INTRODUCTION
Malaria remains one of the largest global health threats, with the greatest burden of mortality and morbidity in developing countries of tropical and sub-tropical regions. Despite considerable efforts to control the disease and block its spread, 3·3 billion people remained at risk of contracting malaria in 2010, resulting in an estimated 660 000 deaths (World Health Organization (WHO), 2015), primarily among young children and women in their first pregnancy. The difficulties in eliminating malaria with currently available tools (Griffin et al. Reference Griffin, Hollingsworth, Okell, Churcher, White, Hinsley, Bousema, Drakeley, Ferguson, Basanez and Ghani2010) and the emergence of artemisinin resistance in Southeast Asia (Dondorp et al. Reference Dondorp, Nosten, Yi, Das, Phyo, Tarning, Lwin, Ariey, Hanpithakpong, Lee, Ringwald, Silamut, Imwong, Chotivanich, Lim, Herdman, An, Yeung, Singhasivanon, Day, Lindegardh, Socheat and White2009; Mbengue et al. Reference Mbengue, Bhattacharjee, Pandharkar, Liu, Estiu, Stahelin, Rizk, Njimoh, Ryan, Chotivanich, Nguon, Ghorbal, Lopez-Rubio, Pfrender, Emrich, Mohandas, Dondorp, Wiest and Haldar2015) highlight the need for interventions that specifically aim to reduce the transmission of the parasites responsible for the disease.
Gametocytes are the highly specialized form of Plasmodium parasites that are infectious to mosquitoes and thus required for successful transmission from humans to mosquitoes. Their formation and infectivity to mosquitoes is presented in Fig. 1. Plasmodium falciparum gametocytes progress through 5 distinct morphological stages, until they reach the specific elongated crescent shape characteristic of falciparum malaria. Male and female parasites are independently differentiated but genetically identical haploid stages that originate from asexual precursor stages; current evidence favours a model where one schizont gives rise to either male or female gametocytes (Smith et al. Reference Smith, Lourenco, Carter, Walliker and Ranford-Cartwright2000; Sinha et al. Reference Sinha, Hughes, Modrzynska, Otto, Pfander, Dickens, Religa, Bushell, Graham, Cameron, Kafsack, Williams, Llinas, Berriman, Billker and Waters2014). The only gametocytes that are observed in peripheral blood are sexually committed ring-stage parasites and fully mature stage V gametocytes (Schneider et al. Reference Schneider, Schoone, Schallig, Verhage, Telgt, Eling and Sauerwein2004; Pelle et al. Reference Pelle, Oh, Buchholz, Narasimhan, Joice, Milner, Brancucci, Ma, Voss, Ketman, Seydel, Taylor, Barteneva, Huttenhower and Marti2015). Intermediate stages II–IV are sequestered mainly in bone marrow compartments (Farfour et al. Reference Farfour, Charlotte, Settegrana, Miyara and Buffet2012; Aguilar et al. Reference Aguilar, Moraleda, Achtman, Mayor, Quinto, Cistero, Nhabomba, Macete, Schofield, Alonso and Menendez2014b; Joice et al. Reference Joice, Nilsson, Montgomery, Dankwa, Egan, Morahan, Seydel, Bertuccini, Alano, Williamson, Duraisingh, Taylor, Milner and Marti2014). Although no genomic replication is present in gametocytes and the digestion of haemoglobin ceases after 6 days of development, gametocyte-specific mRNA is produced and a subset of transcripts is translationally repressed until gametocytes are taken up in the mosquito blood meal and rapidly transform into gametes (Mair et al. Reference Mair, Braks, Garver, Wiegant, Hall, Dirks, Khan, Dimopoulos, Janse and Waters2006, Reference Mair, Lasonder, Garver, Franke-Fayard, Carret, Wiegant, Dirks, Dimopoulos, Janse and Waters2010). The reduction in temperature, rise in pH and exposure to xanthurenic acid inside the mosquito induces gametogenesis, initiating activation and expression of stage-specific proteins (Billker et al. Reference Billker, Shaw, Margos and Sinden1997, Reference Billker, Lindo, Panico, Etienne, Paxton, Dell, Rogers, Sinden and Morris1998). After 3 rounds of DNA replication, male gametocytes exflagellate to release 8 motile microgametes from the host cell that are targeted at the fertilization of female macrogametes (Sinden, Reference Sinden1983a,Reference Sindenb). The resulting zygotes elongate to form ookinetes that traverse the mosquito midgut and develop into oocysts. Sporozoites then develop within oocysts and, approximately 10 days after the blood meal, rupture the oocyst capsule and render the mosquito infectious by invading the insect's salivary glands, completing the cycle of transmission (Vaughan, Reference Vaughan2007).

Fig. 1. Maturation and location of Plasmodium falciparum transmission stage parasites. Timings are given cumulatively as the time since gametocyte formation, or where indicated as time post mosquito infection (PI), and as the stage-specific exposure time in brackets.
Transmission of malaria parasites from humans to mosquitoes is highly effective, with infectious humans capable of giving rise to >100 secondary human infections (Smith et al. Reference Smith, McKenzie, Snow and Hay2007). However, many factors influence the infectivity of gametocytes, and their transmission potential (Bousema and Drakeley, Reference Bousema and Drakeley2011; Stone et al. Reference Stone, Goncalves, Bousema and Drakeley2015). Gametocyte prevalence and density are highest in infants and young children (Shute and Maryon, Reference Shute and Maryon1951), decreasing with age in parallel with cumulative exposure to malaria infection and acquired immunity to the parasite's asexual stages (Doolan et al. Reference Doolan, Dobaño and Baird2009; Ouedraogo et al. Reference Ouedraogo, Bousema, de Vlas, Cuzin-Ouattara, Verhave, Drakeley, Luty and Sauerwein2010). Though once thought to be rare, molecular detection methods have revealed that gametocytes are produced by the majority of infected individuals of all ages (Schneider et al. Reference Schneider, Bousema, Omar, Gouagna, Sawa, Schallig and Sauerwein2006; Shekalaghe et al. Reference Shekalaghe, Teun Bousema, Kunei, Lushino, Masokoto, Wolters, Mwakalinga, Mosha, Sauerwein and Drakeley2007; Bousema et al. Reference Bousema, Okell, Shekalaghe, Griffin, Omar, Sawa, Sutherland, Sauerwein, Ghani and Drakeley2010a; Bousema and Drakeley, Reference Bousema and Drakeley2011; Joice et al. Reference Joice, Narasimhan, Montgomery, Sidhu, Oh, Meyer, Pierre-Louis, Seydel, Milner, Williamson, Wiegand, Ndiaye, Daily, Wirth, Taylor, Huttenhower and Marti2013; Bousema et al. Reference Bousema, Okell, Felger and Drakeley2014) and that onward malaria transmission is not restricted to microscopically detectable gametocyte densities (Schneider et al. Reference Schneider, Bousema, Gouagna, Otieno, van de Vegte-Bolmer, Omar and Sauerwein2007; Ouedraogo et al. Reference Ouedraogo, Bousema, Schneider, de Vlas, Ilboudo-Sanogo, Cuzin-Ouattara, Nebie, Roeffen, Verhave, Luty and Sauerwein2009; Beshir et al. Reference Beshir, Sutherland, Sawa, Drakeley, Okell, Mweresa, Omar, Shekalaghe, Kaur, Ndaro, Chilongola, Schallig, Sauerwein, Hallett and Bousema2013; Gaye et al. Reference Gaye, Bousema, Libasse, Ndiath, Konate, Jawara, Faye and Sokhna2015). In addition to gametocyte maturity and sex ratio, human immune factors targeting the sexual stages of the parasite also highly influence transmission efficiency (Bousema and Drakeley, Reference Bousema and Drakeley2011).
