INTRODUCTION
Swordfish is a cosmopolitan species found in tropical and temperate waters of all the oceans between 45°N and 45°S, including the Mediterranean, Black Sea and the Marmara Sea (Palko et al., Reference Palko, Beardsley and Richards1981).
Most research results based on genetic studies have shown that the Mediterranean swordfish constitutes a single stock, separated from the North Atlantic and South Atlantic stocks (Kotoulas et al., Reference Kotoulas, Mejuto, Tserpes, Garcia-Cortes, Peristeraki, De la Serna and Magoulas2003; Smith et al., Reference Smith, Lu, García-Cortés, Viñas, Yeh and Alvarado Bremer2015). Recent research suggests that there is genetic differentiation among the Mediterranean swordfish population (Viñas et al., Reference Viñas, Pérez-Serra, Vidal, Jaime, Bremer and Pla2010).
Although there is incomplete information on the mixture and boundaries between stocks, it is generally accepted that Mediterranean population swordfish extends beyond the current management boundary at the Strait of Gibraltar to ~10°W. Mixing between the North Atlantic and Mediterranean populations is low with the Strait of Gibraltar as a mixing zone (Smith et al., Reference Smith, Lu, García-Cortés, Viñas, Yeh and Alvarado Bremer2015).
Swordfish perform vertical excursions, reaching depths up to 800 m during daylight and remaining near the surface at night when they stay in the superficial layer between 0 and 10 m; however, their diel vertical excursions are usually discontinuous and frequently interrupted by vertical rises (Carey & Robinson, Reference Carey and Robinson1981; Carey, Reference Carey and Stroud1990; Matsumoto et al., Reference Matsumoto, Saito and Miyabe2003; Canese et al., Reference Canese, Garibaldi, Orsi Relini and Greco2008). Past studies showed that billfishes such as swordfish use an unusual method of catching the prey, by using the bill as shown by the prey status (Tibbo et al., Reference Tibbo, Day and Doucet1961; Stillwell & Kohler, Reference Stillwell and Kohler1985; Romeo et al., Reference Romeo, Consoli, Castriota and Andaloro2009).
In general, stomach content analysis is an important tool to study ecology and fisheries biology (Clarke, Reference Clarke1966). In that sense, several studies on the swordfish diet were previously conducted. In the Atlantic, the diet of swordfish was studied, analysing the frequency of occurrence and the percentages of prey in number and/or in weight (Stillwell & Kohler, Reference Stillwell and Kohler1985; Moreira, Reference Moreira1990, Guerra et al., Reference Guerra, Simún, Gonzalez, Okutani, O'Dor and Kubodera1993; Hernández-García, Reference Hernández-García1995; Chancollon et al., Reference Chancollon, Pusineri and Ridoux2006; Castillo et al., Reference Castillo, Ibáñez, González and Chong2007). In the Mediterranean Sea, Bello (Reference Bello1991), Peristeraki et al. (Reference Peristeraki, Tserpes and Lefkaditou2005) and Romeo et al. (Reference Romeo, Consoli, Castriota and Andaloro2009, Reference Romeo, Battaglia, Peda, Perzia, Consoli, Esposito and Andaloro2011) studied prey preferences of swordfish, using the frequency of occurrence and the percentage in weight and in number of food items as diet indicators.
Nevertheless, information on the diet of the species in the Strait of Gibraltar has remained limited to the study published by Hernández-García (Reference Hernández-García1995). In addition, very few studies have analysed changes in swordfish diet composition in relation to season, sex and body size (Stillwell & Kohler, Reference Stillwell and Kohler1985; Ibáñez et al., Reference Ibáñez, González and Cubillos2004).
The specific purpose of the present study was to assess the diet preferences and improve knowledge on feeding behaviour and ecology of swordfish in the Strait of Gibraltar. It also aimed to examine for the first time the diet variability of the species in relation to season, sex and body size. The analysis utilizes biological and fisheries data obtained from the Moroccan artisanal longline fishery operating in the Strait of Gibraltar.
MATERIALS AND METHODS
Data collection and laboratory analysis
To study the diet of swordfish in the Strait of Gibraltar (Figure 1), a total of 176 stomachs of which 57% were females (100), were collected from the Moroccan commercial artisanal longline fleet landings at the port of Tangier for contents examination. The samples covered a size range from 110 to 237 cm lower jaw–fork length (LJFL) and eviscerated weight from 17 to 243 kg. Sampling took place on a monthly basis during the main swordfish fishing season in the area, from April to September 2015, except for the month of July when there was no fishing activity due to the absence of the species in the study area.
