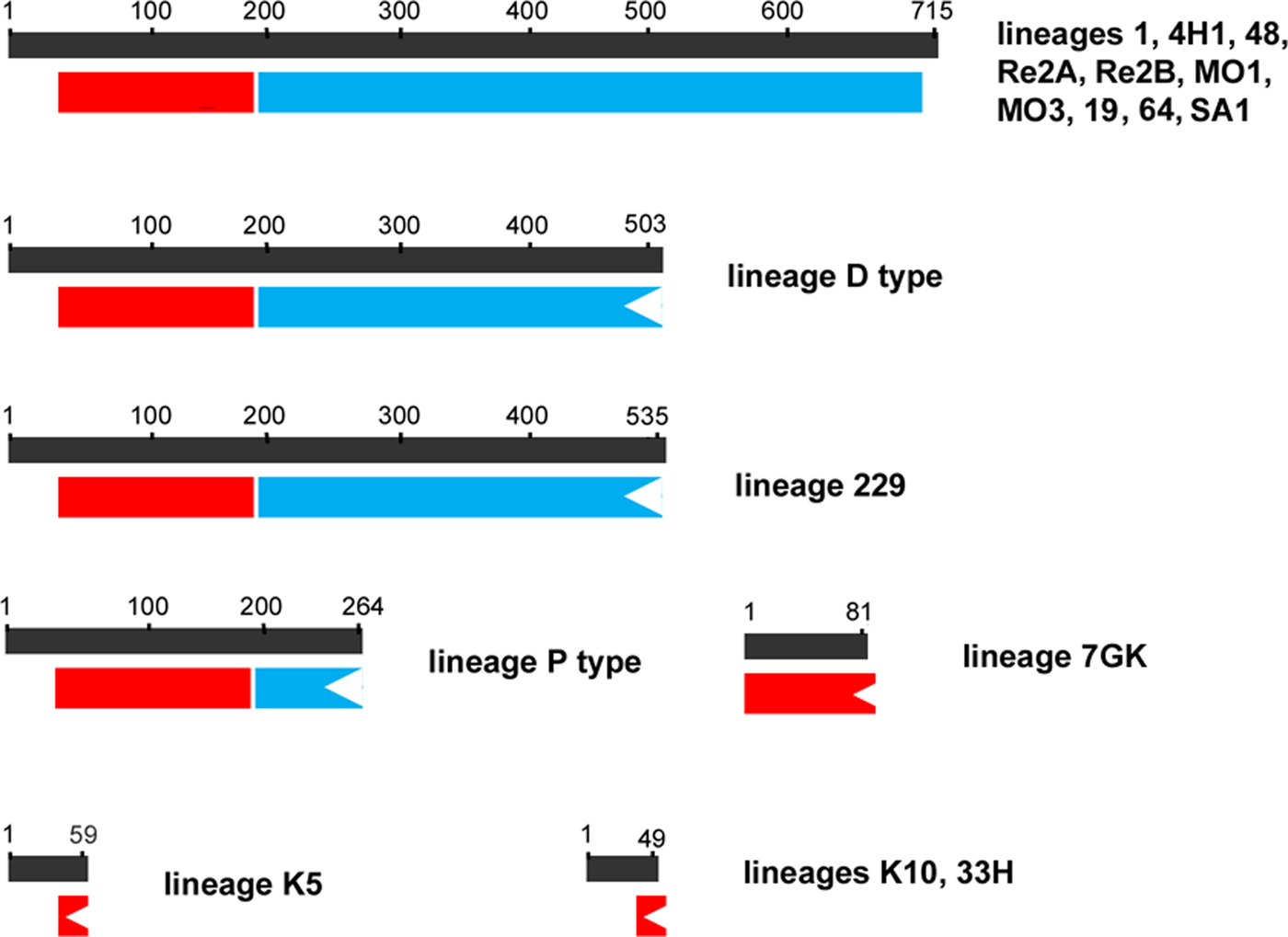
Fig. 2. Schematic representation of the presence, localization, size (amino acids number) and integrity of the histidine kinase-like ATPase (in red) and hsp90 domains (in blue) in the HSP90 proteins coded by the 17 analysed M. persicae clones.
Table 2. Mutations observed for each studied M. persicae clone.

