INTRODUCTION
Cephalopods are highly versatile predators which feed opportunistically on a wide range of prey. The study of cephalopod feeding is complicated by characteristics of digestive process: the prey is bitten into small pieces by the chitinous beaks prior to swallowing. This makes the identification of components in the food lumps difficult (Zuev & Nesis, Reference Zuev, Nesis and Sweeney1971). Despite these problems, a considerable amount of information on the trophic relationships of cephalopods has been collected using direct observations, conventional visual analysis of stomach contents, stable isotope ratios, lipid signatures and serological methods (Boyle & Rodhouse, Reference Boyle and Rodhouse2005). However, only a few of these studies are about feeding in early life stages.
Some authors suggest a high mortality rate during paralarval phase either by predation or failure in the first feeding stage after the yolk absorption (Vecchione, Reference Vecchione and Boyle1987, Reference Vecchione1991; Sakurai et al., Reference Sakurai, Kiyofuji, Saito, Goto and Hiyama2000). After hatching, most cephalopod paralarvae have a buccal mass completely differentiated including the radula and beak. The alimentary canal seems ready to function as soon as the first prey items are ingested (Boletzky, Reference Boletzky2003). The fully developed hatchlings still have sufficient yolk reserves allowing them a brief period to practise and improve their hunting behaviour without requiring that every attack be successful. The remaining yolk also allows them to sustain the high energy consumption required by the active movements involved in prey attack (Boletzky, Reference Boletzky2003). Survival of newly hatched paralarvae depends on their ability to switch successfully from yolk nourishment to active predation when the yolk is exhausted (Uchikawa et al., Reference Uchikawa, Sakai, Wakabayashi and Ichii2009).
Preliminary observations on beak development in Todarodes pacificus paralarvae indicated that they may depend on their yolk reserves for around four days after hatching (Bower et al., Reference Bower, Nakamura, Mori, Yamamoto, Isoda and Sakurai1999). In fact unfed paralarvae of this species die in captivity at about 6–7 days after hactching (Bower & Sakurai, Reference Bower and Sakurai1996). Uchikawa et al. (Reference Uchikawa, Sakai, Wakabayashi and Ichii2009) showed that Ommastrephes bartramii paralarvae can bite prey soon after the beaks protrude, at 3–4 mm mantle length (ML) (13–16 days old).
Ommastrephid paralarvae (‘rhynchoteuthions’) are distinctive and easily recognizable because the two tentacles are fused into a ‘proboscis’ (Shea, Reference Shea2005). The role of the proboscis in feeding is unclear. According to Vidal & Haimovici (Reference Vidal and Haimovici1998) the proboscis may play an important role in the ingestion of mucus which serves as a substrate for microbial growth but also may be a food source. This mucus feeding could provide energy before the paralarvae attain the state necessary for prey capture.
The ommastrephid jumbo flying squid, Dosidicus gigas (D'Orbigny, 1835) is an active predator. It is especially abundant in the Gulf of California, where it constitutes the only squid fishery in Mexico since 1974. Although it is commercially important almost nothing is known on many aspects of the early life stages. Adult squid feed mainly on mesopelagic micronekton, such as myctophids and squids (Markaida & Sosa-Nishizaki, Reference Markaida and Sosa-Nishizaki2003; Markaida, Reference Markaida2006; Markaida et al., Reference Markaida, Gilly, Salinas-Zavala, Rosas-Luis and Booth2008). These prey types also were found in adults from Chilean and Peruvian waters (Ulloa et al., Reference Ulloa, Fuentealba and Ruíz2006; Ibáñez et al., Reference Ibáñez, Arancibia and Cubillos2008; Liu et al., Reference Liu, Chen, Lu, Chen and Quian2010; Rosas-Luis et al., Reference Rosas-Luis, Tafur-Jiménez, Alegre-Norza, Castillo-Valderrama, Cornejo-Urbina, Salinas-Zavala and Sánchez2011). For early life stages, only one report exists (Shchetinnikov, Reference Shchetinnikov1989). He distinguished four ontogenetic stages for D. gigas from Peru and described changes in the food spectrum passing over higher trophic level (from plankton feeders to large-size nyctoepipelagic predators). In this paper, we analysed for first time the food contents in digestive systems of paralarvae and juvenile D. gigas to describe the diet of this species in the Gulf of California.