Fig. 1. A map showing the study area. Shaded area indicates the fishing grounds of the Moroccan artisanal longline fleet targeting swordfish.
For each sampled stomach, the following information was recorded: date of catch, sex, size (LJFL) and eviscerated weight of fish. The sex of fish was determined visually by examining the gonads of fish. The collected samples were frozen at −20°C to halt any further digestive process (Ferraton, Reference Ferraton2007).
In the laboratory, for each examined stomach, the prey were sorted by species, counted and weighed, using an electronic balance with an accuracy of 0.01 g. Given their low level of digestion, most of the prey were macroscopically identified to the species level, using published identification guides for fish, cephalopods and crustaceans (Lagardère, Reference Lagardère1971; Lloris & Rucabado, Reference Lloris and Rucabado1998). One hundred and eighty cephalopod beaks were identified using the guide developed by Clarke (Reference Clarke1986); these beaks were also compared with those from entire cephalopods found in the stomachs. The cephalopod weight was estimated by multiplying the total number of beaks by the mean individual weight of whole individuals of the same species found in the corresponding samples (Hyslop, Reference Hyslop1980).
To analyse the monthly evolution of the catch per unit effort (CPUE), as a relative abundance index of the main prey species of swordfish in the study area, daily catch and effort data from the Moroccan purseiners operating in the Strait of Gibraltar during 2015, were obtained from the fish market at the port of Tangier.
Statistical analysis
The diet of swordfish was characterized by calculating and analysing the following indices (Hureau, Reference Hureau1970; Hyslop, Reference Hyslop1980):
Fullness index: fi = W/We
W: total weight of the various prey
We: eviscerated weight of fish
Frequency of occurrence index: FO = Ni/Np, where
Np: Total number of full stomachs
Ni: Number of stomachs containing prey i
Percentage in number: Cni = Pi × 100/P
Pi: Number of observations of the prey i
P: Number of various preys
Percentage in weight: Cpi = Wi × 100/W
Wi: total weight of the prey i
W: total weight of the various prey
Index of relative importance: IRI
This index combines both the frequency of occurrence of prey, its percentage in number (Cni) and its percentage in weight (Cpi). Thus, it gives a more accurate picture of the diet than that obtained by using a single index, and minimizing the biases caused by each of these percentages (Pinkas et al., Reference Pinkas, Oliphan and Iverson1971). It is expressed as follows:
Per cent index of relative importance: %IRI
It is the ratio between the index value (IRI) of each prey and the sum of all index values (ΣIRI).
IRI% = IRI /ΣIRI
This index allows classification of prey items as preferential (%IRI > 50%), secondary (10 ≤ %IRI ≤ 50%), complementary (1 ≤ %IRI ≤ 10%) and accidental (%IRI < 1%) (Pinkas et al., Reference Pinkas, Oliphan and Iverson1971).
To compare the swordfish diet by size class, sex and month, we used the χ2 statistical test on contingency tables (percentage of prey in number), with rows representing the prey groups/species and columns the levels of different variables (size, sex and month) (Zar, Reference Zar1984). In addition, a linear discriminant analysis was performed employing as the dependent variable the percentage (in terms of weight) of each prey species and as explanatory variables the size classes, sex and month of capture (Quinn & Keough, Reference Quinn and Keough2002).
The F test of ANOVA analysis was used to evaluate if there is any linear correlation between the body size of swordfish (LJFL) and the mean individual weight of prey, and between sex of fish and the mean individual weight of prey (Zar, Reference Zar1984). All statistical analyses were performed using the statistical program XLStat-Pro. 7.5. All statistical inference was based on the 95% significance level.
RESULTS
Fullness index
The mean fullness index values (fi) decreased from April to August, reaching a minimum value of 0.67 in August, and increased in September. The differences among months were statistically significant (F = 27.34, P < 0.01). However, the differences in mean values of this index were not statistically significant between males and females (F = 0.799, P = 0.373) and between individuals of different size classes (F = 0.421, P = 0.657) (Figure 2A–C).