Here, we review the evidence for naturally occurring human immunity targeted to the transmission stages of Plasmodium parasites, discuss hypotheses for the mechanisms of this immunity, and suggest future research directions that may aid the design of transmission reducing interventions.
Immune responses to transmission stage parasites
Transmission reducing immunity (TRI) can be directed against numerous targets during gametocyte formation, maturation and transmission. Within the human host, a humoral response targeting parasite-specific epitopes on the gametocyte-infected erythrocyte (GIE) surface of different developmental stages could contribute to a decreased transmission capacity. As erythrocytes do not express major histocompatibility complex (MHC) molecules and consequently lack organelles required for antigen and MHC class I complex processing, presentation to and activation of CD8 + T cells is thought to play a minor role in immune responses to the red blood cell (RBC) stages of the parasite, including gametocytes (Bousema and Drakeley, Reference Bousema and Drakeley2011). Phagocytosis by monocytes and neutrophils may provide a protective immune mechanism against schizont- and merozoite-infected erythrocytes (Bouharoun-Tayoun et al. Reference Bouharoun-Tayoun, Oeuvray, Lunel and Druilhe1995; McGilvray et al. Reference McGilvray, Serghides, Kapus, Rotstein and Kain2000), and though there is evidence of early stage GIE phagocytosis in vitro (Smith et al. Reference Smith, Serghides, Patel, Febbraio, Silverstein and Kain2003), evidence is limited for a functional role in vivo (Sinden and Smalley, Reference Sinden and Smalley1976; Healer et al. Reference Healer, Graszynski and Riley1999a). Infected RBCs may, however, be sensitive to cytokines released during general inflammatory responses. During periods of peak parasitaemia, non-specific inflammatory responses may kill both asexual blood stage parasites and circulating gametocytes, resulting in substantially reduced infectivity (Naotunne et al. Reference Naotunne, Karunaweera, Del Giudice, Kularatne, Grau, Carter and Mendis1991; Karunaweera et al. Reference Karunaweera, Carter, Grau, Kwiatkowski, Del Giudice and Mendis1992). Clinical immunity to human malaria is associated with reduced induction of cytokines and complementary parasite killing factors (Karunaweera et al. Reference Karunaweera, Carter, Grau, Kwiatkowski, Del Giudice and Mendis1992; Naotunne et al. Reference Naotunne, Karunaweera, Mendis and Carter1993), but there is no evidence that such factors have activity in the mosquito midgut. By comparison, there is substantial evidence that naturally acquired humoral responses to gametocyte proteins play a role in determining transmission efficiency.
Early stage gametocyte infected erythrocyte surface antigens
Early stage gametocytes of P. falciparum (stage I–IV) sequester primarily in the bone marrow (Joice et al. Reference Joice, Nilsson, Montgomery, Dankwa, Egan, Morahan, Seydel, Bertuccini, Alano, Williamson, Duraisingh, Taylor, Milner and Marti2014). Previously, it has been assumed that developing gametocytes are not recognized by the immune system, but more recent evidence provides a rationale for the existence of immunogenic proteins on the GIE surface. Sutherland et al. proposed the involvement of immunogenic gametocyte proteins in GIE adhesion to microvasculature, as well as spatial and temporal signal transduction, in the human host (Sutherland, Reference Sutherland2009). For example, parasite sensing of signals via host receptors or via non-specific permeability pathways could induce changes in the host RBC erythrocyte adhesive phenotype and thus affect the timing of gametocyte release into circulation. In a Thai cohort, naturally acquired antibody responses reduced transmission to mosquitoes and distorted the morphology and maturation of young gametocytes (Tonwong et al. Reference Tonwong, Sattabongkot, Tsuboi, Iriko, Takeo, Sirichaisinthop and Udomsangpetch2012). Some of these plasma antibodies also bound the GIE surface from gametocyte stage II onwards, suggesting that host antibodies can target early gametocytes, and supporting the need for similar studies in other patient cohorts. Initial studies into gametocyte sequestration suggested that gametocytes participate in adhesive interactions with host cell receptors, and therefore adhesins are likely expressed on the GIE surface. Adhesive properties of GIEs to C32 melanoma cells and transformed human bone marrow endothelial cells (trHBMEC) were shown to only partly overlap with those of asexual parasites, indicating gametocyte-specific sequestration mechanisms (Hayward et al. Reference Hayward, Tiwari, Piper, Baruch and Day1999; Rogers et al. Reference Rogers, Hall, Obiero, Targett and Sutherland2000). Recent studies detected limited adhesion of gametocytes towards various endothelial cell types (Silvestrini et al. Reference Silvestrini, Tiburcio, Bertuccini and Alano2012). Building on recent evidence that gametocytes are enriched in the bone marrow parenchyma (Farfour et al. Reference Farfour, Charlotte, Settegrana, Miyara and Buffet2012; Aguilar et al. Reference Aguilar, Magallon-Tejada, Achtman, Moraleda, Joice, Cistero, Li Wai Suen, Nhabomba, Macete, Mueller, Marti, Alonso, Menendez, Schofield and Mayor2014a,Reference Aguilar, Moraleda, Achtman, Mayor, Quinto, Cistero, Nhabomba, Macete, Schofield, Alonso and Menendezb Joice et al. Reference Joice, Nilsson, Montgomery, Dankwa, Egan, Morahan, Seydel, Bertuccini, Alano, Williamson, Duraisingh, Taylor, Milner and Marti2014), Joice et al. showed that the majority of bone marrow gametocytes in a cerebral malaria patient cohort localized specifically at erythroblastic islands, where young erythroblasts develop around a nursing macrophage (Joice et al. Reference Joice, Nilsson, Montgomery, Dankwa, Egan, Morahan, Seydel, Bertuccini, Alano, Williamson, Duraisingh, Taylor, Milner and Marti2014). Transcriptional data suggest that at least a subset of young gametocytes are in the blood circulation (Pelle et al. Reference Pelle, Oh, Buchholz, Narasimhan, Joice, Milner, Brancucci, Ma, Voss, Ketman, Seydel, Taylor, Barteneva, Huttenhower and Marti2015) while presence of asexual parasites in the bone marrow parenchyma and formation of gametocytes in erythroid precursor cells in vitro (Peatey et al. Reference Peatey, Watson, Trenholme, Brown, Nielson, Guenther, Timmins, Watson and Gardiner2013; Joice et al. Reference Joice, Nilsson, Montgomery, Dankwa, Egan, Morahan, Seydel, Bertuccini, Alano, Williamson, Duraisingh, Taylor, Milner and Marti2014) suggests that gametocyte formation could also occur in the bone marrow. In either case, potentially immunogenic surface molecules could be involved both in the processes of (i) parasite binding to endothelial cells, and (ii) erythroblast island binding in the bone marrow parenchyma. At the erythroblastic island, gametocyte