Introduction
The rat lungworm Angiostrongylus cantonensis (Nematoda: Metastrongyloidea) is a parasitic nematode that causes neuroangiostrongyliasis, because of its larvae tropism for the central nervous system (CNS). This can manifest as eosinophilic meningitis with possible severe and fatal outcomes (Wang et al., Reference Wang, Lai, Zhu, Chen and Lun2008; Cowie, Reference Cowie2013; Morassutti et al., Reference Morassutti, Thiengo, Fernandez, Sawanyawisuth and Graeff-Teixeira2014). Humans are accidental hosts, with infection occurring after ingestion of terrestrial gastropods (slugs and snails) infected by third-stage larvae (L3), including accidental consumption of these invertebrates in vegetables; or through the ingestion of paratenic hosts containing L3 (e.g. freshwater shrimp, crabs, planarians) (Thiengo et al., Reference Thiengo, de Oliveira Simões, Fernandez and Júnior2013). Neuroangiostrongyliasis has expanded from southeastern Asia and Pacific Islands, where the disease is considered endemic, to countries and islands around the world (Cowie, Reference Cowie2013), advancing from tropical to temperate zones (Červená et al., Reference Červená, Modrý, Fecková, Hrazdilová, Foronda, Alonso, Lee, Walker, Niebuhr and Malik2019). The disease is well documented in South America (Valente et al., Reference Valente, Robles, Navone and Diaz2018) and since the first reported case in Brazil in 2007 it has continued to emerge in the north (Barbosa et al., Reference Barbosa, Thiengo, Fernandez, Graeff-Teixeira, Morassutti, Mourão, Miranda, Jorge, Costa and Gomes2020), northeastern (Thiengo et al., Reference Thiengo, Maldonado, Mota, Torres, Caldeira, Carvalho, Oliveira, Simões, Fernandez and Lanfredi2010) and southeastern regions (Caldeira et al., Reference Caldeira, Mendonça, Goveia, Lenzi, Graeff-Teixeira, Lima, Mota, Pecora, de Medeiros and Carvalho2007) of the country. Infected intermediate and definitive hosts are found in almost all regions of the country (Carvalho et al., Reference Carvalho, Scholte, de Mendonça, Passos and Caldeira2012; Moreira et al., Reference Moreira, Giese, Melo, Simões, Thiengo, Maldonado and Santos2013; Carvalho-Pereira et al., Reference Carvalho-Pereira, Souza, Santos, Walker, Pertile, de Oliveira, Pedra, Minter, Rodrigues, Bahiense, Reis, Diggle, Ko, Childs, da Silva, Begon and Costa2018).
The transmission cycle involves gastropods as the intermediate host of A. cantonensis and rodents as definitive hosts. Infected rodents release first-stage larvae in their feces which then infect molluscs by ingestion or, perhaps less importantly, by penetrating the body wall or the respiratory pore (Thiengo et al., Reference Thiengo, de Oliveira Simões, Fernandez and Júnior2013). The larvae then develop to the third stage within the molluscs and infect rodents when they ingest infected molluscs. In rats, these larvae develop to the subadult stage (L5) in the CNS, subsequently migrating to the pulmonary arteries, where they develop into adult nematodes, reproduce and complete the cycle (Thiengo et al., Reference Thiengo, de Oliveira Simões, Fernandez and Júnior2013).
In impoverished tropical urban areas, both rats and certain widespread gastropods thrive because of the highly favourable environmental conditions (Panti-May et al., Reference Panti-May, Carvalho-Pereira, Serrano, Pedra, Taylor, Pertile, Minter, Airam, Carvalho, Júnior, Rodrigues, Reis, Ko, Childs, Begon and Costa2016; Onyishi et al., Reference Onyishi, Aguzie, Okoro, Nwani, Ezenwaji, Oluah and Okafor2018). However, the relationship between specific environmental variables and gastropod abundance and diversity has not been studied in detail in this context. The life cycle of A. cantonensis can be maintained by a broad range of intermediate hosts (Kim et al., Reference Kim, Hayes, Yeung and Cowie2014) but the introduced (and invasive) snail species Achatina fulica Bowdich, 1822 and the slug Sarasinula marginata Semper, 1885 are the most commonly found intermediate host species in Brazil (Caldeira et al., Reference Caldeira, Mendonça, Goveia, Lenzi, Graeff-Teixeira, Lima, Mota, Pecora, de Medeiros and Carvalho2007; Carvalho et al., Reference Carvalho, Scholte, de Mendonça, Passos and Caldeira2012). The lack of adequate sanitation and refuse collection offers a habitat with plentiful food sources which enables definitive host populations for A. cantonensis – such as one of its main definitive hosts, Rattus norvegicus Berkenhout, 1769 (Valente et al., Reference Valente, Robles, Navone and Diaz2018) – to proliferate (Masi et al., Reference Masi, Pino, Santos, Genehr, Albuquerque, Bancher and Alves2010). This diversity of abundant host species is therefore likely to facilitate A. cantonensis infection in residents of impoverished urban areas already affected by several other zoonotic agents (Costa et al., Reference Costa, Porter, Rodrigues, Farias, de Faria, Wunder, Osikowicz, Kosoy, Reis, Ko and Childs2014; Carvalho-Pereira et al., Reference Carvalho-Pereira, Souza, Santos, Walker, Pertile, de Oliveira, Pedra, Minter, Rodrigues, Bahiense, Reis, Diggle, Ko, Childs, da Silva, Begon and Costa2018).