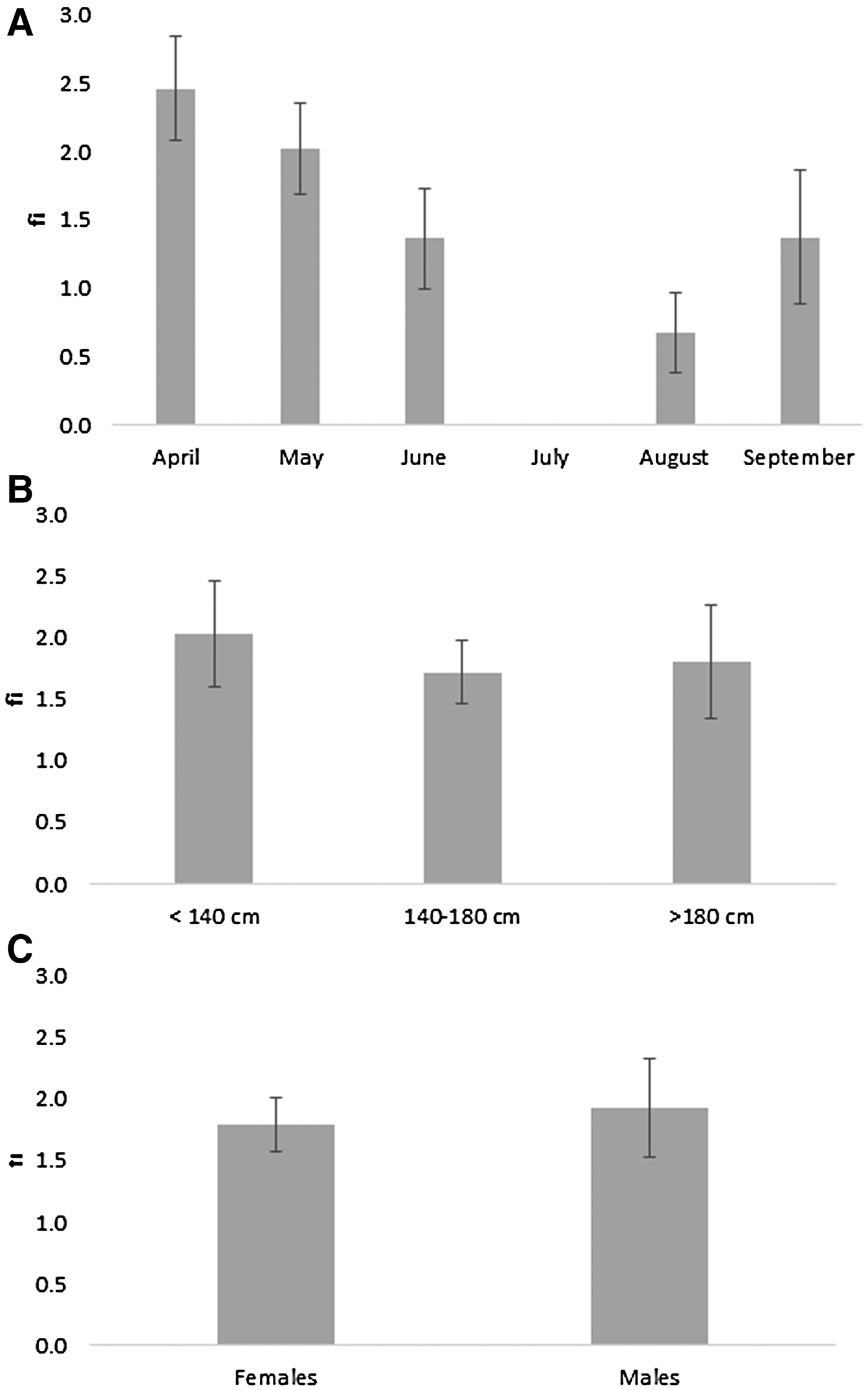
Fig. 2. Evolution of the mean fullness index values (fi) by month (A); size class (B); by sex (C) for swordfish caught in the Strait of Gibraltar (bars represent confidence interval at 95%).
Diet composition
Except for some fragments of unidentified fish that represented about 5% of the total food items in number, all prey items found in the examined stomachs were identified.
In general, the diet of the swordfish caught in the Strait of Gibraltar was quite diverse. It consisted of 13 species of which nine species were fish, two species were cephalopods and two species were crustaceans, belonging to 12 families (Table 1).