MATERIALS AND METHODS
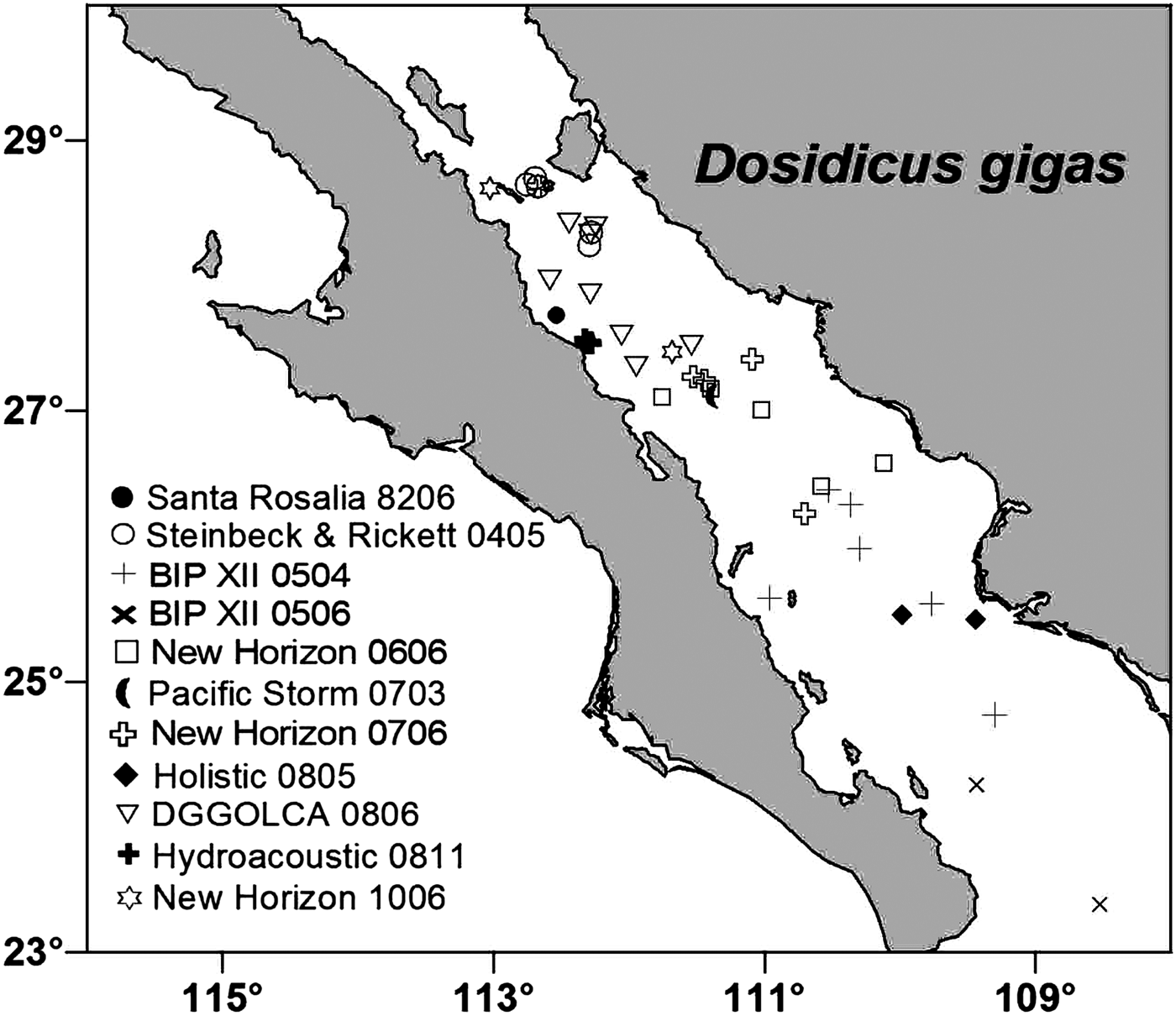
Samples of early life stages of the squid Dosidicus gigas were taken from ten different research cruises carried out from 1982 to 2010 at locations of the central and southern Gulf of California (Figure 1). Paralarvae were collected using a 0.5 m diameter bongo net with 0.5 mm mesh size. One 10-min oblique tow was conducted at each station to about 150 m depth. The ship's towing speed was about 1–1.5 knots. On board, plankton samples from one net were fixed in 75% ethanol and samples from the other net were fixed in a 4% buffered formaldehyde–seawater solution. Juvenile squid were caught with a dipnet from the sides of the ship while at anchor without any attracting lights. Some of the juveniles were fixed in 85–95% ethanol and others with 4% formalin. We distinguished paralarvae from juveniles based on complete separation of the paralarval ‘proboscis’ into two functional tentacles. In the laboratory, D. gigas paralarvae were identified based on the criteria proposed by Nesis (Reference Nesis1979), Wormuth et al. (Reference Wormuth, O'Dor, Balch, Dunning, Forch, Harman, Rowell, Sweeney, Roper, Mangold, Clarke and Boletzky1992), Yatsu (Reference Yatsu1999), Yatsu et al. (Reference Yatsu, Tafur and Maravi1999) and Ramos-Castillejos et al. (Reference Ramos-Castillejos, Salinas-Zavala, Camarillo-Coop and Enriquez-Paredes2010). The juveniles were assumed to belong to D. gigas, as no other ommastrephid has been caught in the study area. Dorsal mantle lengths (MLs) of all D. gigas paralarvae were measure to the nearest 0.1 mm with an ocular micrometer in a Stemi DV4 Zeiss stereomicroscope. Mantle lengths from deformed paralarvae were not measured. Juvenile MLs were measured with an electronic digital caliper.

Fig. 1. Dosidicus gigas early life stages collected in the Gulf of California. The symbols indicate the research cruise (name–year–month) and the sites where specimens used in this work were collected.