A recent study in an impoverished urban area of Brazil estimated that the local population of R. norvegicus had an A. cantonensis prevalence of around 40%, indicating that residents were at risk of exposure to this nematode (Carvalho-Pereira et al., Reference Carvalho-Pereira, Souza, Santos, Walker, Pertile, de Oliveira, Pedra, Minter, Rodrigues, Bahiense, Reis, Diggle, Ko, Childs, da Silva, Begon and Costa2018). However, information on the abundance and diversity of intermediate host species and the prevalence of A. cantonensis within these host populations is scarce for impoverished urban settings, complicating efforts to understand the risk of exposure in human populations. Consequently, the main objective of this study was to describe the occurrence and abundance of terrestrial gastropods present in a Brazilian impoverished urban area and to identify the associated environmental factors. Our secondary objective was to identify whether environmental and demographic factors, body condition or coinfection with other helminths were associated with the presence and intensity of A. cantonensis infection in R. norvegicus, using long-term monitoring data. This study seeks to identify factors facilitating the maintenance of this helminth's life cycle, which can be used to identify high-risk areas and to guide future targeting of public health interventions.
Materials and methods
Study area
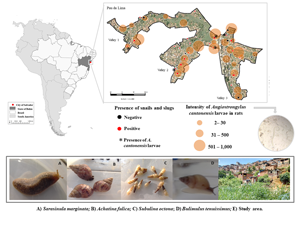
We conducted our study in the neighbourhood of Pau da Lima in the municipality of Salvador-Bahia (northeastern Brazil), in an area (0.17 km2) geographically stratified in three valleys, with approximately 3717 inhabitants (Panti-May et al., Reference Panti-May, Carvalho-Pereira, Serrano, Pedra, Taylor, Pertile, Minter, Airam, Carvalho, Júnior, Rodrigues, Reis, Ko, Childs, Begon and Costa2016) (Fig. 1). The community lives at the intersection of poverty and the environment, with poor-quality housing and inadequate sanitation infrastructure (Panti-May et al., Reference Panti-May, Carvalho-Pereira, Serrano, Pedra, Taylor, Pertile, Minter, Airam, Carvalho, Júnior, Rodrigues, Reis, Ko, Childs, Begon and Costa2016; Carvalho-Pereira et al., Reference Carvalho-Pereira, Souza, Santos, Walker, Pertile, de Oliveira, Pedra, Minter, Rodrigues, Bahiense, Reis, Diggle, Ko, Childs, da Silva, Begon and Costa2018). Transmission of other zoonotic diseases is well-documented within the community (Kikuti et al., Reference Kikuti, Cunha, Paploski, Kasper, Silva, Tavares, Cruz, Queiroz, Rodrigues, Santana, Lima, Calcagno, Takahashi, Gonçalves, Araújo, Gauthier, Diuk-Wasser, Kitron, Ko, Reis and Ribeiro2015; Hagan et al., Reference Hagan, Moraga, Costa, Capian, Ribeiro, Wunder, Felzemburgh, Reis, Nery, Santana, Fraga, Dos Santos, Santos, Queiroz, Tassinari, Carvalho, Reis, Diggle and Ko2016), for example, leptospirosis, a rodent-borne zoonosis had an incidence of 35.4 cases per 1000 people/year (Hagan et al., Reference Hagan, Moraga, Costa, Capian, Ribeiro, Wunder, Felzemburgh, Reis, Nery, Santana, Fraga, Dos Santos, Santos, Queiroz, Tassinari, Carvalho, Reis, Diggle and Ko2016).