Table 1. The frequency occurrence, the percentage in number, the percentage in weight and the %IRI of each prey species in swordfish stomachs.

The analysis of per cent index of relative importance (%IRI), for the whole fishing season, showed that there was no preferential prey species for swordfish caught in the Strait of Gibraltar (%IRI for all prey items were <50%) (Table 1).
Analysis of the diet according to the size of predator
The analysis of per cent index of relative importance (%IRI) by size classes showed that there was no preferential prey for any given size class (%IRI < 50). From the values of this index, the Atlantic horse mackerel (Trachurus trachurus), the southern shortfin squid (Illex coindetii) and the silver scabbard fish (Lepidopus caudatus) were all secondary prey for the three size classes considered (10 < %IRI < 50%) (Figure 3).

Fig. 3. Per cent index of relative importance (%IRI) by size class for the main prey species of swordfish caught in the Strait of Gibraltar.
The percentage by number of prey groups (fish, cephalopods and crustaceans) in the diet varied significantly among the three size classes for crustaceans (χ2 = 9488, P = 0.002). Indeed, the percentage of kangaroo shrimp (Glyphus marsupialis) that essentially made up this group was relatively higher (6.9%) in individuals less than 140 cm LJFL compared with fish larger than this size (<0.1%). However the differences in the percentages by number of the main prey species were not statistically significant among size classes at 5% (χ2 = 9488, P = 0.062) (Figure 4).

Fig. 4. Percentage in number by size class of the main prey groups and prey species of swordfish caught in the Strait of Gibraltar.
The results of the linear discriminant analysis confirmed those based on the %IRI and the percentage by number of each prey species. Indeed, according to the results of this analysis, it was not possible to discriminate among individuals of the three size classes considered based on the percentage by weight of their prey species (F = 2.147, P = 0.152).
The F test result showed that there is a significant linear correlation between the size of swordfish and the mean individual weight of prey (F = 11.82, P = 0.00069). Thus, the size of prey tends to increase with the size of predator (Figure 5).

Fig. 5. Relationship between the size of swordfish and individual mean weight of prey species.
Analysis of the diet according to the sex of predator
The prey species ingested by swordfish remain generally similar between males and females. The analysis of the per cent index of relative importance (%IRI) by sex indicated that Atlantic horse mackerel (T. trachurus) and the southern shortfin squid (I. coindetii) were secondary prey for both sexes, while the silver scabbard fish (L. caudatus) is secondary prey for females (%IRI = 24.07%), but only a complementary prey for males (%IRI = 6.36%). It seems that females consume more silver scabbardfish than males, while the latter consume more shortfin squid than females (Figure 6).

Fig. 6. Per cent index of relative importance (%IRI) by sex for the main prey species of swordfish caught in the Strait of Gibraltar.
Statistical analysis showed that individual mean weight of prey species varies significantly among sexes (F = 10.79, P = 0.00115). It seems that females tend to feed on larger prey size species than males (Figure 7).

Fig. 7. Individual mean weight distribution of prey species in male and female swordfish caught in the Strait of Gibraltar.
The differences in percentage by number of prey groups were not statistically significant between males and females (χ2 = 5.991, P = 0.067). Neither are the differences in percentage by number of the main prey species (χ2 = 7.81, P = 0.054) (Figure 8).

Fig. 8. Percentage in number by sex of the main prey groups and prey species of swordfish caught in the Strait of Gibraltar.
The results of linear discriminant analysis showed also that there were no significant differences in the percentages by weight of the main prey species between males and females (F = 1.390, P = 0.248).
Analysis of the diet according to the month
The monthly analysis of per cent index of relative importance (%IRI) revealed that the Atlantic horse mackerel (T. trachurus) is the preferential prey of swordfish during the months of April and May, with a %IRI of 73.30% and 60.80%, respectively, while the silver scabbard fish (L. caudatus) and the southern shortfin squid (I. coindetii) are only secondary prey. The situation was reversed during August–September where the shortfin squid clearly became the preferential food of swordfish (%IRI = 92%), while the Atlantic horse mackerel and silver scabbard fish were only complementary prey of the predator with a %IRI of 2.96% and 2.01%, respectively.
It should be noted that sardine (Sardina pichardus) was also a complementary prey of swordfish in June with a %IRI of 2.37%. It is the same for the Kangaroo shrimp (G. marsupialis) during the month of August (%IRI = 1.32%) (Figure 9).