Holocyclic lineages 1 and 4H1 presented an identical hsp90 coding sequence, whereas lineages 48, 64 and SA1 possessed four, three and two mutations, respectively, when compared with the hsp90 isolated from lineage 1. These mutations were neutral or silent and consequently they did not affect the presence neither the structure of the hsp90 functional domains (evaluated using the CDD tool in NCBI). Similarly, holocyclic lineages RE2a and RE2b possessed an identical missense mutation that had no effect on the structure of the conserved histidine kinase-like ATPase and HSP domains. Holocyclic lineages MO1 and MO3 possessed the same missense mutations that did not alter the structure of the coded HSP90 protein. Similarly, lineage 19 showed four missense mutations that did not affect the structure of the coded protein.
The asexual lineage D type possessed five mutations including two frameshift mutations resulting in the synthesis of a truncated HSP90 protein (503 aa long) with an uncompleted hsp90 domain. Similarly lineage 229 coded for a truncated HSP90 protein (535 aa) possessing an uncompleted hsp90 domain. Lineage K5 showed three severe mutations, including a frameshift mutation at the beginning of the coding sequence resulting in a 59 aa long protein devoid of any conserved functional domain. A truncated HSP90 protein was also coded by the asexual lineages 33H and K10 that showed the same frameshift mutation that brought to a not functional hsp90 (Table 2, fig. 2).
A truncated HSP90 protein (81 aa) was also coded by the asexual lineage 7 that possessed a non-sense mutation that introduced a premature stop codon into the hsp90 gene (Table 2, fig. 2). Similarly, a non-sense mutation introduced a premature stop codon into the hsp90 gene in the lineage P type that resulted coding for a 264 aa long protein.
As a whole, among the considered strains, all the M. persicae asexual lineages (229, D type, P type, K5, 33H, 7 and K10) coded for not functional HSP90 proteins suggesting that hsp90s could be functionally involved in the maintenance of the cyclical parthenogenesis.
In vivo functional assays with the hsp90 inhibitor geldanamycin showed that lineages 4H1, 1 and 48 maintained their ability to induce sexuales in the absence of active hsp90s (at both the analysed geldanamycin concentrations) with male numbers similar to those observed in the control experiments (fig. 3). Differently, lineages MO1 and 64 showed males in the control experiments only, whereas geldanamycin-treated aphids never gave birth to males (fig. 3).
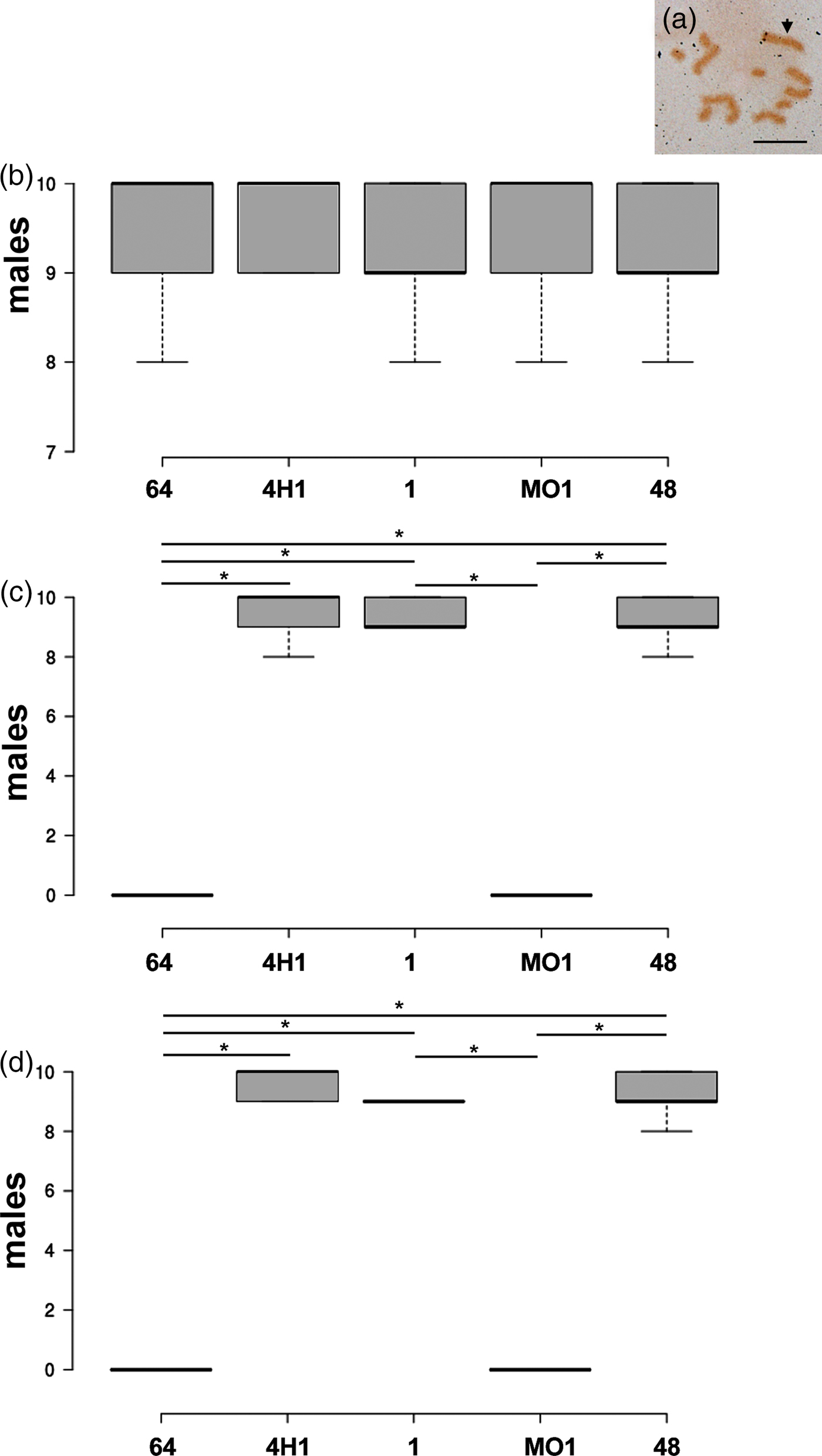