proteins could be involved in binding either to erythroid precursor cells or macrophages. To further test these possibilities, binding experiments with different cell types could be performed and the ability of patient sera to inhibit these binding interactions examined. As cytokines and other innate immune factors likely influence endothelial permeability, it will be important to discern what role antibodies play compared with other immune components during both extravasation into and development within the bone marrow. Recent advances in distinguishing gametocyte stages in vitro (Aingaran et al. Reference Aingaran, Zhang, Law, Peng, Undisz, Meyer, Diez-Silva, Burke, Spielmann, Lim, Suresh, Dao and Marti2012; Tiburcio et al. Reference Tiburcio, Silvestrini, Bertuccini, Sander, Turner, Lavstsen and Alano2012) and in flow cytometry assays examining antibody binding (Saeed et al. Reference Saeed, Roeffen, Alexander, Drakeley, Targett and Sutherland2008; Ajua et al. Reference Ajua, Engleitner, Esen, Theisen, Issifou and Mordmuller2012) will enable identification of early stage gametocyte specific immune responses in diverse patient populations. The functionality of these immune responses could then be characterized in different clinical situations and the identity of the proteins targeted by early stage gametocyte-specific antibodies defined. Epitopes on erythrocytes infected with early stage gametocytes may form attractive novel targets for transmission blocking strategies. Functional recognition of early stage gametocytes could be exploited in vaccination strategies that interfere with homing to the bone marrow, maturation at this immunoprotective site and/or release of mature gametocytes into the blood stream
Late stage gametocyte infected erythrocyte surface antigens
In the first study of its kind, Saeed et al. observed naturally acquired humoral immunity against fully mature stage V gametocytes in serum samples of gametocyte-carrying Gambian children by flow cytometry (Saeed et al. Reference Saeed, Roeffen, Alexander, Drakeley, Targett and Sutherland2008). This late GIE surface recognition increased with age and was most pronounced in serum samples taken at later time points during the infection when mature gametocytes appear in peripheral blood, but was unrelated to anti-asexual stage humoral immunity. Interestingly, immune responses to GIE were associated with decreased gametocyte density, suggesting that this type of humoral immunity may have consequences for gametocyte production or longevity. If confirmed in other studies, these findings hold great promise for the identification of novel mature gametocyte vaccine targets. These confirmatory studies should take great care in preventing (low level) activation of these stage V gametocytes that would result in the accessibility of gamete antigens to which antibody responses may be highly prevalent in endemic settings (Mulder et al. Reference Mulder, Lensen, Tchuinkam, Roeffen, Verhave, Boudin and Sauerwein1999; Drakeley et al. Reference Drakeley, Eling, Teelen, Bousema, Sauerwein, Greenwood and Targett2004; Gouagna et al. Reference Gouagna, Bonnet, Gounoue, Verhave, Eling, Sauerwein and Boudin2004).
The identification of gametocyte-specific epitopes on the GIE surface and their function remain to be established. Possible targets include multigene families located in the subtelomeric regions, which encode variant surface antigens known for their role in sequestration of asexual parasites and are also expressed in late stage gametocytes. STEVOR proteins have been identified as localized at the membrane of erythrocytes infected with late stage gametocytes (McRobert et al. Reference McRobert, Preiser, Sharp, Jarra, Kaviratne, Taylor, Renia and Sutherland2004) but a subset of the variant RIFIN antigens may also be targets of naturally acquired immunity against epitopes on the stage V GIE (Sharp et al. Reference Sharp, Lavstsen, Fivelman, Saeed, McRobert, Templeton, Jensen, Baker, Theander and Sutherland2006). Another late stage gametocyte specific protein containing binding motifs is pSLAP (Delrieu et al. Reference Delrieu, Waller, Mota, Grainger, Langhorne and Holder2002). Furthermore, the existence of signalling molecules expressed on the GIE and involved in spatial and temporal tropism (tropins and circadins) has been hypothesized (Sutherland, Reference Sutherland2009).
Human immune responses active against mosquito-stage parasites
Since the majority of mature gametocytes die within the human host and fail to transmit to mosquitoes, proteins that are expressed by gametocytes but are only functional in activated gametes and later developmental stages are released and presented to the immune system. Indeed, many antigens present on the surface of these parasite stages (gametes, zygotes, ookinetes, oocysts) are already expressed in gametocytes in the human host, in preparation for gametogenesis (Pradel, Reference Pradel2007). Immune components that are taken up in the blood meal together with the gametocytes can inhibit fertilization in the mosquito midgut (Mulder et al. Reference Mulder, Tchuinkam, Dechering, Verhave, Carnevale, Meuwissen and Robert1994). Once gametocytes leave the erythrocyte in the mosquito midgut, they are exposed to human cellular and humoral immune factors including leukocytes, antibodies and complement. In the avian malaria P. gallinaceum, transmission from chickens to mosquitoes was significantly reduced after vaccination of the birds with formalin-treated or irradiated gametes (Carter and Chen, Reference Carter and Chen1976; Gwadz, Reference Gwadz1976; Carter et al. Reference Carter, Gwadz and McAuliffe1979). Also in the primate malaria Plasmodium knowlesi, evidence of induced TRI was observed upon immunization with gametocytes (Gwadz and Koontz, Reference Gwadz and Koontz1984). These animal models revealed for the first time that TRI could be induced, and was active against the parasite developmental stages occurring in the mosquito. Research into TRI and transmission blocking vaccine (TBV) development has since focused on immune responses to the mosquito stages of parasite development.
Mechanisms of mosquito-stage transmission blocking immunity
Leukocytes are capable of phagocytizing Plasmodium gametes, though the efficiency of this process appears lower in the mosquito midgut than during in vitro experiments, possibly because of lower temperature and enzymes present in the mosquito midgut (Sinden and Smalley, Reference Sinden and Smalley1976; Healer et al. Reference Healer, Graszynski and Riley1999a). However, there is little evidence for the direct involvement of leukocytes in TRI, and conflicting evidence for a synergistic effect of cellular and antibody mediated immunity (Lensen et al. Reference Lensen, Bolmer-Van de Vegte, van Gemert, Eling and Sauerwein1997, Reference Lensen, Mulder, Tchuinkam, Willemsen, Eling and Sauerwein1998).