Fig. 9. Per cent index of relative importance (%IRI) by month of the prey species of swordfish caught in the Strait of Gibraltar.
The differences in the percentages by number of prey groups are statistically significant between months (χ2 = 15.5, P < 0.0001). It is the same for the percentages by number of the main prey species (χ2 = 31.41, P < 0.0001). As shown by %IRI analysis, there was a slight supremacy of Atlantic horse mackerel in April–May in number (51%), however the southern shortfin squid became the dominating prey during the period August–September (71%) (Figure 10).

Fig. 10. Percentage in number by month of the main prey groups and species of swordfish caught in the Strait of Gibraltar.
The results of the discriminant analysis confirmed the previous analyses results, showing that the differences in the percentages by weight of prey species are statistically significant among months (F = 1.59, P < 0.0001). The southern shortfin squid and the Atlantic horse mackerel were the two species that essentially discriminate the diet of swordfish by months (Figure 11).

Fig. 11. Results of the discriminant analysis based on the percentage in weight of the main prey species of swordfish caught in Strait of Gibraltar.
DISCUSSION
The findings of the present study confirmed the predominance of teleost fish in the diet of swordfish in the Strait of Gibraltar, as previously reported by Hernández-García (Reference Hernández-García1995). Similar results were also found in the Eastern Atlantic and in the Mediterranean Sea (Moreira, Reference Moreira1990; Salman, Reference Salman2004; Romeo et al., Reference Romeo, Consoli, Castriota and Andaloro2009). On the contrary, many authors clearly highlighted the importance of cephalopods in the diet of the species in the Atlantic (Toll & Hess, Reference Toll and Hess1981; Guerra et al., Reference Guerra, Simún, Gonzalez, Okutani, O'Dor and Kubodera1993; Hernández-García, Reference Hernández-García1995; Chancollon et al., Reference Chancollon, Pusineri and Ridoux2006) as well as in the Mediterranean Sea (Bello, Reference Bello1991; Peristeraki et al., Reference Peristeraki, Tserpes and Lefkaditou2005).
The differences in the diet composition of swordfish among different geographic areas, confirm the opportunistic feeding behaviour of the species, foraging a wide range of abundant prey in their distribution area (Stillwell & Kohler, Reference Stillwell and Kohler1985; Guerra et al., Reference Guerra, Simún, Gonzalez, Okutani, O'Dor and Kubodera1993; Velasco & Quintans, Reference Velasco and Quintans2000; Chancollon et al., Reference Chancollon, Pusineri and Ridoux2006; Young et al., Reference Young, Lansdell, Riddoch and Revill2006). The variations in the swordfish diet seem to be due to the proximity to the coast (longitudinal variation) rather than to latitudinal variation, since all prey species whose abundance varies from one area to another, have a wide geographic distribution. Thus, Hernández-García (Reference Hernández-García1995), Chancollon et al. (Reference Chancollon, Pusineri and Ridoux2006) and Young et al. (Reference Young, Lansdell, Riddoch and Revill2006) found different patterns of predation in swordfish between neritic and oceanic areas. Indeed, in neritic areas, as is the case in our study, these authors found that the diet of swordfish was primarily based on pelagic and benthic fish and squid, while in the oceanic areas, this species feeds mainly on cephalopods.
In the Strait of Gibraltar, the diet composition of swordfish determined by this study (13 species) is quite diverse. It is comparable to that obtained by Toll & Hess (Reference Toll and Hess1981) from the Florida Straits (15 species); Moreira (Reference Moreira1990) off the Portuguese coast (14 species); and Peristeraki et al. (Reference Peristeraki, Tserpes and Lefkaditou2005) in the southern Aegean Sea (11 species). However, this figure is lower than that found by Stillwell & Kohler (Reference Stillwell and Kohler1985) in the north-western Atlantic Ocean (36 species), Hernández-García (Reference Hernández-García1995) in the central-east Atlantic (38 species), Salman (Reference Salman2004) in the Aegean Sea (34 species) and Romeo et al. (Reference Romeo, Consoli, Castriota and Andaloro2009) in the Central Mediterranean Sea (40 species). The differences in number of prey species ingested by swordfish are related to their abundance and availability in a given area (Stillwell & Kohler, Reference Stillwell and Kohler1985). It is generally accepted that large predators such as swordfish eat all that is abundant in their immediate environment (Palko et al., Reference Palko, Beardsley and Richards1981).