Fig. 1. Distribution of the presence of molluscs and intensity of Angiostrongylus cantonensis in rats, in Brazil. (A) The northeastern region of Brazil, where the state of Bahia and the city of Salvador are located. (B) Study area, health district of Pau da Lima, which has an elevated topography divided into three geographic valleys. B also represents the distribution of the presence of terrestrial molluscs and the intensity of Angiostrongylus cantonensis found in rats. Angiostrongylus cantonensis larvae were confirmed in terrestrial gastropods by molecular tests.
Study design
Sampling sites were selected from 108 locations that had been established for a previous study in the area with a minimum distance between locations of 20 m (Panti-May et al., Reference Panti-May, Carvalho-Pereira, Serrano, Pedra, Taylor, Pertile, Minter, Airam, Carvalho, Júnior, Rodrigues, Reis, Ko, Childs, Begon and Costa2016). From these 108 locations, we randomly selected 40 gastropod sampling sites and 45 R. norvegicus sampling sites, 17 of which coincided, stratifying by the three valleys to ensure a similar number of sites in each valley. The sampling area for both taxa at each site consisted of a 15 m radius circle centred at the geotagged location.
Molluscs were sampled during a single sampling period between November 2016 and January 2017. Rat data were obtained from four sampling periods spanning 2014–2016: two periods in the rainy season (March–July 2014 and March–April 2016) and two in the dry season (October–December 2014 and November–December 2015).
Terrestrial gastropod sampling
Exhaustive mollusc sampling was performed by visual searching of the sampling sites, following the protocol developed by the Brazilian Ministry of Health (Ministério da Saúde, 2008). Sampling was carried out in the early morning (08:00–10:00) because the molluscs are more active and exposed during this time of day. The individuals collected were taken to the laboratory, where they were then identified using morphological characteristics, based on the literature (Salgado and dos Santos Coelho, Reference Salgado and dos Santos Coelho2003; Simone, Reference Simone2006; Colley, Reference Colley2012; Breure and Ablett, Reference Breure and Ablett2014).
Processing of terrestrial mollusc samples and larval extraction
At each site, gastropods of the same species with a length of <2 cm were pooled for analysis (Carvalho et al., Reference Carvalho, Scholte, de Mendonça, Passos and Caldeira2012), while individuals >2 cm were dissected and analysed separately. Gastropods from each site were processed separately. The animals (or pools) were macerated and digested following Wallace and Rosen (Reference Wallace and Rosen1969), and adaptations of the technique of Rugai et al. (Reference Rugai, Mattos and Brisola1954) for faecal samples, as it follows: the mollusc macerate was divided into portions of approximately 3 g, laid in six layers of gauze and distributed in sedimentation cones, for sedimentation with pepsin/HCl solution (4% pepsin 0.7% hydrochloric acid, in de-chlorinated water) at 42°C; then, the cones were incubated at 37°C for 2 h; after incubation, the sediment in the sediment cones was aspirated using a pipette, transferred to a Petri dish and observed under a binocular microscope at 40×. All larvae found were counted and fixed in 70% ethanol, with a subsample stored at −20°C for molecular analysis by PCR-RFLP (polymerase chain reaction-restriction fragment length polymorphism).
Morphometrics and identification of A. cantonensis larvae by PCR-RFLP
Larvae with metastrongyloid characteristics [based on their morphology, mainly the tail conformation (Ash, Reference Ash1970)] were identified, with 10% randomly selected for morphometric analysis. The following measurements were taken for each specimen: total body length, length of oesophagus, distance between excretory pore and anterior extremity, distance between genital primordium and tail, length of genital primordium and distance between anus and tail. The measurements were compared with the measurements from reference specimens of A. cantonensis and A. costaricensis, in Instituto Adolfo Lutz (IAL) by calculating the percentage of similar samples. All samples were sent for molecular confirmation (PCR-RFLP) of species identification as A. cantonensis, A. costaricensis or A. vasorum, following the method of Caldeira et al. (Reference Caldeira, Carvalho, Mendonça, Graeff-Teixeira, Silva, Ben, Maurer, Lima and Lenzi2003).