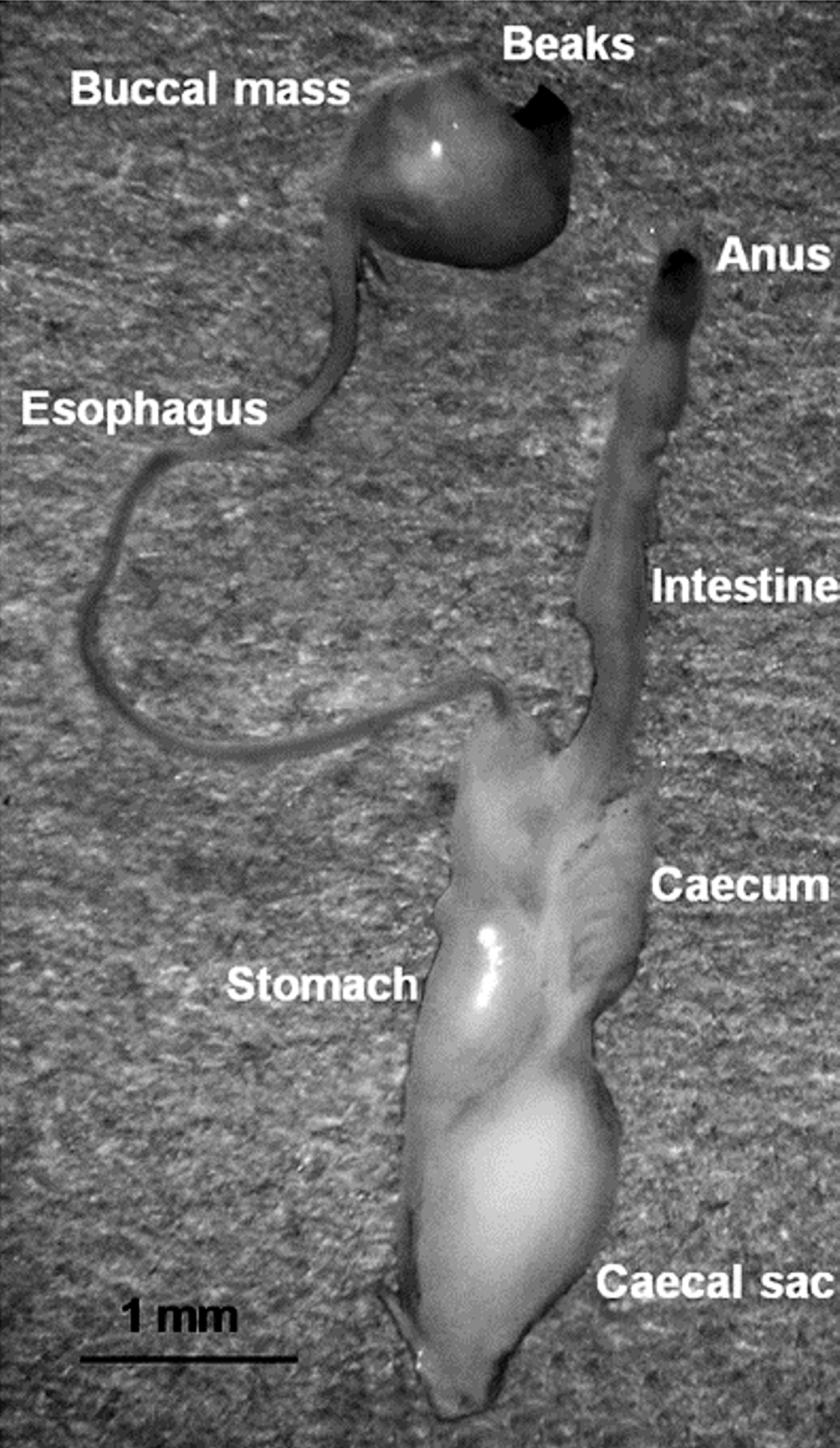
The digestive system comprises the buccal complex, oesophagus, salivary glands, stomach, digestive gland, digestive-gland duct appendages, caecum, intestine and anus (Mangold & Bidder, Reference Mangald and Bidder1989). All these parts (Figure 2) were placed on a hole-slideglass, dissected with fine dissecting needles and examined under a Meiji stereomicroscope at 75× magnification and a Zeiss Stemi stereomicroscope 2000-C at 100× magnification.
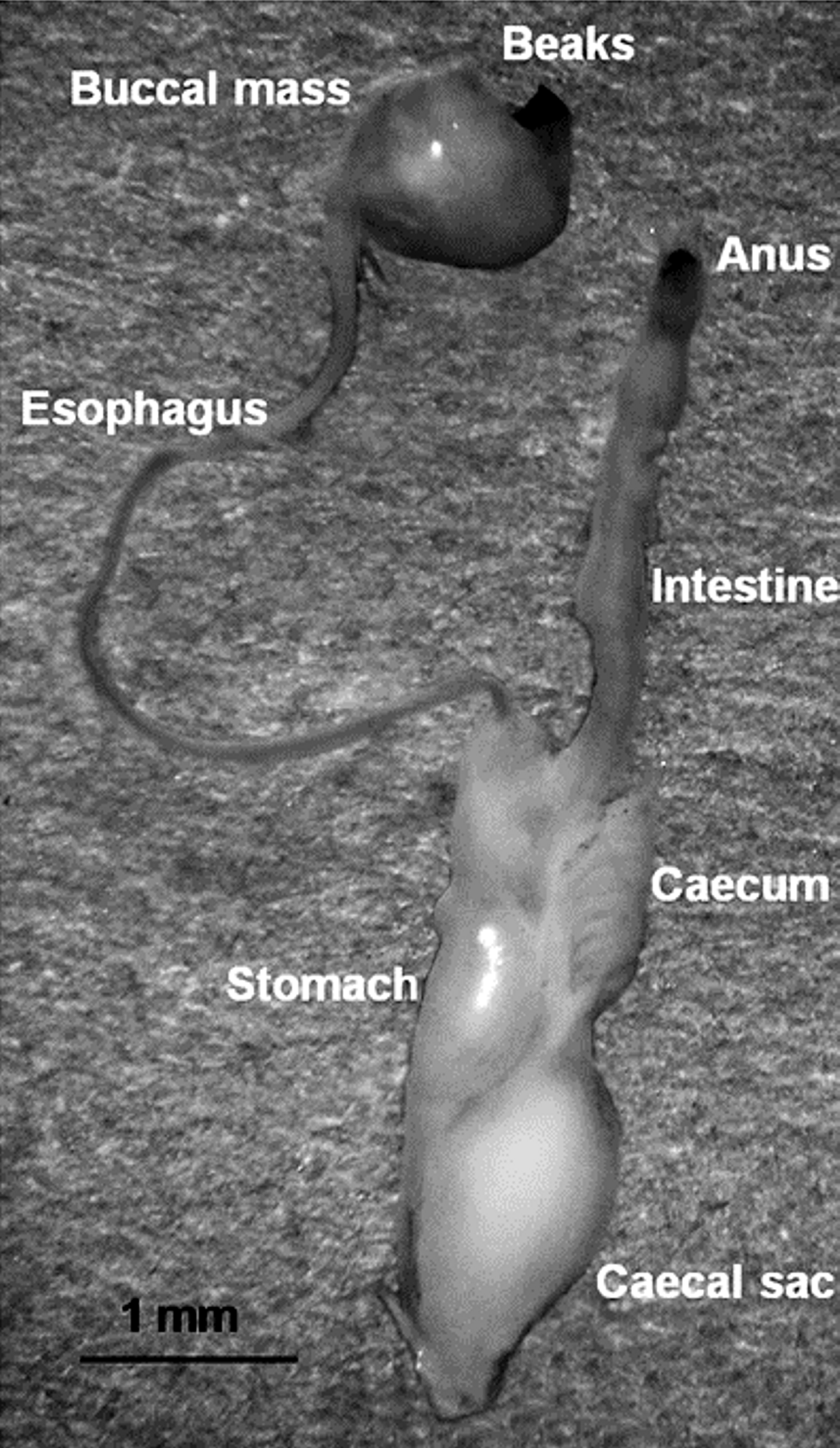
Fig. 2. Digestive system from a juvenile of Dosidicus gigas (13.2 mm mantle length). All the structures indicated were examined for food content.
The food content was separated first into identifiable material (IM) and non-identifiable material (NIM). In some cases of hard structures covered with much NIM material, a few drops of distilled water with sodium hydroxide (NaOH) were added for 3–5 min or even one day to remove exceeding NIM. The chitin fragments were rinsed with distilled water, photographed and stored in Eppendorf vials with the original preservative solution.
The stomach fullness index (FI) proposed by Breiby & Jobling (Reference Breiby and Jobling1985) was used which assigns values between 0 and 4. We modified the scale to 0–3 for juveniles: (0) empty, (1) scarce remains, (2) half full and (3) completely full. The frequency of occurrence (FO) was also estimated as the number of jumbo squid that fed on a certain prey. The number of each prey type per squid examined was not counted.