Humoral immunity appears to be the dominant mechanism of natural TRI. The intra-host death of gametocytes that are not successfully transmitted releases a multitude of epitopes, including many present on the surface of the female macro- or male micro-gamete upon activation within the blood meal of a mosquito. Humoral immunity remains functional within the mosquito midgut and can inhibit transmission through a range of mechanisms. Specific antibodies may inhibit gamete fertilization either by agglutination resulting in inhibited gamete mobility, coating the micro- or macro-gamete causing reduced cell–cell contact, opsonization for immune mediated lysis, or activation of the complement system resulting in gamete lysis (Vermeulen et al. Reference Vermeulen, Ponnudurai, Beckers, Verhave, Smits and Meuwissen1985; Grotendorst et al. Reference Grotendorst, Carter, Rosenberg and Koontz1986; Kaslow et al. Reference Kaslow, Bathurst and Barr1992; Ranawaka et al. Reference Ranawaka, Alejo-Blanco and Sinden1994;). Evidence for the dominance of antibody mediation in TRI development comes from its induction by vaccination with whole parasites (Carter and Chen, Reference Carter and Chen1976; Carter et al. Reference Carter, Gwadz and McAuliffe1979; Gwadz and Koontz, Reference Gwadz and Koontz1984) or from the activity of mAb specific to major sexual stage proteins (Rener et al. Reference Rener, Graves, Carter, Williams and Burkot1983). The threshold at which humoral immunity significantly impacts transmission reduction, and the longevity of these responses are currently insufficiently explored.
Assessing immune responses that block mosquito infection
The well-standardized method of determining the presence of TRI is the standard membrane feeding assay (SMFA) in which gametocytes are cultured in vitro and fed to laboratory-reared mosquitoes (Ponnudurai et al. Reference Ponnudurai, Lensen, Van Gemert, Bensink, Bolmer and Meuwissen1989). The direct membrane feeding assay (DMFA) is an alternative assay applicable in field situations, where gametocytes from naturally infected individuals are fed to mosquitoes either in the whole blood of the donor (to assess infectivity in the presence of other blood components), or after the removal and re-addition of autologous plasma or addition of plasma from a naive donor (to assess the impact of plasma components on transmission efficiency). These assays are described in detail elsewhere (Ponnudurai et al. Reference Ponnudurai, Lensen, Van Gemert, Bensink, Bolmer and Meuwissen1989; Bousema et al. Reference Bousema, Dinglasan, Morlais, Gouagna, van Warmerdam, Awono-Ambene, Bonnet, Diallo, Coulibaly, Tchuinkam, Mulder, Targett, Drakeley, Sutherland, Robert, Doumbo, Toure, Graves, Roeffen, Sauerwein, Birkett, Locke, Morin, Wu and Churcher2012; Ouédraogo, Reference Ouédraogo, Guelbéogo, Cohuet, Morlais, King, Gonçalves, Bastiaens, Vaanhold, Sattabongkot, Wu, Coulibaly, Ibrahima, Jones, Morin, Drakeley, Dinglasan and Bousema2013). In both assays, the transmission reducing activity (TRA) of test sera/autologous plasma is generally determined one week after feeding when oocysts lining the mosquito midgut are visible by microscopy (Lensen et al. Reference Lensen, van Druten, Bolmer, van Gemert, Eling and Sauerwein1996). Evidence of functional TRI has generally been reported when the mean number of oocysts in test mosquitoes is decreased to less than 10% (TRA ⩾ 90%) of that in control mosquitoes (mosquitoes fed the same gametocyte source with control serum [SMFA] or naïve plasma [DMFA]), while TRA < 50% is considered evidence of limited or non-existent TRI (Bousema et al. Reference Bousema, Roeffen, van der Kolk, de Vlas, van de Vegte-Bolmer, Bangs, Teelen, Kurniawan, Maguire, Baird and Sauerwein2006; Bousema and Drakeley, Reference Bousema and Drakeley2011).
Advantages of the DMFA include the assessment of a multitude of locally relevant strains and more physiological gametocyte densities; however, large variation in these experiments does limit its predictive value and correlation with SMFA results. Transmission reduction in the DMFA was also correlated with gametocyte carriage, age above 5 years, and late season sampling, while it was inversely correlated with gametocyte density (Bousema et al. Reference Bousema, Sutherland, Churcher, Mulder, Gouagna, Riley, Targett and Drakeley2011). Several population-based screens using the DMFA in Cameroon, Kenya and the Gambia found that transmission efficiency is enhanced by 14–66% after replacement of autologous plasma with naive control serum (Drakeley et al. Reference Drakeley, Eling, Teelen, Bousema, Sauerwein, Greenwood and Targett2004; Gouagna et al. Reference Gouagna, Bonnet, Gounoue, Verhave, Eling, Sauerwein and Boudin2004; Mulder et al. Reference Mulder, Lensen, Tchuinkam, Roeffen, Verhave, Boudin and Sauerwein1999; Bousema et al. Reference Bousema, Sutherland, Churcher, Mulder, Gouagna, Riley, Targett and Drakeley2011). Although complete transmission blockage is rare, these findings suggest a relatively high prevalence of functional TRI in endemic populations. However, non-specific factors (e.g. chemicals) present in the serum may contribute to the observed effect on TRA, and methodological issues including maintaining sample temperature during serum replacement may also affect transmission efficiency (Bousema et al. Reference Bousema, Churcher, Morlais and Dinglasan2013). Non-specific factors can be excluded in the SMFA, as not only whole serum, but also specifically purified antibodies can be added to the infectious blood-meal. Naturally acquired transmission-reducing immunity has been detected using the SMFA with whole serum and purified IgG (Mulder et al. Reference Mulder, Lensen, Tchuinkam, Roeffen, Verhave, Boudin and Sauerwein1999; Drakeley et al. Reference Drakeley, Eling, Teelen, Bousema, Sauerwein, Greenwood and Targett2004, Reference Drakeley, Bousema, Akim, Teelen, Roeffen, Lensen, Bolmer, Eling and Sauerwein2006; van der Kolk et al. Reference van der Kolk, De Vlas, Saul, van de Vegte-Bolmer, Eling and Sauerwein2005; Bousema et al. Reference Bousema, Drakeley, Kihonda, Hendriks, Akim, Roeffen and Sauerwein2007), although the proportion of individuals with reproducible TRI is generally much lower than observed in the DMFA with serum replacement. Variations in the intensity of mosquito infection arising from feeds on natural gametocyte carriers or cultured gametocytes make the assay outcomes difficult to compare, and may lead to a biological bias, as higher gametocyte density in the SMFA may increase epitope availability (Churcher et al. Reference Churcher, Blagborough, Delves, Ramakrishnan, Kapulu, Williams, Biswas, Da, Cohuet and Sinden2012). Efforts must therefore be made to standardize the conditions of the DMFA and SMFA. DMFA experiments require a better parameterization of inter-assay variability and procedural artefacts that may be interpreted as TRI. In turn, the SMFA can be optimized to better resemble natural conditions. The incorporation of multiple strains originating from various geographical locations could help avoid false-negative results due to sequence variation of gamete-specific epitopes in endemic strains while the use of gametocyte densities representative of natural mosquito infection rates would improve estimation of the impact of TRI on the likelihood of secondary infections (Bousema et al. Reference Bousema, Dinglasan, Morlais, Gouagna, van Warmerdam, Awono-Ambene, Bonnet, Diallo, Coulibaly, Tchuinkam, Mulder, Targett, Drakeley, Sutherland, Robert, Doumbo, Toure, Graves, Roeffen, Sauerwein, Birkett, Locke, Morin, Wu and Churcher2012). Ideally, aiming to more accurately resemble the in vivo situation and avoid exhaustion of essential antibodies by non-specific binding to asexual and immature gametocytes currently present in the feeding material, only mature stage V gametocytes with sex ratios comparable with natural infections would be fed to mosquitoes.