Rat sampling and determination of A. cantonensis infection
Rats were captured using live rat-trapping, with two traps left at each location for 24 h on two consecutive days following previously established protocols (Panti-May et al., Reference Panti-May, Carvalho-Pereira, Serrano, Pedra, Taylor, Pertile, Minter, Airam, Carvalho, Júnior, Rodrigues, Reis, Ko, Childs, Begon and Costa2016; Carvalho-Pereira et al., Reference Carvalho-Pereira, Souza, Santos, Walker, Pertile, de Oliveira, Pedra, Minter, Rodrigues, Bahiense, Reis, Diggle, Ko, Childs, da Silva, Begon and Costa2018).
Demographic data (sex, age, reproductive status) and body condition [presence and severity of wounds and amount of fat deposits, as well as the Scaled Mass Index (SMI) (Peig and Green, Reference Peig and Green2009) – which accounts for the effect of age] were recorded.
Feces were collected directly from the large intestine and stored in 10% formaldehyde for subsequent identification of first-stage larvae of A. cantonensis following the protocol of Carvalho-Pereira et al. (Reference Carvalho-Pereira, Souza, Santos, Walker, Pertile, de Oliveira, Pedra, Minter, Rodrigues, Bahiense, Reis, Diggle, Ko, Childs, da Silva, Begon and Costa2018). Faecal sample analyses consisted of a qualitative stage followed by a quantitative stage. During the qualitative stage, we employed an adapted sedimentation method (Hoffman et al., Reference Hoffman, Pons and Janer1934), with larval identification based on morphological characteristics observed under light microscopy, with probable metastrongyloid nematodes indicated by observing the tail conformation, a particular characteristic of this group (Anderson, Reference Anderson1978). We also identified eggs of other parasites based on the morphology described in the literature (Vicente et al., Reference Vicente, Rodrigues, Gomes and Pinto1997). To confirm the species of identified parasite, we also searched for adult worms by collecting target tissues from the rodents captured during one of the sampling periods. Samples were taken from the intestines, stomach, heart and lungs, fixated in formalin–acetic acid–alcohol at 50°C and stored at 4°C. This allowed for the confirmation of occurrence of adults of A. cantonensis in captured R. norvegicus pulmonary arteries, as well as the other helminth species included in the study (see Additional file 1: Table S2). For the quantitative analysis, larvae per gram of feces were counted for A. cantonensis and eggs per gram of feces were counted for all other helminths. This was conducted using an adapted flotation method (Gordon et al., Reference Gordon and Whitlock1939), using a saturated zinc sulphate solution with a density of 1.18 g cm−3 (Faust et al., Reference Faust, D'Antoni, Odom, Miller, Peres, Sawitz, Thomen, Tobie and Walker1938). Co-infection variables were created as the intensity of infection for each parasite species.
Environmental variables
Environmental variables were selected for the survey by considering factors associated with mollusc abundance that reflects their physiological and ecological needs (Nunes and Santos, Reference Nunes and Santos2012). At all mollusc and rat sampling locations, environmental data were recorded. We observed data on the presence of food access, garbage access, rubbish, construction material, open sewage and water (standing or running). Variables related to habitat were also collected: type of ground (permeable/impermeable) and vegetation (absent/herbaceous/shrubbery). Additionally, during the capture of rodents, we recorded whether food and harbour sites for rats (pet food, human garbage, water sources and vegetation coverage by number of trees) were present within a radius of 10 m of the site. Additional information including the valley in which the samples were collected, the number of rat collection events and mollusc collection days (sampling effort: 2 or 3 days by two field technicians) was included as variables in this study. Daily pluviometry data were obtained from the station in the study area, managed by the Institute of Environment and Hydric Resources of Bahia (INEMA).
Statistical analysis
The occurrence and abundance of mollusc species at each study site were determined, as was the prevalence and intensity (number of larvae) of A. cantonensis in each mollusc species identified. The prevalence of A. cantonensis in rats was then estimated for each sampling period, with comparisons between periods made by χ2 tests.
To model the association between gastropod abundance and environmental factors, we used zero-inflated negative binomial models using the R function (R Core Team, 2013) pscl (Loeys et al., Reference Loeys, Moerkerke, De Smet and Buysse2012) to account for zero-inflation in the data. This analysis was performed separately for each gastropod species. Univariate analyses were performed to explore the relationship between each environmental variable and the abundance outcome, with variables statistically significant at the P ⩽ 0.1 level included in the final multivariate model for each species. To account for the difference in the sampling effort between sites, the mollusc collection days was included in the models as a fixed effect.