Prey diversity was recorded into major taxonomic groups. Crustaceans were recognized from remains of exoskeletons, appendages and eyes; fish from scales, otoliths, vertebrae, spines and jaw elements, and cephalopods from beaks, lenses, suckers, statolith and arm pieces. Crustaceans were subdivided into four groups: amphipods, copepods, euphausiids and unidentified crustacean. Copepods species were identified by consulting Ricardo Palomares (Centro Interdisciplinario de Ciencias Marinas, IPN La Paz, Mexico), euphausiids following Brinton (Reference Brinton, Gasca and Suárez-Morales1996) and amphipods based on Vinogradov et al. (Reference Vinogradov, Volkov and Semenova1996) and Gasca (Reference Gasca2009).
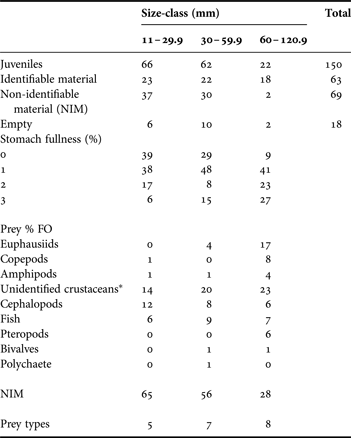
Food contents data from paralarvae and juveniles were analysed separately as the contents of paralarvae were so well chopped that no comparison could be done with juvenile's food content. Mantle lengths of juveniles were ranked in three size-classes for an ontogenetic analysis: 11–29.9 mm, 30–59.9 mm and 60–120.9 mm ML (N = 66, 62 and 22 squid, respectively).
All images were composed and edited for publication using Corel PhotoshopTM.
RESULTS
In total, 186 digestive systems were analysed from 36 paralarvae and 150 juveniles. The paralarvae considered in this paper measured between 2.8 and 10.4 mm ML, most of them with proboscis splitting. Proboscis division started at 5.3 mm ML and was completed at 10.4 mm ML. Juveniles squid with tentacles completely separated were all ≥11 mm ML, except the three early juveniles ranging from 9 to 9.8 mm ML.