Fig. 3. The presence of males in the progeny has been evaluated by the analysis of their karyotype presenting a single X chromosomes (an example from lineage 48 in panel a). Male counts in control (b), geldanamycin 1.5 µg ml−1 (c) and geldanamycin 3 µg ml−1 (d) evidenced a stable presence/amount of males in lineages 4H1, 1 and 48, differently from lineages 64 and MO1, where male were present only in the control experiments and absent after geldanamycin treatment (at both concentrations). Asterisk indicates that values are significantly different at the 5% level in the Student's t-test. Arrow indicates the X chromosome. Bar corresponds to 10 µm.
Discussion
Hsp90s have been widely studied in humans since they are involved in cellular defence against cancer by directly interacting and stabilizing the tumour suppressor protein p53 (Muller et al., Reference Muller, Ceskova and Vojtesek2005). Hsp90 chaperone activity is indeed of high importance and mutations in hsp90 coding genes were identified in more than half of all human tumours studies (Bagatell & Whitesell, Reference Bagatell and Whitesell2004; Sangster et al., Reference Sangster, Lindquist and Queitsch2004). More generally, hsp90 plays a pivotal role by buffering mutations that could have morphological effects (Rutherford & Lindquist, Reference Rutherford and Lindquist1998; Queitsch et al., Reference Queitsch, Sangster and Lindquist2002; Jarosz & Lindquist, Reference Jarosz and Lindquist2010).
In insects, HSP90 proteins have been deeply studied in D. melanogaster only, where they have been denominated as HSP83 (Xiao & Lis, Reference Xiao and Lis1989). According to the literature data, fly hsp83 gene is expressed at high levels during development; it increases several fold in response to heat shock (Xiao & Lis, Reference Xiao and Lis1989) and it supports diverse (but specific) signal transducers laying at the interface of several developmental pathways (van der Straten et al., Reference van der Straten, Rommel, Dickson and Hafen1997; Bandura et al., Reference Bandura, Jiang, Nickerson and Edgar2013). For instance, hsp83 interacts with various cellular signalling proteins, such as steroid hormone receptors, src-like kinases and the serine/threonine kinase Raf (van der Straten et a l., Reference van der Straten, Rommel, Dickson and Hafen1997). A further function of fly hsp83 is related to the control of transcription and mobilization of transposable elements in the fly germ cells by affecting piRNA biogenesis (Specchia et al., Reference Specchia, Piacentini, Tritto, Fanti, D'Alessandro, Palumbo, Pimpinelli and Bozzetti2010). In particular, it has been suggested that the reduction of hsp83 causes a stress-response-like activation and transposition of mobile elements (Specchia et al., Reference Specchia, Piacentini, Tritto, Fanti, D'Alessandro, Palumbo, Pimpinelli and Bozzetti2010).