To identify factors associated with the intensity of A. cantonensis in rats, we conducted a model selection process for the three groups of (i) environmental, (ii) demographic and (iii) co-infection variables separately (see Additional file 1: Table S2). We fitted a zero-inflated generalized linear model with a Gamma error distribution to the intensity response variable, transformed to the base 2 logarithm to facilitate the interpretation of results (Carvalho-Pereira et al., Reference Carvalho-Pereira, Souza, Santos, Walker, Pertile, de Oliveira, Pedra, Minter, Rodrigues, Bahiense, Reis, Diggle, Ko, Childs, da Silva, Begon and Costa2018). Modelling followed three steps: (1) univariate analysis of the environmental variables with variables included in the multivariate model if P ⩽ 0.1; (2) then each rat demography and body condition variable was added one at a time into the multivariate environmental model and the same process of variable selection was repeated; (3) finally, each helminth co-infection intensity variable was added into the multivariate model selected in (2) and the same process was repeated for these variables. Final models of each step were defined for terrestrial molluscs and rats using model selection criteria based on Akaike's Information Criterion corrected for small samples (AICc) (Zuur and Ieno, Reference Zuur and Ieno2016), with the help of the MuMIn package, which ranks models by AICc based on the importance of each explanatory variable included (Bartón, Reference Bartón2013). Thus, it was possible to identify all plausible models (ΔAICc ⩽ 2.00) and the most parsimonious, this being the model with the fewest explanatory variables among the plausible models.
Results
We collected 577 gastropods, widely distributed in all valleys of the area studied, representing eight species from seven families. The most common species were Subulina octona (47.5%), S. marginata (30%), A. fulica (25%) and Bulimulus tenuissimus (22.5%) with the co-occurrence of the four species common in the sampled areas. Only these four species were found to be infected with A. cantonensis. Bradybaena similaris, Drymaeus papyraceus, Helicina sp. and a representative of the family Streptaxidae occurred at fewer sites and in lower abundance, with an average of five individuals per occurrence site.
The prevalence of A. cantonensis was 33, 11, 8 and 5% in A. fulica, B. tenuissimus, S. marginata and S. octona, respectively. Achatina fulica exhibited the highest number of larvae per individual, with an average of 22.7 ± 22.5. The remaining species exhibited lower levels of infection, with only one individual of S. marginata infected, with four larvae. Bulimulus tenuissimus and S. octona, predominantly analysed in pooled samples, exhibited the means of 0.5 and 0.3 larvae per individual, respectively.
Occurrence and abundance of mollusc species in the study area were associated with several local environmental variables, with the presence of water an important factor (see Additional file 1: Tables S3 and S4). Humidity and accumulated rain exhibited positive associations with the abundance of A. fulica and S. octona, respectively. The presence of construction materials was positively correlated with the number of S. marginata, which was significantly less abundant in valleys 2 and 3 compared to valley 1. In the univariate models (see Additional file 1: Table S3), the presence of herbaceous vegetation was positively correlated with the abundance of S. octona. On the other hand, areas with shrub vegetation exhibited higher B. tenuissimus numbers. The model including solely sampling effort was significantly associated with the presence of B. tenuissimus, indicating that a 3-day sampling effort was associated with a higher chance of finding individuals of B. tenuissimus, compared to 2-day efforts (Table 1). Only seven of the gastropod samples were infected with A. cantonensis. However, the infected samples occurred among four species (A. fulica, B. tenuissimus, S. marginata and S. octona) and in five sampling sites. Given the small number of infected gastropod samples, analysis of the risk factors associated with the presence and intensity of infection by A. cantonensis in the gastropod species was disregarded. All 17 sites sampled for both rats and molluscs were positive for both taxa. In 45% of these sites, rats were positive for A. cantonensis (Fig. 1B).
Table 1. Final zero-inflated negative binomial regression models for terrestrial molluscs

IRR, incidence rate ratios; CI, confidence intervals; Sig., significance codes: 0; ‘***’ 0.001; ‘**’ 0.01; ‘*’ 0.05; ‘#’ 0.1; ‘ ’ 1. The significative variables are shown in bold.