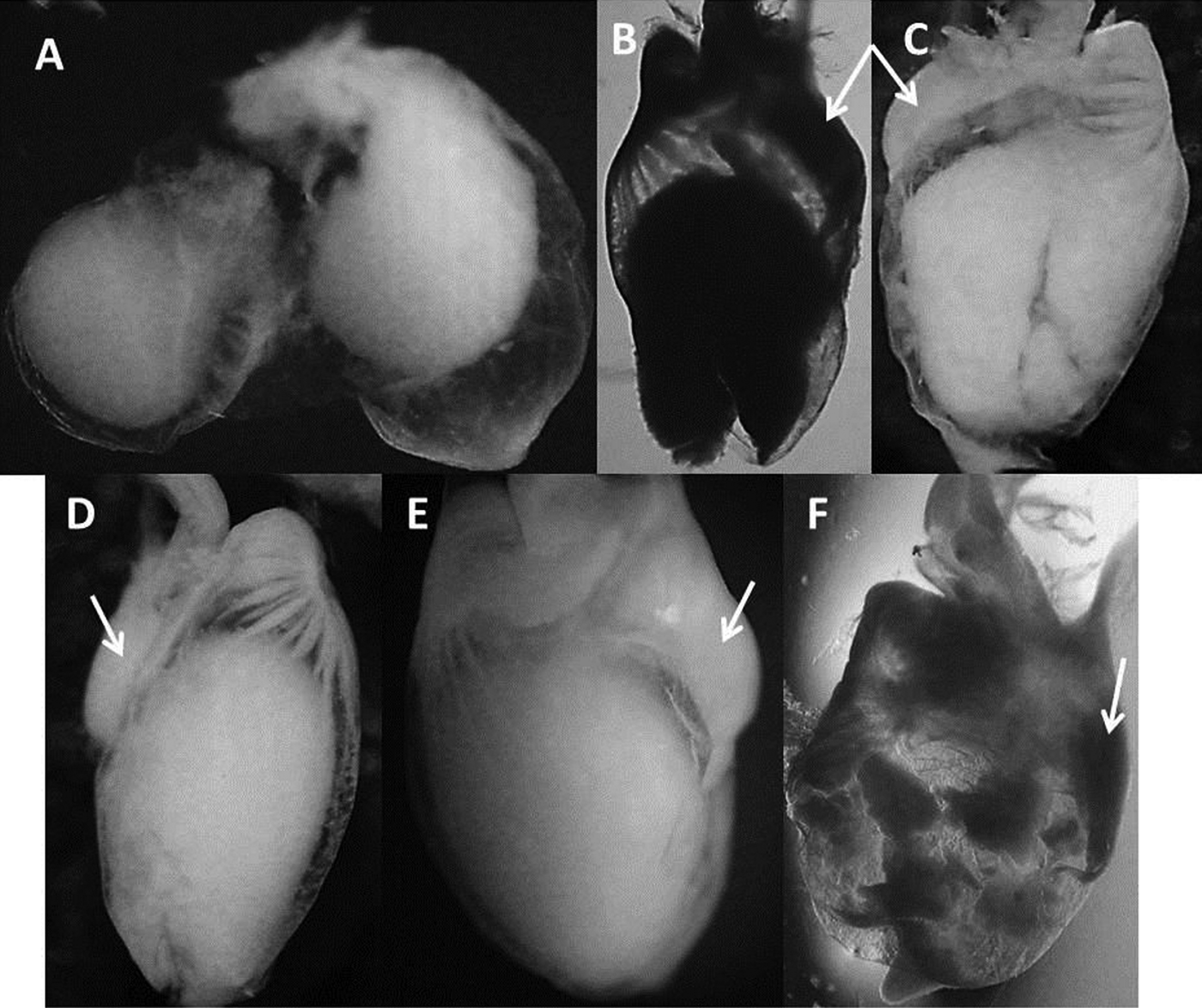
All paralarvae presented non-identifiable food content without any hard structure. This NIM was contained mostly in the caecum (Figure 3).

Fig. 3. Caecum and stomach of Dosidicus gigas paralarvae. All paralarvae presented non-identifiable material stored in the caecum rather than in the stomach. White arrows indicate the position of the stomach. Pictures are ordered by increasing mantle length of the dissected paralarvae: (A) 2.8 mm; (B) 8.2 mm; (C) 8.8 mm; (D) 9.2 mm; (E) 9.4 mm; (F) 9.8 mm.
From 150 juvenile digestive systems, 63 had identifiable material, 69 had only non-identifiable material and 18 were empty (Table 1). Squids in the largest size-class were underrepresented due to the low number of specimens with mantle lengths >50 mm collected.
Table 1. Summary data for Dosidicus gigas juveniles captured from 1982 to 2010 in the Gulf of California. The content of digestive systems, stomach fullness and frequency of occurrence percentage (% FO) of prey types are presented for the three mantle length size-classes of juveniles.

*All fragments and remains do not correspond to euphausiids, copepods and amphipods.
Stomach fullness
The size-class of 11–29.9 mm ML comprised 66 juvenile squid showing high percentages of empty stomachs or with scarce remains, and low percentages of half full to completely full stomachs. The next size-class (30–59.9 mm ML) contained 62 juveniles. They showed an increase in the percentages with scarce remains and completely full stomachs. Only 22 juveniles were in the largest size-class (60–120.9 mm ML). These had a notable decrease of empty stomachs, an increase of scarce remains, half full and, completely full stomachs (Table 1).
Diet per size-class
Unidentified crustacean were the main prey type with the highest frequency of occurrence (57%) and were present in all size-classes. Other prey items found in all size-classes were cephalopods (26% FO), fish (22% FO) and amphipods (6% FO) although their FO between size-classes were more variable than that of unidentified crustacean.