As a consequence of the numerous functions, a reduced amount of hsp83 or the presence of hsp83 mutants can induce abnormal developmental phenotypic variations in flies (Rutherford & Lindquist, Reference Rutherford and Lindquist1998). Defects ranged from subtle to severe, involving either one or both sides of the body, and included body-part transformations, disrupted abdominal patterning, bristle duplications, deformed eyes or legs and changes in wing shape or venation (Rutherford & Lindquist, Reference Rutherford and Lindquist1998).
The current hypothesis is that Drosophila hsp83 buffers pre-existing genetic variations that are not expressed and accumulate in neutral conditions. As a consequence, when hsp83 buffering is compromised, for example, by mutations or by specific drugs, cryptic variants are expressed thus leading to mutant phenotypes and pronounced genomic instability (Rutherford & Lindquist, Reference Rutherford and Lindquist1998; Specchia et al., Reference Specchia, Piacentini, Tritto, Fanti, D'Alessandro, Palumbo, Pimpinelli and Bozzetti2010).
Sequencing of the hsp90 gene in 17 lineages of the peach potato aphid M. persicae revealed the presence of seven lineages (229, D type, P type, K5, 33H, 7 and K10) with severe mutations, resulting in not functional HSP90s. Interestingly, all these lineages did not show any morphological aberration, but have in common the presence of a reproduction mode based on obligate parthenogenesis. A possible role of hsp90 in wing presence was also evaluated, but no relationship has been observed between the occurrence of mutations in the hsp90 gene and wing presence/absence in M. persicae.
The presence of mutations in the hsp90 gene in the M. persicae lineages 33H and 7 is interesting considering that these lineages have a genomic instability resulting in recurrent intra-individual chromosomal rearrangements involving chromosomes A1, A3 and X (Monti et al., Reference Monti, Lombardo, Loxdale, Manicardi and Mandrioli2012; Kati et al., Reference Kati, Mandrioli, Skouras, Malloch, Voudouris, Venturelli, Manicardi, Tsitsipis, Fenton and Margaritopoulos2014; Manicardi et al., Reference Manicardi, Nardelli and Mandrioli2015). The occurrence of these karyotype variants in M. persicae has been explained considering that the holokinetic structure of aphid chromosomes can facilitate the inheritance of chromosomal fragments since they can be attached to microtubules and furthermore they can be inherited without the constraint of homologous pairing typical of meiosis (Manicardi et al.,Reference Manicardi, Nardelli and Mandrioli2015). At the same time, the apomictic mode of the aphid parthenogenesis, characterized by the absence of both homologous chromosome pairing and genetic recombination, could further make possible that rearranged karyotypes can be passed to offspring. Actually, these suggestions explain how fragmented chromosomes may be inherited and not their origin, but the study of hsp90-mutated lineages could help to suggest possible experimental perspectives. Indeed, as reported in the literature (Hoeijmakers, Reference Hoeijmakers2001; Chistiakov et al., Reference Chistiakov, Voronova and Chistiakov2008; Bridge et al., Reference Bridge, Rashid and Martin2014), cells respond to DNA damage by activating the complex DNA damage response (DDR) pathway that includes the cell cycle arrest, the transcriptional and post-translational activation of a subset of genes including those associated with DNA repair, and, under some circumstances, the triggering of programmed cell death (Hoeijmakers, Reference Hoeijmakers2001; Chistiakov et al., Reference Chistiakov, Voronova and Chistiakov2008; Bridge et al., Reference Bridge, Rashid and Martin2014). The inability to repair DNA damage leads to genetic instability, which in turn may enhance the rate of cancer development as reported in human cells (Hoeijmakers, Reference Hoeijmakers2001; Chistiakov et al., Reference Chistiakov, Voronova and Chistiakov2008; Bridge et al., Reference Bridge, Rashid and Martin2014). Although the precise cellular circumstances that incorporate hsp90 into an optimal DDR mechanism remains unknown, multiple components of the double-strand break (DSB) repair machinery, including BRCA1, BRCA2, RAD51, CHK1, DNA-PKcs, members of the FA pathway, histones, and components of the MRE11/RAD50/NBN complex, have been reported to be hsp90 clients (as recently reviewed by Pennisi et al., Reference Pennisi, Ascenzi and di Masi2015). The importance of a robust DNA-damage surveillance network involving hsp90 has been repeatedly reported in the literature since defects in sensing, signalling and repair of DNA damage are linked to the development of inherited chromosome instability (Pennisi et al., Reference Pennisi, Ascenzi and di Masi2015). Starting from these literature data, it could be therefore suggested that hsp90-mutated M. persicae lineages cannot buffer the presence of mutations in genes involved in the DSB repair resulting in rearranged karyotypes.