A major drawback of both assays is their labour intensiveness, as the readout requires the dissection and midgut analysis of minimally 20 mosquitoes per condition after maintaining mosquitoes for a week. Recently, the use of a transgenic parasite line expressing luciferase enabled high throughput analysis of infection rates in mosquito midguts, which is a fundamental improvement for performing SMFA at a large scale (Stone et al. Reference Stone, Churcher, Graumans, van Gemert, Vos, Lanke, van de Vegte-Bolmer, Siebelink-Stoter, Dechering, Vaughan, Camargo, Kappe, Sauerwein and Bousema2014).
Immune targets of transmission blocking immunity
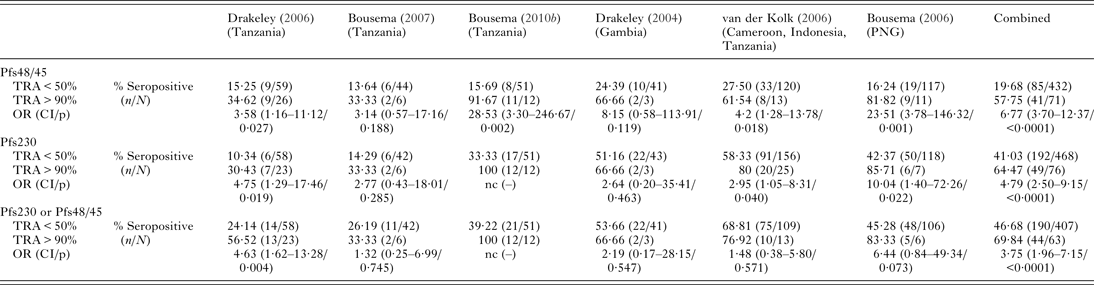
Until the turn of the century, our understanding of protein expression during P. falciparum sexual development was limited to a selection produced in abundance at the onset of gametocytogensis (Pfg27/25 and Pfs16) (Carter et al. Reference Carter, Graves, Creasey, Byrne, Read, Alano and Fenton1989; Bruce et al. Reference Bruce, Carter, Nakamura, Aikawa and Carter1994) or present on the gametocyte/gamete (Pfs230, Pfs48/45, Pfs41) (Rener et al. Reference Rener, Graves, Carter, Williams and Burkot1983) or zygote/ookinete (Pfs25, Pfs28) (Duffy and Kaslow, Reference Duffy and Kaslow1997) surface. Many more proteins have now been identified as sexual stage specific (Silvestrini et al. Reference Silvestrini, Lasonder, Olivieri, Camarda, van Schaijk, Sanchez, Younis Younis, Sauerwein and Alano2010; Joice et al. Reference Joice, Narasimhan, Montgomery, Sidhu, Oh, Meyer, Pierre-Louis, Seydel, Milner, Williamson, Wiegand, Ndiaye, Daily, Wirth, Taylor, Huttenhower and Marti2013; Tao et al. Reference Tao, Ubaida-Mohien, Mathias, King, Pastrana-Mena, Tripathi, Goldowitz, Graham, Moss, Marti and Dinglasan2014; Pelle et al. Reference Pelle, Oh, Buchholz, Narasimhan, Joice, Milner, Brancucci, Ma, Voss, Ketman, Seydel, Taylor, Barteneva, Huttenhower and Marti2015), with the best characterized described in previous reviews (Pradel, Reference Pradel2007; Nikolaeva et al. Reference Nikolaeva, Draper and Biswas2015; Wu et al. Reference Wu, Sinden, Churcher, Tsuboi and Yusibov2015). Pfs25 and Pfs28 are post-transcriptionally repressed until the parasite's development in the mosquito midgut (Pradel, Reference Pradel2007), so it is unlikely that they would elicit functional TRI (Miura et al. Reference Miura, Takashima, Deng, Tullo, Diouf, Moretz, Nikolaeva, Diakite, Fairhurst, Fay, Long and Tsuboi2013). Naturally occurring antibody mediated TRI is more likely the result of exposure to proteins expressed in the human parasite stages (so called pre-fertilization antigens). Pfs230 and Pfs48/45 expression is initiated during gametocyte development and proceeds until fertilization of the micro- and macro-gametes, with both proteins appearing to have an important role in male microgamete fertility (van Dijk et al. Reference van Dijk, Janse, Thompson, Waters, Braks, Dodemont, Stunnenberg, van Gemert, Sauerwein and Eling2001; Eksi et al. Reference Eksi, Czesny, van Gemert, Sauerwein, Eling and Williamson2006). Antibodies specific to both proteins are naturally acquired after malaria infection (Ong et al. Reference Ong, Zhang, Eida, Graves, Dow, Looker, Rogers, Chiodini and Targett1990; Bousema et al. Reference Bousema, Roeffen, van der Kolk, de Vlas, van de Vegte-Bolmer, Bangs, Teelen, Kurniawan, Maguire, Baird and Sauerwein2006), and associated with varying levels of TRI; 14 studies have attempted to correlate TRI measured in MFA with immune recognition of Pfs230 and Pfs48/45, or sub-units thereof (Graves et al. Reference Graves, Carter, Burkot, Quakyi and Kumar1988; Premawansa et al. Reference Premawansa, Gamage-Mendis, Perera, Begarnie, Mendis and Carter1994; Roeffen et al. Reference Roeffen, Lensen, Mulder, Teelen, Sauerwein, Eling, Meuwissen and Beckers1994, Reference Roeffen, Mulder, Teelen, Bolmer, Eling, Targett, Beckers and Sauerwein1996; Healer et al. Reference Healer, McGuinness, Carter and Riley1999b; Mulder et al. Reference Mulder, Lensen, Tchuinkam, Roeffen, Verhave, Boudin and Sauerwein1999; Drakeley et al. Reference Drakeley, Mulder, Tchuinkam, Gupta, Sauerwein and Targett1998, Reference Drakeley, Eling, Teelen, Bousema, Sauerwein, Greenwood and Targett2004, Reference Drakeley, Bousema, Akim, Teelen, Roeffen, Lensen, Bolmer, Eling and Sauerwein2006; Bousema et al. Reference Bousema, Roeffen, van der Kolk, de Vlas, van de Vegte-Bolmer, Bangs, Teelen, Kurniawan, Maguire, Baird and Sauerwein2006, Reference Bousema, Drakeley, Kihonda, Hendriks, Akim, Roeffen and Sauerwein2007, Reference Bousema, Roeffen, Meijerink, Mwerinde, Mwakalinga, van Gemert, van de Vegte-Bolmer, Mosha, Targett, Riley, Sauerwein and Drakeley2010b; van der Kolk et al. Reference van der Kolk, de Vlas and Sauerwein2006; Jones et al. Reference Jones, Grignard, Nebie, Chilongola, Dodoo, Sauerwein, Theisen, Roeffen, Singh, Singh, Singh, Kyei-Baafour, Tetteh, Drakeley and Bousema2015). Of these 14 studies, 9 determined the presence of antibody responses to Pfs230 and Pfs48/45 using enzyme-linked immunosorbent assay (ELISA), and related this data to serum TRA determined in the SMFA. Data were available for 6 studies, allowing a combined analysis of reactivity to these antigens in individuals with and without functional TRI (Table 1).