The first size-class of juveniles (11–29.9 mm ML) fed on five prey types. According with the FO, the preys ingested were mainly unidentified crustaceans (eyes, appendages, no identifiable chitin fragments), cephalopods, fish, amphipods and copepods. At the size-class of 30–59.9 mm ML the prey types increased to seven, with the most frequent being unidentified crustaceans, fish, cephalopods and euphausiids. Less frequent were amphipods, bivalves and a single polychaete. Squid from the largest size-class presented eight prey types. Unidentified crustaceans, euphausiids, and copepods were the main prey types by FO, followed by fish, pteropods, cephalopods, amphipods and bivalves (Table 1).
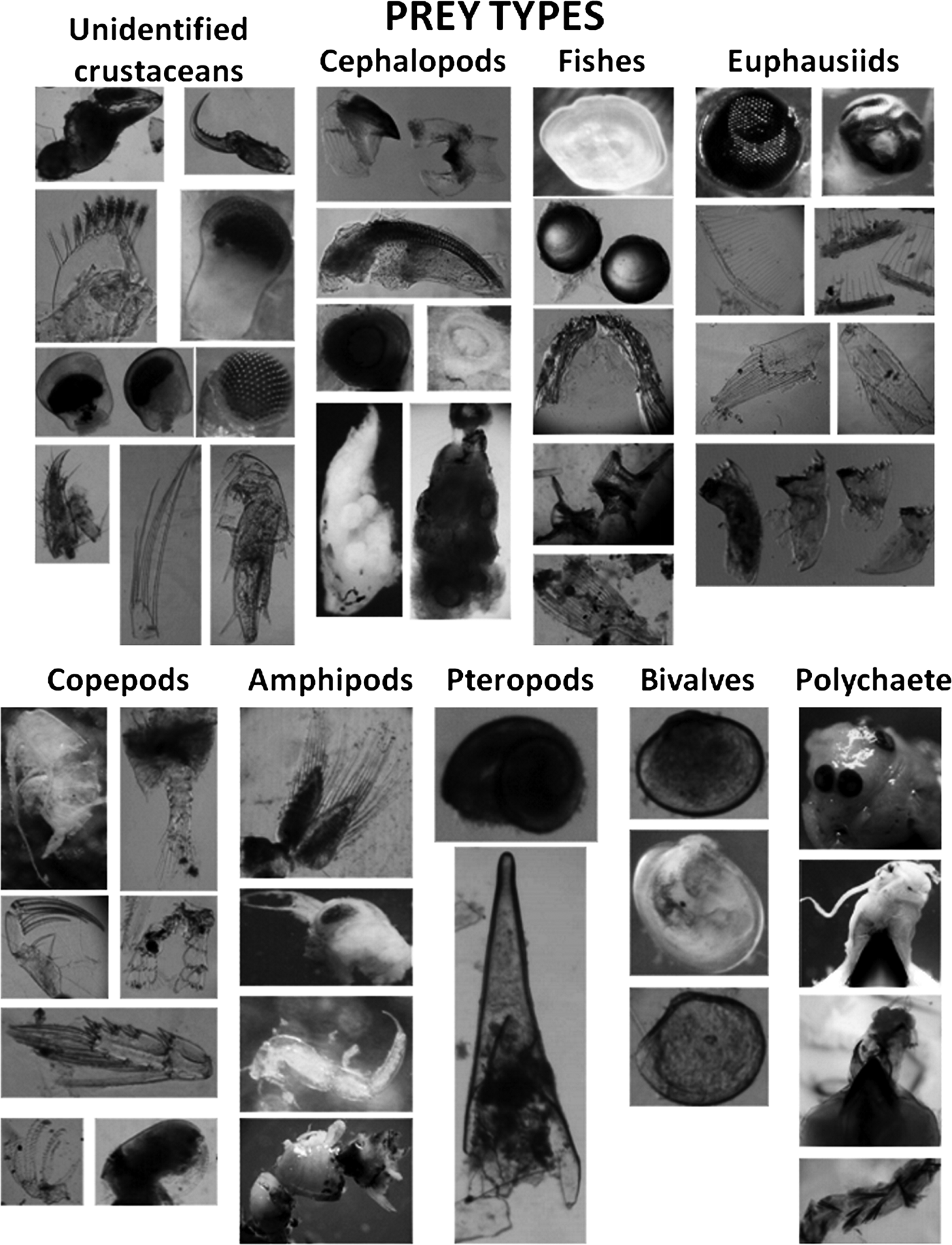
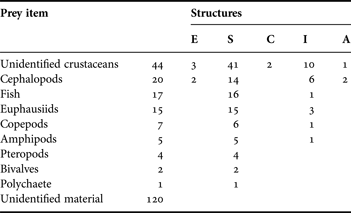
A total of nine prey types were found in juvenile squid (Table 2; Figure 4). Eyes, appendages, and unidentifiable chitin fragments belonging to unidentified crustacean were present in 44 juvenile, mainly in the stomach and intestine (41 and 10, respectively). Euphausiids were represented by cephalothorax, eyes, thoracic leg, mandibles, pleopod and uropod in the stomach and intestine of 15 juveniles. Some eyes were almost intact, without any degree of digestion. The diameter of 13 eyes was measured to estimate the total length (TL) of the euphausiids based on measurements on Nyctiphanes simplex (Jaime Gómez Gutiérrez, unpublished data). The sizes ranged between 8.5 and 12.8 mm TL. Copepods were identifiable by fragments such as prosome, urosome or appendages and were found exclusively in the stomach. Amphipods were identified by the presence of recently ingested specimens, most found in stomachs although two were found inside the brachial crown and beak (not considered in Table 2) of the juvenile. Cephalopod remains were the second most frequent prey. They were found in stomachs, intestines and in degraded state inside the oesophagus and anus of 20 specimens. This prey group was identified by the presence of radulae, beaks, suckers, lenses, one statolith, and arm pieces. The third prey group were fish (spines, rays, lenses, scales, otoliths), mostly found in the stomachs. Of 19 otoliths, the largest measured 0.5 mm length whereas the smallest 0.06 mm length. Two genera of pteropods (probably Limacina and Clio) were observed in four juveniles. Bivalves were found in better condition than the rest of the groups. Finally, one large polychaete was found on the beak, oesophagus and some setae in the stomach of a juvenile squid (Table 2; Figure 4).