In addition, it should be noted that the number of cephalopod species identified by the present study (two species) is lower than that reported by Hernández-García (Reference Hernández-García1995) in the Strait of Gibraltar (13 species), Salman (Reference Salman2004) in the Aegean Sea (19 species) and Romeo et al. (Reference Romeo, Battaglia, Peda, Perzia, Consoli, Esposito and Andaloro2011) in the central Mediterranean Sea (20 species). These discrepancies could be explained by the fact that in the study area, the Moroccan artisanal longline fleet targets swordfish in surface waters at depths not exceeding generally 30 m, where the few abundant epipelagic squid prey such as I. coindetii are available to swordfish at night when these cephalopods rise near the surface to feed. In contrast, in the Mediterranean Sea, large longliners fished for swordfish in deeper waters (10–100 m) (García-Barcelona et al., Reference García-Barcelona, Ortiz de Urbina, de la Serna, Alot and Macías2010; Cambie et al., Reference Cambiè, Muino, Mingozzi and Freire2013). The mesopelagic longliners can even target large swordfish at depths ranging between 100 and 500 m (Cambie et al., Reference Cambiè, Muino, Mingozzi and Freire2013), which could explain the higher number of cephalopod species found in the swordfish stomachs contents. Furthermore, a recent study conducted in the central western Mediterranean Sea showed that the presence of cephalopods in the diet of large pelagics is strictly related to the water layer where the predator usually feeds (Romeo et al., Reference Romeo, Battaglia, Peda, Perzia, Consoli, Esposito and Andaloro2011). Unfortunately, the rocky aspect and the irregular bottom topography of the Strait of Gibraltar, make it difficult to assess the cephalopod biodiversity in this particular area by means of bottom trawl surveys (Sánchez-Garrido et al., Reference Sánchez-Garrido, Sannino, Liberti, García Lafuente and Pratt2011).
The results from this study confirm that cephalopods, especially the ommastrephidae, play an important role in the diet of swordfish in the Strait of Gibraltar, contributing 43.36% of total prey in number. This fact was previously highlighted by several authors (Stillwell & Kohler, Reference Stillwell and Kohler1985; Bello, Reference Bello1991; Guerra et al., Reference Guerra, Simún, Gonzalez, Okutani, O'Dor and Kubodera1993; Clarke et al., Reference Clarke, Clarke, Martins and Silva1995; Hernández-García, Reference Hernández-García1995; Velasco & Quintans, Reference Velasco and Quintans2000; Young et al., Reference Young, Lansdell, Riddoch and Revill2006; Romeo et al., Reference Romeo, Consoli, Castriota and Andaloro2009, Reference Romeo, Battaglia, Peda, Perzia, Consoli, Esposito and Andaloro2011). Moreover, it is well known that these muscular, fast-swimming squids are high-speed, growing and active predators, which efficiently convert prey into biomass (Clarke, Reference Clarke1996).
The analysis of the depth distribution of prey species showed that in the Strait of Gibraltar swordfish feeds primarily on pelagic species such as the Atlantic horse mackerel (T. trachurus), but also on some demersal species like the southern shortfin squid (I. coindetii) and silver scabbard fish (L. caudatus). It can even occasionally forage some deep crustacean species such as the striped red shrimp (Aristeus varidans) (300–1134 m) (Holthuis, Reference Holthuis1980) and kangaroo shrimp (G. marsupialis) (500–1100 m) (Fischer et al., Reference Fischer, Bianchi and Scott1981). The last two prey species have been reported for the first time by the present study. The presence of these two crustacean species in the swordfish stomachs confirms that swordfishes are able to dive during the day to depths reaching up to 800 m (Takahashi et al., Reference Takahashi, Okamura, Yokawa and Okazaki2003; Matsumoto et al., Reference Matsumoto, Saito and Miyabe2003; Canese et al., Reference Canese, Garibaldi, Orsi Relini and Greco2008). This can also be justified by the bathymetric characteristics of the study area, in which the maximum depth reaches up to 900 m (Malanotte-Rizzoli & Robinson, Reference Malanotte-Rizzoli and Robinson1994).