In total, 168 R. norvegicus were trapped during the four sampling periods. Among them, sub-adults (103; 61.3%) were captured more frequently than the young (35; 20.8%) and adults (30; 17.9%), with more females (90; 53.9%) than males (77; 46.1%); and a similar number of captures in dry (85; 50.6%) and rainy seasons (83; 49.4%). First-stage A. cantonensis larvae were detected in 56 (33.3%) individuals, presenting an average of 90.00 (11.00) (Fig. 1) and with no significant difference between sampling periods. In addition to A. cantonensis, another seven helminth species already recorded by Carvalho-Pereira et al. (Reference Carvalho-Pereira, Souza, Santos, Walker, Pertile, de Oliveira, Pedra, Minter, Rodrigues, Bahiense, Reis, Diggle, Ko, Childs, da Silva, Begon and Costa2018) were identified. Of these, the soil-transmitted nematode Strongyloides spp. (160; 95%) and Nippostrongylus brasiliensis (52; 30.9%) were the most prevalent.
Accumulated rainfall in the previous 2 weeks, as well as the presence of construction materials, was positively associated with a higher prevalence of A. cantonensis in R. norvegicus (Table 2). These relations were maintained when controlling for demographic and body condition variables (Table 2 – step 2; accumulated rain: rate 1.000, IC 95% 0.999–1.001; construction materials: 1.333, IC 95% 1.056–1.693). In this model, rat body condition (SMI) was significantly negatively associated with the probability of infection by A. cantonensis. Contrary to what was expected, however, the increase of age (in days) was negatively associated with infection intensity (Table 2). The final model, including coinfection variables, retained body condition as the sole significantly associated factor linked to the probability of infection. However, in terms of A. cantonensis infection intensity in rats, besides the accumulated rain, which was positively associated with greater A. cantonensis intensity, the intensity of A. cantonensis exhibited a negative association with that of N. brasiliensis and a positive association with that of Strongyloides spp.
Table 2. Final zero-inflated regression models with Gamma family for A. cantonensis infection in Rattus norvegicus

IRR, incidence rate ratios; CI, confidence intervals; Db, ‘double’; Sig., significance codes: 0; ‘***’ 0.001; ‘**’ 0.01; ‘*’ 0.05; ‘#’ 0.1; ‘ ’ 1. The significative variables are shown in bold.
Discussion
This study demonstrated that in the impoverished urban area, studied environmental conditions that are correlated with the occurrence of terrestrial gastropods (intermediate hosts) are also indicators that rats (definitive hosts) are infected with A. cantonensis. The transmission cycle of A. cantonensis can be established and maintained in these contexts because these environmental conditions (available water and accumulation of inorganic matter such as construction materials) create microhabitats that favour both mollusc and rat abundance, with molluscs a food source for rats within these shared environments. The occurrence of several mollusc species in most of the studied area and lack of seasonality in rat prevalence across the two study years suggest that transmission of A. cantonensis is stable within the study area. This demonstrates the need for preventive action given the severity of human neuroangiostrongyliasis, in spite of the current lack of recorded cases of the disease in the community. This may, in turn, be a result of misdiagnosis because the main symptoms (e.g. headache, pain in the neck and fever) (Morassutti et al., Reference Morassutti, Thiengo, Fernandez, Sawanyawisuth and Graeff-Teixeira2014) can be easily mistaken for other illnesses that co-occur in impoverished urban areas with abundant rats and molluscs.
Many mollusc species have been reported as susceptible to A. cantonensis infection and capable of maintaining transmission (Kim et al., Reference Kim, Hayes, Yeung and Cowie2014). However, usually only a few species are considered as the primary intermediate hosts in a given region (Wang et al., Reference Wang, Lai, Zhu, Chen and Lun2008). The presence of the species S. octona, A. fulica, S. marginata, B. tenuissimus and B. similaris (all characterized as A. cantonensis hosts in the literature) may indicate the occurrence of more contamination foci, given that these species can occupy different habitats. This is the first record of B. tenuissimus as a natural intermediate host of A. cantonensis in the state of Bahia. Bradybaena similaris, although previously reported as a host in Brazil (Carvalho et al., Reference Carvalho, Scholte, de Mendonça, Passos and Caldeira2012), was not positive for A. cantonensis infection in this study, which is consistent with the findings of Kim et al. (Reference Kim, Hayes, Yeung and Cowie2014). Overall, the study demonstrated a diversity of positive intermediate host species and that the co-occurrence of more than one species infected by A. cantonensis in a sampling site is common. This could favour the development of larvae to the infective phase, which could promote continuous dissemination.
