Over the past four decades, advances in medical and surgical interventions for infants with complex CHD have led to significantly improved outcomes in this population. Reference Pace, Oster, Forestieri, Enright, Knight and Meyer1–Reference Gordon, Rodriguez, Lee and Chang3 As more infants are expected to survive to adulthood, growth and development has become a primary focus of the healthcare team, with feeding an area of particular concern. Complex CHD increases metabolic and myocardial demand, Reference Medoff-Cooper and Ravishankar4–Reference Mangili, Garzoli and Sadou6 with surgical and neurological complications further putting these infants at risk for feeding-related morbidity and mortality. Reference Gaynor, Stopp and Wypij7–Reference Ghanayem, Allen and Tabbutt11 Furthermore, family caregivers report a high level of stress and uncertainty related to infant feeding, Reference Hartman and Medoff-Cooper12,Reference Imms13 and feeding problems often become so concerning to families that they overshadow all other cardiac issues. Reference Tregay, Wray and Crowe14 Yet, feeding is a key variable, amenable to treatment, with increased interventions, monitoring, and family support all associated with improved outcomes for these vulnerable infants. Reference Edwards and Spatz15–Reference Hehir, Rudd and Slicker19
In recent years, there have been efforts to address a lack of high-quality evidence Reference Demirci, Caplan, Brozanski and Bogen20–Reference Ehrmann, Mulvahill and Harendt22 on best practices for feeding infants with complex CHD. In particular, there is growing interest in the provision of human milk for this vulnerable population, with compelling benefits described in the literature, including reduced risk of necrotising enterocolitis, infection, and sepsis; improved weight gain; and greater cardiorespiratory stability while feeding. Reference Davis and Spatz23-Reference Cognata, Kataria-Hale and Griffiths29 This emerging evidence, however, is not consistently translated into practice. Instead, there remains centre- and provider-dependent variation in feeding practice. Reference Tregay, Wray and Crowe14,Reference Slicker, Sables-Baus and Lambert30–Reference Alten, Rhodes and Tabbutt34 Many of these variations can lead to suboptimal outcomes for growth and development, Reference Lambert, Pike and Medoff-Cooper33,Reference Ehrmann, Harendt and Church35–Reference Furlong-Dillard, Miller and Sward37 and can result in inconsistent communication between the healthcare team and family caregivers. Reference Demirci, Caplan, Brozanski and Bogen20,Reference Lambert, Pike and Medoff-Cooper33,Reference Lambert and Watters38 Healthcare providers, family caregivers, and affected children would all benefit from increased clarity of understanding in regard to feeding.
The aim of this study was to bring to light areas of consensus and non-consensus in regard to evidence-based statements about feeding infants with complex CHD, with a particular focus on the provision of human milk for this population. For our purposes, infants are defined as ≤ 12 months of age, and complex CHD is defined as CHD that requires surgical intervention within the first year of life. A modified Delphi survey of healthcare experts in complex CHD feeding management is an ideal method to determine the level of clinical agreement on key feeding topics. Areas of non-consensus may indicate discrepancies between research findings and practice, with consequent variation in feeding management. Moreover, by understanding which areas of practice are most vulnerable to uncertainty or disagreement, the healthcare team is better positioned to support family caregivers in flexibly navigating feeding concerns.
Materials and methods
Modified Delphi method
This study used a modified Delphi method. The Delphi method is a multistep survey technique that aims to transform individual opinions into group consensus, based on the assumption that group opinions are more accurate than those of individuals. Reference Rowe and Wright39,Reference Akins, Tolson and Cole40 The collective knowledge that unfolds through the Delphi process prompts areas of consensus and non-consensus to emerge. A modified Delphi method is similar to the original Delphi technique in that a group of panelists is surveyed through multiple rounds, with a goal of achieving consensus. However, the modified Delphi technique used in this study began with a comprehensive literature review to develop items for the survey, Reference Iqbal and Pipon-Young41 rather than eliciting open-ended suggestions from clinical experts or stakeholders. This ensured that the survey was evidence-based, and satisfied the objective of determining clinician agreement with the research literature. Figure 1 shows the process used in the study.

Figure 1. Modified Delphi survey process.
Search
To develop an understanding of the current evidence on feeding infants with complex CHD, a search was carried out by KE and ACM, with the assistance of an experienced research librarian. The search strategy can be found in Table 1. After an initial prescreening by title, two authors (KE and ACM) reviewed abstracts following the inclusion and exclusion criteria outlined in Table 2. After the abstract review, it was noted that two authors, Diane Spatz and Barbara Medoff-Cooper, had published widely on the topic of human milk feeding and breastfeeding for infants with complex CHD. To ensure that all relevant evidence was included, the reviewers surveyed all publications generated by these authors, and also examined reference lists for additional studies. A total of 128 abstracts met the inclusion criteria for full-text review, after which 8 articles were excluded, resulting in 120 included articles (Supplementary Table S1). The study selection process can be seen in Fig 2.