Table 1. Summary of studies correlating TRI assessed in the SMFA, with the presence of antibody responses specific to antigens Pfs48/45 and Pfs230

CI, confidence interval; OR, odds ratio; SMFA, standard membrane feeding assay; TRA, transmission reducing activity; TRI, transmission reducing immunity.
Sample/population size, total number of samples for which membrane feeding assay (MFA) and enzyme-linked immunosorbent assay (ELISA) data for anti-Pfs230 or anti-Pfs48/45 responses were available/number of individuals from which samples were obtained. Three studies (Bousema et al. Reference Bousema, Roeffen, van der Kolk, de Vlas, van de Vegte-Bolmer, Bangs, Teelen, Kurniawan, Maguire, Baird and Sauerwein2006, Reference Bousema, Drakeley, Kihonda, Hendriks, Akim, Roeffen and Sauerwein2007, Reference Bousema, Roeffen, Meijerink, Mwerinde, Mwakalinga, van Gemert, van de Vegte-Bolmer, Mosha, Targett, Riley, Sauerwein and Drakeley2010b) were conducted longitudinally, giving multiple viable observations (n = 1–4) for each individual. Exact sample sizes which vary between antigens: TRA ⩾ 90%, sera reducing test mosquito mean oocyst intensity in the SMFA by ⩾90% relative to oocyst intensity in control mosquitoes; TRA < 50%, sera reducing test mosquito mean oocyst intensity in the SMFA by <50% relative to oocyst intensity in control mosquitoes; % seropositive (n/N), percentage of sera reducing transmission that are seropositive for antibodies specific to Pfs230 or Pfs48/45/(number seropositive/total sample number); OR (CI/p), OR for functional TRI and seropositivity against Pfs48/45 or Pfs230 (CI/P-value). TRI was considered evident if participant sera gave ⩾90% TRA in the SMFA. Seropositivity in this ‘transmission blocking’ group was compared with seropositivity in individuals whose sera had <50% TRA. Sera reducing oocyst intensity by 50–90% were excluded from this analysis. For cross-sectional studies (Drakeley et al. Reference Drakeley, Eling, Teelen, Bousema, Sauerwein, Greenwood and Targett2004, Reference Drakeley, Bousema, Akim, Teelen, Roeffen, Lensen, Bolmer, Eling and Sauerwein2006; van der Kolk et al. Reference van der Kolk, de Vlas and Sauerwein2006) OR was calculated using standard logistic regression. For studies with multiple observations per individual, study subject was incorporated into a generalized estimating equation (GEE) model as a random effect. For the combined analysis, study id was incorporated into a GEE model as a fixed effect and an exchangeable working correlation matrix was used for observations on the same subject. Robust standard errors were used. Adjustment for the age of sampled individuals did not affect estimates, nor was age an independent statistically significant predictor of TRI; Pfs230 or Pfs48/45, to conduct analysis based on the recognition of either Pfs48/45 or Pfs230, only samples for which antibody responses to both antigens were assessed were included; combined, the results of all 6 studies for which data were available were combined to provide summary statistics for the association of anti-Pfs48/45 anti-Pfs230 responses and TRI; nc, OR not calculable (100% of transmission blockers were reactive to the specified protein).
There is growing evidence from studies utilizing recombinant Pfs230 and Pfs48/45 that antibody responses to sexual stage antigens are acquired with age (Drakeley et al. Reference Drakeley, Bousema, Akim, Teelen, Roeffen, Lensen, Bolmer, Eling and Sauerwein2006; Bousema et al. Reference Bousema, Roeffen, Meijerink, Mwerinde, Mwakalinga, van Gemert, van de Vegte-Bolmer, Mosha, Targett, Riley, Sauerwein and Drakeley2010b; Jones et al. Reference Jones, Grignard, Nebie, Chilongola, Dodoo, Sauerwein, Theisen, Roeffen, Singh, Singh, Singh, Kyei-Baafour, Tetteh, Drakeley and Bousema2015) in parallel with the development of blood-stage immunity (Stewart et al. Reference Stewart, Gosling, Griffin, Gesase, Campo, Hashim, Masika, Mosha, Bousema, Shekalaghe, Cook, Corran, Ghani, Riley and Drakeley2009). Evidence for the age relatedness of TRI has been limited by age biased sampling. In the only study that assessed TRI for individuals of all ages, antigen-specific responses increased with age while the functionality of this response appeared to decrease (Drakeley et al. Reference Drakeley, Bousema, Akim, Teelen, Roeffen, Lensen, Bolmer, Eling and Sauerwein2006). More research is needed to improve our understanding of the acquisition and maturation of the TRI response in individuals of different ages across a range of transmission intensities.
Variability in the efficacy of sera to inhibit the transmission of different gametocyte isolates from naturally infected individuals suggests strain specific effects (Drakeley et al. Reference Drakeley, Mulder, Tchuinkam, Gupta, Sauerwein and Targett1998). This might be due to antigenic variation, although Pfs48/45 and Pfs230 encoding genes show very limited variability in coding sequences (Jones et al. Reference Jones, Grignard, Nebie, Chilongola, Dodoo, Sauerwein, Theisen, Roeffen, Singh, Singh, Singh, Kyei-Baafour, Tetteh, Drakeley and Bousema2015). Recently, immune sera from individuals in Mali were shown to recognize the male gamete protein HAP2, indicating expression by gametocytes in the human host. HAP2 specific mAb block transmission to mosquitos in Plasmodium berghei (Blagborough and Sinden, Reference Blagborough and Sinden2009) and P. falciparum (Miura et al. Reference Miura, Takashima, Deng, Tullo, Diouf, Moretz, Nikolaeva, Diakite, Fairhurst, Fay, Long and Tsuboi2013), but it has yet to be shown if the presence of anti-HAP2 Ab in endemic sera correlates with functional TRI.