Fig. 4. Prey types identified from 63 digestive systems examined of Dosidicus gigas juveniles from the Gulf of California.
Table 2. Frequency of occurrence by prey items found in different structures of digestive systems of Dosidicus gigas juveniles. Structures are: (E) oesophagus; (S) stomach; (C) caecum; (I) intestine; (A) anus.

Prey items
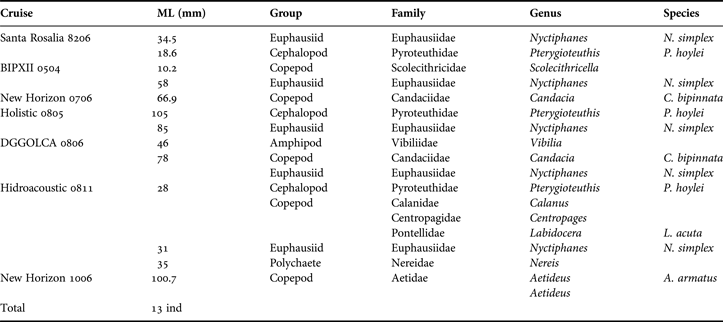
Prey species were identified in more detail from juveniles of seven cruises (Table 3). Euphausiids, amphipods and cephalopods were each represented by one family and one species, whereas copepods included six families.
Table 3. Prey items found in digestive systems of Dosidicus gigas juveniles from seven surveys in the Gulf of California.

DISCUSSION
We assume that the contents identified in this work from the digestive systems of juvenile Dosidicus gigas reflect the natural diet of this species as they were collected with a dipnet while they were swimming at sea surface in the Gulf of California. However we recognize that some incidental feeding on the zooplankton attracted to lights while fishing these squid could have happened.
The early post-hatching phase is marked by the coexistence of two nutritive systems: (1) an embryonic energy source in the form of yolk, in a brief endotrophic phase; and (2) a post-embryonic energy supply using captured food that is rapidly digested (Boletzky, Reference Boletzky2003). The U-shaped digestive system (Ivanovic & Brunetti, Reference Ivanovic and Brunetti2002) appears to be developed, functional and equipped with digestive enzymes in smallest ommastrephid paralarvae of 1–2 mm ML (Boucaud-Camou & Roper, Reference Boucaud-Camou and Roper1998). Once the yolk is consumed paralarvae must begin to feed and therefore the survival of newly hatched paralarvae depends on their ability to successfully switch to active predation (Uchikawa et al., Reference Uchikawa, Sakai, Wakabayashi and Ichii2009). The development of in vitro fertilization techniques have been widely used to obtain paralarvae in laboratory (Villanueva et al., Reference Villanueva, Staaf, Argüelles, Bozzano, Camarillo-Coop, Nigmatullin, Petroni, Quintana, Sakai, Sakurai, Salinas-Zavala, De Silva-Dávila, Tafur, Vidal and Yamashiro2012). Until now all attempts at rearing ommastrephid paralarvae have failed due to starvation at the time of yolk absorption, stressing the need for further feeding studies from wild early life stages.
Studies conducted on wild paralarvae showed similar results in three ommastrephid species: Sthenoteuthis oualaniensis, Illex argentinus and Ommastrephes bartramii (Vecchione, Reference Vecchione1991; Vidal & Haimovici, Reference Vidal and Haimovici1998; Uchikawa et al., Reference Uchikawa, Sakai, Wakabayashi and Ichii2009, respectively). Paralarvae started to feed on crustacean and copepods when ML reach 3.7 mm in I. argentinus, 4.2 mm in O. bartramii and 5.4 mm in S. oualaniensis, suggesting that ommastrephid paralarvae feed on small crustaceans zooplankton after the onset of active predation. Our results did not indicate evidence of hard structures in the digestive system of Dosidicus gigas paralarvae but all examined specimens had well-chopped, unidentified food in the caecum. This NIM was usually present as compact lump in the oesophagus, in the intestine entrance, and mainly in the caecum which sometimes was full. Unlike in adult Dosidicus, the size of the paralarval caecum was always bigger than the stomach which was empty in most of the paralarvae. This interesting feature indicates that D. gigas paralarvae feed on certain type of ‘initial food’ (not determined in this study). Probably this ‘initial food’ may be easy to degrade and its presence in the caecum is the result of the final digestion and the absorption that take place there (Boucaud-Camou & Roper, Reference Boucaud-Camou and Roper1995), indicating that paralarvae were feeding successfully.