The wide bathymetric distribution of prey species, and the presence of entire food items in swordfish stomachs, indicate that swordfish feeds throughout the water column, especially at night when some species, such the southern shortfin squid and silver scabbard fish, rise near the surface in search of prey (Roper & Young, Reference Roper and Young1975). Moreover, the presence in the majority of examined stomachs of many individuals belonging to the same prey species and with similar size confirms that swordfish prefers chasing schools of fish and cephalopods on which to feed (Tibbo et al., Reference Tibbo, Day and Doucet1961; Carey & Robinson, Reference Carey and Robinson1981).
The findings from this study revealed that swordfish of both sexes feed generally on the same prey species. Similarly, the percentages of the main prey groups and species (both in number and in weight) did not vary significantly according to the sex and the size of predator. Our results are in agreement with those found by Stillwell & Kohler (Reference Stillwell and Kohler1985) and Ibáñnez et al. (Reference Ibáñez, González and Cubillos2004).
Our results showed for the first time that there are significant differences in the diet composition of swordfish between months, depending on the abundance of prey species in the study area. In fact, the high proportion of Atlantic horse mackerel in the stomach contents in April, suggests their abundance in the area, as shown by the monthly evolution of catches and abundance index of this species from the Moroccan purse seiners operating in the Strait of Gibraltar (Figure 12A, B). The high relative abundance of T. trachurus in the Strait of Gibraltar from January to May could be due to the fact that this species spawns during that period in the study area. Indeed, previous studies showed that in the Moroccan Atlantic coast adjacent to the Strait of Gibraltar, Atlantic horse mackerel spawns from January to May (Sedletskaya, Reference Sedletskaya1971).

Fig. 12. Monthly evolution of the catches (A) and the CPUE (B) for Trachurus trachurus caught by Moroccan purseiners in the Strait of Gibraltar.
The predominance of small size shortfin squid (average weight of about 107 g) in the swordfish stomachs during August–September is worth noting. Results from the most recent bottom trawl surveys in the Atlantic area adjacent to the Strait of Gibraltar showed that the southern shortfin squid was the most abundant species of cephalopod in the area during that period (Benchoucha et al., unpublished).
The results from this study revealed also that there is a significant linear correlation between the body length of the predator and the size of its prey species. This means that large swordfish tend to feed on larger prey than small ones. These results are in agreement with those obtained by other authors (Velasco & Quintans, Reference Velasco and Quintans2000; Chancollon et al., Reference Chancollon, Pusineri and Ridoux2006).
The remarkable decrease in the fullness index (fi) from June to August may indicate that this period corresponds to the spawning season of swordfish caught in the area. Thus, the observed decline in the fullness index would be associated with a change in the physiological state of the fish owing to the process of gonad maturation (Berg, Reference Berg1979). This hypothesis is supported by previous studies suggesting that swordfish spawns in the Mediterranean Sea in the summer time (Tserpes et al., Reference Tserpes, Peristeraki and Somarakis2001). Furthermore, a more recent growth study suggests that a fraction of swordfish catches in the Strait of Gibraltar would be of Mediterranean origin (Abid et al., Reference Abid, Bakkali, Tserpes and Idrissi2013), supporting results of the most recent genetic studies that showed that the Mediterranean swordfish population extends beyond the current management boundary at the Strait of Gibraltar to ~10°W (Smith et al., Reference Smith, Lu, García-Cortés, Viñas, Yeh and Alvarado Bremer2015).
In the light of current results and given that the diet of swordfish depends considerably on the abundance of prey species in their distribution area, more studies will be needed in the future to depict any changes in the prey species composition due to fishing activities and environmental factors. Indeed, research has shown that variations in the abundance of marine organisms depend on the combined effect of the sea surface temperature and fishing (Ware, Reference Ware1995; O'Brien et al., Reference O'Brien, Fox, Planque and Casey2000). The sea temperature can also have a direct effect on spawning and survival of larvae and juveniles as well as on fish growth, by acting on physiological processes. It also affects the biological production rate, thus food availability in the ocean, which is a powerful regulator of fish abundance and distribution (Lehodey et al., Reference Lehodey, Bertignac, Hampton, Lewis and Picaut1997).
ACKNOWLEDGEMENTS
This study would not have been possible without the help of many people. I would like to thank especially my colleague Dr Said Benchoucha for having helped in the identification of crustacean prey species. My thanks go also to Dr George Tserpes and Dr John Neilson for their useful comments.