We observed in this study similarity in the distribution of highly infected rats and of infected molluscs, although geospatial analyses are still needed. Other characteristics of each study valley that were not measured could also contribute to the uneven distribution of mollusc species. For example, compared to valleys 2 and 3, valley 1 exhibited (based on unquantified observation) more poor sanitary conditions and more potential faunal refuges (e.g. construction material), which could contribute to the mollusc's abundance in the environment. Achatina fulica, a generalist species, was the most abundant and an important host of A. cantonensis, as found in other studies (Barratt et al., Reference Barratt, Chan, Sandaradura, Malik, Spielman, Lee, Marriott, Harkness, Ellis and Stark2016; Thiengo et al., Reference Thiengo, Maldonado, Mota, Torres, Caldeira, Carvalho, Oliveira, Simões, Fernandez and Lanfredi2010). The abundance of A. fulica may be due to it being a generalist invasive species in Brazil. Humid habitats may contribute to its increased abundance (Miranda et al., Reference Miranda, Fontenelle and Pecora2015), which may increase the chances of infection by A. cantonensis.
The prevalence of A. cantonensis in our samples of the R. norvegicus in our study (33% of 116 specimens) was moderate, similar to a previous study in the area (Carvalho-Pereira et al., Reference Carvalho-Pereira, Souza, Santos, Walker, Pertile, de Oliveira, Pedra, Minter, Rodrigues, Bahiense, Reis, Diggle, Ko, Childs, da Silva, Begon and Costa2018), but additionally stable among seasons. Other studies in Brazil recorded prevalences of 11% of 19 rats in the state of Pará (Moreira et al., Reference Moreira, Giese, Melo, Simões, Thiengo, Maldonado and Santos2013) and of 71% of 114 rats in the state of Rio de Janeiro (Simões et al., Reference Simões, Júnior, Olifiers, Garcia, Bertolino and Luque2014). Stable prevalences between seasons indicate that there may be continuous contamination of the environment with A. cantonensis through rat feces, and potential year-round transmission, similar to the results of Simões et al. (Reference Simões, Júnior, Olifiers, Garcia, Bertolino and Luque2014) – which considered a larger sampling scale. Both regions of Bahia and Rio de Janeiro have tropical climates, with limited variation in temperature and rainfall throughout the year (Climate-Data.org), which may explain both the prevalences found and their stability between the dry and rainy seasons.
The majority of relationships found in the demographic and body condition analysis were expected (Carvalho-Pereira et al., Reference Carvalho-Pereira, Souza, Santos, Walker, Pertile, de Oliveira, Pedra, Minter, Rodrigues, Bahiense, Reis, Diggle, Ko, Childs, da Silva, Begon and Costa2018, Reference Carvalho-Pereira, Souza, Santos, Pedra, Minter, Bahiense, Reis, Ko, Childs, Silva, Costa and Begon2019). However with regard to the association with age, as the present study used cross-sectional data, a possibility is that older rats had been previously exposed to the nematode, and, consequently, had developed the capacity to modulate the parasite load when re-infected, thus exhibiting a lower infection rate (Au and Ko, Reference Au and Ko1979). In terms of coinfection associations, there was a different pattern than that previously found given that, when analysing rats stratified by sex, females presented with a positive association between the intensity of N. brasilienses and A. cantonenis (see Carvalho-Pereira et al., Reference Carvalho-Pereira, Souza, Santos, Pedra, Minter, Bahiense, Reis, Ko, Childs, Silva, Costa and Begon2019). This may be because pregnant or lactating females allocate fewer resources to fending off parasites, thus being more prone to infections and coinfections (Spickett et al., Reference Spickett, Junker, Krasnov, Haukisalmi and Matthee2017; Castillo and Paller, Reference Castillo and Paller2018).
This study was carried out in an impoverished urban community, which is an environment characterized by precarious sanitation services, a lack of urban infrastructure such as pavement, and the presence of points with rubbish containing construction materials and trash. Although the accumulation of construction materials was not retained in the final model for A. cantonensis infection in rats, this was considered a factor associated with gastropod abundance, probably by fostering the formation of preferred shelter. The absence of pavement was not identified as a risk factor for rat infection or for the abundance of molluscs, despite it being previously described as an important factor for the establishment of rodent populations, given their habit of digging burrows in soil (Cavia et al., Reference Cavia, Cueto and Suárez2009). The absence of pavement can also contribute to the accumulation of rainwater in the soil, a risk factor detected by our study, which may explain why accumulated rain of the previous 2 weeks was an important predictor of infection intensity by A. cantonensis in rats and abundance of molluscs. This is likely to be because it creates humid, favourable environments for the molluscs. This could be related to a potential contribution of this phenomenon to the viability of A. cantonensis larvae in the environment and may favour infection in intermediate hosts (by contact with water contaminated with rodent feces) and rats (by ingestion of contaminated water or infected molluscs).