In addition to copepods and other crustaceans, Vidal & Haimovici (Reference Vidal and Haimovici1998) found squid, dinoflagellates and other flagellates as well as mucus in the digestive tract of I. argentinus paralarvae. According to Ivanovic & Brunetti (Reference Ivanovic and Brunetti2002), abundant mucus is secreted by the anterior and posterior salivary glands and acts as a lubricant facilitating the passage of food through the oesophagus. A detailed examination of the mucus showed decreasing presence of microbes such as bacteria, flagellates, ciliates, cysts with increasing paralarval size (Vidal & Haimovici, Reference Vidal and Haimovici1998). No mucus was observed in I. argentinus paralarvae higher than 5.6 mm ML (Vidal & Haimovici, Reference Vidal and Haimovici1998). Uchikawa et al. (Reference Uchikawa, Sakai, Wakabayashi and Ichii2009) identified Amphipoda and Branchipoda in O. bartramii paralarvae but did not observe microorganisms (flagellates, dinoflagellates or ciliates) because their samples were preserved in ethanol, which rapidly disintegrates the cells of microorganisms. However, they found a relationship between the presence of solid food in paralarvae of 4.2 mm ML and the time at beak protrusion, occurring at 3–4 mm ML. Paralarvae examined in the present study were also preserved with ethanol and no mucus was detected in digestive systems analysed.
Dosidicus gigas juveniles showed several prey types. Nine zooplankton groups were identified in their diet and increasing prey diversity was observed with increasing squid size. The first preys identifiable in smallest juveniles were cephalopods, crustaceans and fish. Unidentified crustaceans appeared in early juvenile (11 mm ML) squid. Euphausiids showed 21% FO, and they appeared at 30 mm ML. The presence of fish in the food content suggest an improvement of swimming and hunting behaviour as the squid grow because fish are good swimmers and have a fast escape response, although there is not a clear difference between the size-classes with respect to fish. Almost all prey items showed an increase in the frequency of occurrence in juveniles at 60–120 mm ML, with exception of cephalopods and fish. Results obtained from the low number of stomachs available for the last size-class should be considered with caution. However, three of the prey items identified here, were also found in juvenile Illex argentinus gut contents (Santos & Haimovici, Reference Santos and Haimovici1997). These authors found mainly crustaceans and cephalopods as prey items in juveniles between 34–50 mm ML, whereas at 50–100 mm ML the main prey was cephalopods, followed by fish and in a low percentage of occurrences for crustaceans. They also found that the occurrence of fish increased regularly with squid size.
The results of the present study are coincident with observation of Dosidicus gigas juveniles from Peruvian waters, which concentrated mainly in the shallower thermocline (25–35 m depth) at night for active feeding on dense aggregations of pelagic crustaceans and myctophid fry and juveniles (Arkhipkin & Parfenyuk, Reference Arkhipkin, Parfenyuk and Ivanov1986). In the same region, Shchetinnikov (Reference Shchetinnikov1989) found also that D. gigas between 21–100 mm ML mainly fed on mesoplankton including large copepods, euphausiids, amphipods, fish larvae, fry of myctophids and Vinciguerria sp. The amphipod Vibilia sp. and copepods Labidocera sp. and Candacia bipinnata were found also in that study. However some items, such as small bivalves that have been reported in stomachs of adult D. gigas (Markaida and & Sosa-Nishizaki, Reference Markaida and Sosa-Nishizaki2001) could have been ingested in the stomachs of fish prey.
The ontogenetic change in D. gigas food spectrum proposed by Shchetinnikov (Reference Shchetinnikov1989) was partially demonstrated in this study. For the first group (0.9–10 mm ML) the type of food remains unknown. However, for the first transition period (10–15 mm ML) besides the NIM two types of prey were included: unidentified crustaceans and cephalopods. For the second size group (15–100 mm ML), an increase in the planktonic prey such as amphipods, euphausiids, copepods, unidentified crustaceans, cephalopods and fish was indicated. However, for the second transition period (100–150 mm ML) the small number of observations here was not adequate to verify the dietary shift from planktonic crustaceans to myctophids.
The Gulf of California is a highly productive zone due to the seasonal wind-driven upwelling system (Gaxiola-Castro et al., Reference Gaxiola-Castro, Álvarez-Borrego, Lavín, Zirino and Nájera-Martínez1999). The upwelling provides the food supply for Dosidicus gigas all year-round (Markaida & Sosa-Nishizaki, Reference Markaida and Sosa-Nishizaki2001). However, in a previous study, Camarillo-Coop et al. (Reference Camarillo-Coop, Salinas-Zavala, Manzano-Sarabia and Aragón-Noriega2010) found paralarvae and juveniles only during the warm season (June and September). Their presence was related to high sea surface temperature (27.7–29.5°C) and a high secondary productivity derived from chlorophyll-a fronts in the western Guaymas basin and central Gulf area. There available zooplankton and high temperatures could be major factors contributing to a rapid squid growth resulting in a short paralarval phase and minimizing mortality by starvation.
The feeding patterns in the early life stages of D. gigas are partly understood. Considering the results of previous studies, early paralarvae may feed on microorganisms such as flagellates, dinoflagellates, ciliates, to shift to crustacean zooplankton. Juveniles also feed across zooplankton groups. As they grows the food spectrum is broad with the incorporation of more prey types to the diet, passing to higher trophic level without discarding organisms from the initial food web; this is an important feature of the trophic ecology of this species.
ACKNOWLEDGEMENTS
We thank the captains and crews of RV ‘BIP XII’, ‘New Horizon’, ‘Pacific Storm’, and FV ‘Gus D’, for their support on all cruises. We are grateful to Federico García Domínguez, Martín Hernández Rivas and Diane Gendron who donated some juveniles of Dosidicus gigas.
FINANCIAL SUPPORT
This work was supported by the Consejo Nacional de Ciencia y Tecnología (grant number 165-C).