The presence of a role for the HSP90 proteins in the maintenance of the cyclical parthenogenesis has been also evaluated by performing in vivo functional assays with the hsp90 inhibitor geldanamycin. These experiments assessed that MO1 and 64 lineages produced males in the control experiments only, whereas geldanamycin-treated specimens never gave birth to males. Both lineages seem therefore to possess cryptic mutations that could result in the loss of sexuales in the absence of the active hsp90 chaperone. Differently, the absence of effects of the geldanamycin treatments on lineages 4H1, 1 and 48 suggested that no mutations in genes involved in the establishment of the cyclical parthenogenesis are present in their genome.
As reported in the literature (Simon et al., Reference Simon, Rispe and Sunnucks2002), cyclical parthenogens and obligate asexual lineages have been found in different aphid species (including M. persicae) and climate is the major determinant of the distribution of sexual and asexual lines. Indeed, only cyclical parthenogens have the ecological advantage of producing frost-resistant eggs so that cyclical parthenogenetic lineages only can maintain their adaptive potential in cold climate. In view of this assumption, the determination of sexuales needs to be based on a robust gene network in aphids making hsp90 a possible buffering mechanism for the maintenance of cyclical parthenogenesis.
As a whole, hsp90 genes could be very important in the aphid survival rate since a large number of lineages can produce sexuales and winter-resistant eggs in the presence of functional HSP90 proteins. On the contrary, the presence of mutations in the hsp90 gene could make evident the presence of mutations that prevent the birth of males and females so bringing to a bottleneck in the aphid genetic variability in spring. Hsp90 could therefore be relevant not only for the survival during winter, but also to favour the presence of a wider genetic variability in the aphid populations.
Furthermore, as clearly assessed by Simon et al. (Reference Simon, Carrel, Hebert, Dedryver, Bonhomme and Gallic1996) for the aphid Rhopalosiphum padi, a diminished level of heterozygosity is generally present in anholocyclic lineages in comparison to holocyclic ones so that a spread of anholocyclic reproduction should be negatively selected in the aphid populations or buffered by molecular mechanisms.
In examining the forces maintaining sexual reproduction, special attention has been directed toward species with sexual and asexual lineages and past studies have examined fish (Simon et al., Reference Simon, Carrel, Hebert, Dedryver, Bonhomme and Gallic1996; Stöck et al., Reference Stöck, Lampert, Möller, Schlupp and Schartl2010; Barbuti et al., Reference Barbuti, Mautner, Carnevale, Milazzo, Rama and Sturmbauer2012), insects (Harshman & Futuyama, Reference Harshman and Futuyama1985), nemerteans (Ament-Velásquez et al., Reference Ament-Velásquez, Figuet, Ballenghien, Zattara, Norenburg, Fernández-Álvarez, Bierne, Bierne and Galtier2016) and cladocerans (Innes et al., Reference Innes, Schwartz and Hebert1986). However, surprisingly little attention has been directed towards aphids which are excellent candidates for these studies (Delmotte et al., Reference Delmotte, Leterme, Gauthier, Rispe and Simon2002). Our results suggest that different and more intriguing scenarios could regulate the presence of sexual and asexual lineages and an improved knowledge about hsp90 ‘clients’ in aphids could favour the identification of genes involved in the holocyclic reproduction.
Acknowledgements
M. persicae clone G006 genomic DNA sequence data were downloaded from AphidBase (http://www.aphidbase.com), where they have been made available in advance of analysis. Funding for M. persicae clone G006 genomic sequencing was provided by USDA-NIFA Award 2010-65105-20558.