While sera containing antibodies specific to Pfs48/45 or Pfs230 block transmission more commonly than sera with no effect on mosquito infection (Pfs48/45: OR 6·62 (3·61–12·15); Pfs230: OR 4·69 (2·50–8·81), recognition of these proteins does not predict blockage absolutely, nor does it confirm a mechanistic link with TRI. Several studies show no association between Ab response to Pfs230 and/or Pfs48/45 and transmission reduction (Premawansa et al. Reference Premawansa, Gamage-Mendis, Perera, Begarnie, Mendis and Carter1994; Graves et al. Reference Graves, Carter, Burkot, Quakyi and Kumar1988, Mulder et al. Reference Mulder, Lensen, Tchuinkam, Roeffen, Verhave, Boudin and Sauerwein1999, van der Kolk et al. Reference van der Kolk, de Vlas and Sauerwein2006). The presence of TRI in the absence of Pfs48/45 or Pfs230 specific antibodies, and a lack of TRI in their presence (Graves et al. Reference Graves, Carter, Burkot, Quakyi and Kumar1988; Healer et al. Reference Healer, McGuinness, Carter and Riley1999b; Mulder et al. Reference Mulder, Lensen, Tchuinkam, Roeffen, Verhave, Boudin and Sauerwein1999; Drakeley et al. Reference Drakeley, Bousema, Akim, Teelen, Roeffen, Lensen, Bolmer, Eling and Sauerwein2006; van der Kolk et al. Reference van der Kolk, de Vlas and Sauerwein2006; Bousema et al. Reference Bousema, Roeffen, Meijerink, Mwerinde, Mwakalinga, van Gemert, van de Vegte-Bolmer, Mosha, Targett, Riley, Sauerwein and Drakeley2010b) suggests that both may be partially functional, and that responses to multiple proteins could be necessary to elicit transmission inhibition. Combining the data in Table 1, 46·68% (190/407) of samples with <50% TRA recognized either Pfs48/45 or Pfs230, while 30·16% (19/63) of samples with TRA ⩾ 90% recognized neither antigen. To definitively establish the role of naturally occurring anti-Pfs48/45 and anti-Pfs230 antibodies in the development of TRI, specific antibodies should be affinity purified from immune sera using recombinant antigen and tested in the SMFA.
The association of sera transmission enhancement (TE) with anti-gamete immune responses has been a long standing discussion among researchers involved in TBV development. TE appears to occur naturally in a small proportion of gametocyte exposed individuals (Mendis et al. Reference Mendis, Munesinghe, de Silva, Keragalla and Carter1987; Graves et al. Reference Graves, Carter, Burkot, Quakyi and Kumar1988; Peiris et al. Reference Peiris, Premawansa, Ranawaka, Udagama, Munasinghe, Nanayakkara, Gamage, Carter, David and Mendis1988; Gamage-Mendis et al. Reference Gamage-Mendis, Rajakaruna, Carter and Mendis1992; Premawansa et al. Reference Premawansa, Gamage-Mendis, Perera, Begarnie, Mendis and Carter1994; Drakeley et al. Reference Drakeley, Mulder, Tchuinkam, Gupta, Sauerwein and Targett1998; Healer et al. Reference Healer, McGuinness, Carter and Riley1999b), though at lower frequency (van der Kolk et al. Reference van der Kolk, de Vlas and Sauerwein2006) (7% TE/48% TR) and relative intensity than naturally occurring transmission reduction. The cause of serum TE remains obscure: low antibody titres, from diluted immune sera or anti-gamete mAb have been linked with enhanced P. vivax transmission (Mendis et al. Reference Mendis, Munesinghe, de Silva, Keragalla and Carter1987; Peiris et al. Reference Peiris, Premawansa, Ranawaka, Udagama, Munasinghe, Nanayakkara, Gamage, Carter, David and Mendis1988; Gamage-Mendis et al. Reference Gamage-Mendis, Rajakaruna, Carter and Mendis1992), and these findings are supported by the observation of enhancment during periods of low antibody carriage in the early and late stages of Plasmodiun cynomolgi infection in Macaques (Naotunne et al. Reference Naotunne, Rathnayake, Jayasinghe, Carter and Mendis1990). Low titres of anti-gamete antibodies have also been linked with TE for P. falciparum (Graves et al. Reference Graves, Carter, Burkot, Quakyi and Kumar1988; Carter et al. Reference Carter, Graves, Keister and Quakyi1990; Healer et al. Reference Healer, McGuinness, Carter and Riley1999b), however low titre responses to specific gamete and ookinete antigens (Pfs230, Pfs48/45, Pfs25, Pbs21) appear non-significantly (Healer et al. Reference Healer, McGuinness, Carter and Riley1999b) or not associated (Ponnudurai et al. Reference Ponnudurai, van Gemert, Bensink, Lensen and Meuwissen1987; Ranawaka et al. Reference Ranawaka, Alejo-Blanco and Sinden1993; van der Kolk et al. Reference van der Kolk, de Vlas and Sauerwein2006) with enhanced transmission, indicating that TE may be caused by a concurrent response to un-characterized gamete antigens, or by other serum factors. Investigating subtle reduction or enhancement of mosquito infection rate is made difficult by the SMFA's inherent variability, but in light of its potential impact on the efficacy of TBVs TE requires further investigation.
Antibody responses to candidate TBVs provide an incomplete image of the immune signature of natural TRI, and if involved, these proteins probably represent only part of a larger range of immune responses contributing to TRI. The publishing of the Plasmodium genome in 2002 set the course for rapid advances in the fields of transcriptomics and proteomics. A wealth of studies employing high accuracy mass-spectrometry and mRNA microarrays identified proteins expressed specifically in gametocytes (Silvestrini et al. Reference Silvestrini, Bozdech, Lanfrancotti, Di Giulio, Bultrini, Picci, Derisi, Pizzi and Alano2005; Young et al. Reference Young, Fivelman, Blair, de la Vega, Le Roch, Zhou, Carucci, Baker and Winzeler2005), male and female gametocytes separately (Khan et al. Reference Khan, Franke-Fayard, Mair, Lasonder, Janse, Mann and Waters2005; Tao et al. Reference Tao, Ubaida-Mohien, Mathias, King, Pastrana-Mena, Tripathi, Goldowitz, Graham, Moss, Marti and Dinglasan2014), gametes, and ookinetes (Le Roch et al. Reference Le Roch, Johnson, Florens, Zhou, Santrosyan, Grainger, Yan, Williamson, Holder, Carucci, Yates and Winzeler2004; Hall et al. Reference Hall, Karras, Raine, Carlton, Kooij, Berriman, Florens, Janssen, Pain, Christophides, James, Rutherford, Harris, Harris, Churcher, Quail, Ormond, Doggett, Trueman, Mendoza, Bidwell, Rajandream, Carucci, Yates, Kafatos, Janse, Barrell, Turner, Waters and Sinden2005). The results of studies using P. berghei have been reviewed in detail (Wass et al. Reference Wass, Stanway, Blagborough, Lal, Prieto, Raine, Sternberg, Talman, Tomley, Yates and Sinden2012). Recently, high yield purification techniques allowed P. falciparum proteomic expression at the blood, early gametocyte and late gametocyte stages to be disentangled, revealing the expression of >1400 proteins in early gametocytes, and >2000 in late gametocytes. Of these proteins, 1055 appear to be expressed in gametocytes but not in trophozoites, with 637 specific to stage IV and V gametocytes (Silvestrini et al. Reference Silvestrini, Lasonder, Olivieri, Camarda, van Schaijk, Sanchez, Younis Younis, Sauerwein and Alano2010). Proteomics approaches have confirmed the expression of known