The integration of ecological mollusc and rodent data may be the key to understanding the establishment and transmission of A. cantonensis in human populations. The variation in environmental conditions within the urban landscape, especially in areas with precarious sanitation, poor living and infrastructure conditions, may facilitate the existence of heterogeneous microhabitats (Sólymos et al., Reference Sólymos, Farkas, Kemencei, Páll-Gergely, Vilisics, Nagy, Kisfali and Hornung2009; Astor et al., Reference Astor, Proschwitz, Strengbom, Berg and Bengtsson2017) affecting the composition, abundance and distribution of the terrestrial mollusc assemblage. Microhabitats that favour an elevated abundance of molluscs may contribute to an increase in the invertebrate hosts' contact with the parasite, and consequently increase the number of infected rats. In contrast, if environmental changes occur, the equilibrium of the microhabitats may be upset, affecting the target animal populations (Feng and Himsworth, Reference Feng and Himsworth2014), and probably resulting in reduced A. cantonensis transmission. This highlights the importance of carrying out structural and sanitation interventions to help prevent the transmission of A. cantonensis between hosts and to the people of the area. Local and short-term measures (e.g. cleaning yards and empty lots, reduction of construction material) and long-term actions (e.g. structural urban interventions that reduce water accumulation in the environment, such as street paving) may disrupt the parasite cycle already established in areas lacking urban infrastructure and with poor sanitary conditions.
One potential limitation of this study is the time interval between the collection of rat and mollusc data. However, we believe that this will have had a limited impact on our results because rat abundance did not vary seasonally (Panti-May et al., Reference Panti-May, Carvalho-Pereira, Serrano, Pedra, Taylor, Pertile, Minter, Airam, Carvalho, Júnior, Rodrigues, Reis, Ko, Childs, Begon and Costa2016). Additionally, other studies suggest that mollusc abundance does not vary through time in the tropical urban context (Oliveira et al., Reference Oliveira, Gentile, Maldonado Júnior, Lopes Torres and Thiengo2015). Another factor that could have limited the identification of environmental factors associated with the mollusc species – among the ones defined in this study – is that some of the collected species are generalists, occupying several habitats that vary from humid to dry and hot, while others may depend on specific variables that were not accounted for in this study (Hylander et al., Reference Hylander, Nilsson, Jonsson and Göthner2005; Dias et al., Reference Dias, Bessa and D’Ávila2007; Nunes and Santos, Reference Nunes and Santos2012).
In conclusion, this study indicates that the transmission cycle of the rat lungworm, A. cantonensis, is well established in an impoverished urban community in Brazil with evidence of its presence in definitive hosts and five species of intermediate hosts. Overall, our findings indicate the necessity for the involvement of government entities in the execution of structural and sanitation interventions, as well as the involvement of public health and zoonoses control agencies that can inform and guide residents with educational and participative actions regarding the risks associated with A. cantonensis and the potential of molluscs and rats as hosts of an array of zoonotic parasites. Thus, not only would neuroangiostrongyliasis, but also other relevant zoonosis, such as leptospirosis (transmitted by rat urine) and schistosomiasis (transmitted by snails) be prevented.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182021000597
Acknowledgements
We thank the residents of the Pau da Lima community, Salvador, Bahia, Brazil, and anonymous reviewers for their valuable recommendations.
Financial support
This research was supported by projects funded by the Oswaldo Cruz Foundation and Secretariat of Health Surveillance, Brazilian Ministry of Health; the Federal University of Bahia; the National Institutes of Health of the United States (grant numbers F31 AI114245, R01 AI052473, U01 AI088752, R01 TW009504 and R25 TW009338); Medical Research Council (MR/P024084/1 and MR/T029781/1); and by the Wellcome Trust (102330/Z/13/Z and 218987/Z/19/Z). F.N.S. and C.G.Z. participated in this study under a FAPESB doctorate scholarship.
Conflict of interest
None.
Ethical standards
The rodent sampling component of the study used protocol 003/2012, which was approved by the Ethical Committee of the Animal Use (CEUA), of the Gonçalo Moniz Institute (IGM) – Oswaldo Cruz Foundation (Fiocruz). The license for the collection of terrestrial molluscs in an urban area of Salvador-Bahia was provided by the Chico Mendes Institute for Biodiversity Conservation (ICMBio), a federal agency linked to the Brazilian Ministry of the Environment. None of the specimens collected belong to threatened or protected species.