gametocyte surface proteins (Florens et al. Reference Florens, Washburn, Raine, Anthony, Grainger, Haynes, Moch, Muster, Sacci, Tabb, Witney, Wolters, Wu, Gardner, Holder, Sinden, Yates and Carucci2002; Lasonder et al. Reference Lasonder, Ishihama, Andersen, Vermunt, Pain, Sauerwein, Eling, Hall, Waters, Stunnenberg and Mann2002), and identified hypothetical proteins likely to contain export sequences that may indicate surface expression (Silvestrini et al. Reference Silvestrini, Lasonder, Olivieri, Camarda, van Schaijk, Sanchez, Younis Younis, Sauerwein and Alano2010). Combined with analyses which have enabled the ranking of the P. falciparum proteome by the likelihood of possessing transmembrane domains or a glycophosphatidylinositol-anchor (Gilson et al. Reference Gilson, Nebl, Vukcevic, Moritz, Sargeant, Speed, Schofield and Crabb2006), proteomics enables the prioritization of key candidates for involvement in antibody mediated TRI. With high throughput protein expression arrays the recognition by immune sera of thousands of proteins can now be assessed simultaneously (Doolan et al. Reference Doolan, Mu, Unal, Sundaresh, Hirst, Valdez, Randall, Molina, Liang, Freilich, Oloo, Blair, Aguiar, Baldi, Davies and Felgner2008; Crompton et al. Reference Crompton, Kayala, Traore, Kayentao, Ongoiba, Weiss, Molina, Burk, Waisberg, Jasinskas, Tan, Doumbo, Doumtabe, Kone, Narum, Liang, Doumbo, Miller, Doolan, Baldi, Felgner and Pierce2010), and the results, as for previous studies with recombinant Pfs230 and Pfs48/45, correlated to functional TRI measured in mosquito feeding assays. As the activity of many proteins (Pfs230 and Pfs48/45 included) is conformation dependent (Outchkourov et al. Reference Outchkourov, Roeffen, Kaan, Jansen, Luty, Schuiffel, van Gemert, van de Vegte-Bolmer, Sauerwein and Stunnenberg2008; Chowdhury et al. Reference Chowdhury, Angov, Kariuki and Kumar2009), and as high throughput protein expression platforms are currently unable to produce tertiary protein structures, such an analysis is unlikely to provide a definitive list of antigens involved in functional TRI. However, it may still reveal new targets with less conformation-dependent activity, which would significantly further our understanding of TRI development and aid the rational design of malaria TBVs.
Transmission blocking vaccines
In recent years the development of the RTS,S malaria vaccine has received significant investment, with the results of a recent Phase 3 cluster randomized trial indicating that the vaccine may reduce the number of severe and un-complicated clinical cases in areas of high transmission (RTS'S Clinical Trials Partnership, 2015). Though highly effective pre-erythrocytic vaccines may be classified as vaccines that interrupt malaria transmission by preventing parasite multiplication and thereby gametocyte production, the limited longevity and efficacy of the response of RTS,S may be insufficient to significantly affect transmission in all endemic settings (RTS'S Clinical Trials Partnership, 2015), and antibodies elicited by the vaccine have no direct impact on mosquito infection rate (Miura et al. Reference Miura, Jongert, Deng, Zhou, Lusingu, Drakeley, Fay, Long and Vekemans2014). TBV development, reviewed by (Nikolaeva et al. Reference Nikolaeva, Draper and Biswas2015; Sauerwein and Bousema, Reference Sauerwein and Bousema2015; Wu et al. Reference Wu, Sinden, Churcher, Tsuboi and Yusibov2015) has remained focused on 6 proteins against which antibodies (monoclonal, or from immunized sera) have been empirically shown to inhibit mosquito infection; Plasmodium proteins Pfs25 (Duffy and Kaslow, Reference Duffy and Kaslow1997; Wu et al. Reference Wu, Ellis, Shaffer, Fontes, Malkin, Mahanty, Fay, Narum, Rausch, Miles, Aebig, Orcutt, Muratova, Song, Lambert, Zhu, Miura, Long, Saul, Miller and Durbin2008), Pfs28 (Duffy and Kaslow, Reference Duffy and Kaslow1997), Pfs230 (Farrance et al. Reference Farrance, Rhee, Jones, Musiychuk, Shamloul, Sharma, Mett, Chichester, Streatfield, Roeffen, van de Vegte-Bolmer, Sauerwein, Tsuboi, Muratova, Wu and Yusibov2011), Pfs48/45 (Outchkourov et al. Reference Outchkourov, Roeffen, Kaan, Jansen, Luty, Schuiffel, van Gemert, van de Vegte-Bolmer, Sauerwein and Stunnenberg2008; Chowdhury et al. Reference Chowdhury, Angov, Kariuki and Kumar2009) and more recently HAP2 (Blagborough and Sinden, Reference Blagborough and Sinden2009; Miura et al. Reference Miura, Takashima, Deng, Tullo, Diouf, Moretz, Nikolaeva, Diakite, Fairhurst, Fay, Long and Tsuboi2013), and mosquito antigen AgAPN-1 (Dinglasan et al. Reference Dinglasan, Kalume, Kanzok, Ghosh, Muratova, Pandey and Jacobs-Lorena2007). If responses to pre-fertilization antigens considered for TBV development are dominant effectors of naturally occurring TRI, vaccination efforts with these candidates may be aided by immune boosting from natural parasite exposure (Nunes et al. Reference Nunes, Woods, Carter, Raphael, Morin, Diallo, Leboulleux, Jain, Loucq, Kaslow and Birkett2014). More research is required to improve our understanding of the temporal dynamics of sexual stage immunity, but limited evidence that TRI may be short-lived after gametocyte exposure (Jones et al. Reference Jones, Grignard, Nebie, Chilongola, Dodoo, Sauerwein, Theisen, Roeffen, Singh, Singh, Singh, Kyei-Baafour, Tetteh, Drakeley and Bousema2015) highlights the importance of prioritizing vaccine/adjuvant formulations that elicit long-lived immunity.
Whilst the advancement of Pfs25 to clinical trials is promising, examination of the WHO ‘Rainbow tables’ for malaria vaccine candidates currently in development makes it clear that candidate vaccines targeting transmission stage parasite remain vastly overshadowed by the number targeting alternative life cycle stages (pre-erythrocytic and asexual parasites). The value of a sexual stage, TBV in the campaign to eliminate malaria is increasingly acknowledged, and the clinical testing of candidates other than Pfs25 and addition of novel targets are therefore urgently required. In addition to mosquito stage targets able to stimulate immune responses inhibiting parasite fertilization, increased focus should be on identifying targets integral to gametocyte development in the human host. Recent work providing insight into gametocyte development in the bone marrow parenchyma opens up new possibilities for immune responses targeting immature gametocytes while field studies highlight the importance of responses targeting mature gametocytes in inducing TRI. Novel tools to prevent gametocyte maturation and longevity could contribute significantly to the interruption of malaria transmission. A better understanding of naturally occurring TRI and of the gametocyte's interaction with its human host is fundamental to the development of new TBV approaches.
ACKNOWLEDGEMENTS
W. S. and S. R. are supported by PATH Malaria Vaccine Initiative (MVI). W. S. and T. B. are supported by a Marie Curie Career Integration Grant from the European Community's Seventh Framework Programme (SIGNAL, PCIG12-GA-2012-333936).