Figure 2. PRISMA flow diagram of the study selection process.
Table 1. Search parameters for literature review
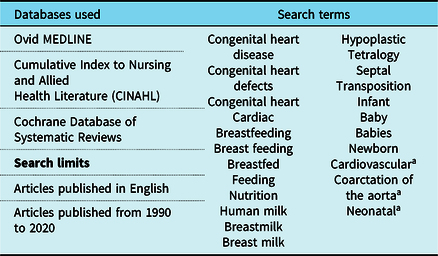
a Search terms truncated for maximum results
Table 2. Inclusion and exclusion criteria for search

CCHD = complex congenital heart disease; CHD = congenital heart disease; NEC = necrotising enterocolitis
Patients
An interdisciplinary group of experts from across the United States of America was sought. These experts fulfilled one of the two possible inclusion criteria: (1) A minimum of 5 years of clinical experience feeding infants with complex CHD in an ICU setting (e.g., neonatal ICU, paediatric ICU, or cardiovascular ICU); or (2) Expertise on nutrition and feeding for vulnerable infants in an ICU setting, including infants with complex CHD, as evidenced by (a) at least three first-author publications on a topic relevant to feeding infants with complex CHD; or (b) a national and/or international profile as an organisational leader, journal editor, or presenter on topics relevant to feeding infants with complex CHD. Experts who agreed to take part were not provided information about any other patients. All data were collected through Qualtrics (Provo, UT, USA) survey software. Patients were provided a $25 Amazon.com gift card upon completion of each survey round, in recognition of their time and effort.
Round 1
The first round focused on the development and validation of content to inform subsequent rounds. A full review of the included literature was conducted, and findings determined to have relevance for both the healthcare team and family caregivers of infants with complex CHD were represented by evidence-based statements (e.g., statements taken directly from the results of the literature review). All statements were generated and organised by subtopic and topic by the first author (KE), and reviewed by the second (ACM) and third (TG) authors. A full list of topics, subtopics, and statements can be seen in Supplementary Table S2.
To validate the topics and subtopics, a subgroup of six patients was surveyed. These experts were asked to indicate the extent of agreement or disagreement in regard to the importance of each topic. A 6-point Likert scale was used, with 1=strongly agree [about the importance of the topic or subtopic] and 6=strongly disagree. Additionally, an open-ended response option queried whether any topics or subtopics had been overlooked.
Round 2
The results of round 1 were analysed, and topics or subtopics determined to be of lower importance (a mean greater than or equal to 3) were excluded. The remaining 5 topics, 38 subtopics, and 206 evidence-based statements comprised the round 2 survey. This survey was sent to the full group of 25 clinical experts, who were asked to indicate how strongly they agreed with each statement, using the same 6-point Likert scale. To preserve the anonymity of respondents’ answers, surveys were not linked to patients’ contact information, and patients were not provided with their responses in subsequent rounds.
Round 3
After analysis of round 2, statements that did not lean strongly towards an agree (mean ≤ 2) or disagree (mean ≥ 4) response or those with a wide range (standard deviation > 1) were included in round 3, which was sent to patients approximately 1 month after round 2. Each statement was presented with its mean, standard deviation, and range, and respondents were asked to answer again using the same 6-point Likert scale, considering the mean group response. If a patient selected a response option that differed in valence from the mean (e.g., choosing a “disagree” answer of 4, 5, or 6 when the mean was 2.4, or “agree”), explanatory comments were elicited. This qualitative response offered insight when there were quantitative outliers.
Data analysis
For each round, Qualtrics data were exported to IBM SPSS Statistics (Macintosh, Version 26.0) to calculate descriptive statistics. Data from round 2 were analysed in a multistep, iterative process to determine the most meaningful approach for including statements in round 3. The mean and standard deviation, median and interquartile range, and percentage of consensus were considered. It was determined that mean (standard deviation) 2.01–4.99 (> 1) resulted in the highest and most inclusive number of statements of non-consensus.
In round 3, the top 10 statements of greatest consensus were considered to be those closest to a mean of 1 (“strongly agree”) or 6 (“strongly disagree”). Statements with the lowest level of consensus were identified in two ways. First, the 10 statements with means closest to the middle (3.5) were considered to show a lack of definitive agreement or disagreement. Second, the ten statements with the highest standard deviation demonstrated the widest range of opinion. Qualitative data from round 3 were analysed through two cycles of coding. Reference Miles, Huberman and Saldaña42 The first coding cycle focused on theming the data to identify pertinent information or meaning within a comment. The second cycle involved pattern coding to reduce findings and form higher order themes along with corresponding illustrative quotations.
Results
Patient description
A cohort of 25 experts agreed to participate in this study. The patients were affiliated with 13 academic and clinical sites across the United States of America, and located in the (n,%) Midwest (15, 60), East (5, 20), Southwest (3, 12), and Southeast (2, 8) regions. The experts represented a variety of disciplines, including (n, %) physicians (7, 28), International Board Certified Lactation Consultants (IBCLC; 6, 24), advanced practice nurses (4, 16), skilled feeding therapists (e.g., speech-language pathologist, occupational therapist, physical therapist) (4, 16), dieticians (4, 16), nurse scientists (3, 12), and registered nurses (2, 8). Several experts held multiple certifications (e.g., registered nurse and IBCLC). Fifteen of the 25 had more than 10 years of clinical experience working directly in feeding infants with complex CHD in an ICU setting.
Of the 13 patients’ academic and clinical sites, 8 are urban teaching hospitals. One is a > 400-bed adult and paediatric academic medical centre. The remaining 7 are dedicated children’s hospitals, with 100–200 beds (n=2), 201–300 beds (n=1), 300–400 beds (n=2), and > 400 beds (n=2). Six of these children’s hospitals include a Level IV neonatal ICU.
Round 1
Based on a full review of the evidence, the authors identified five key topics addressed in the literature: (1) human milk, (2) developing oral feeding skills, (3) clinical feeding practice, (4) growth failure, and (5) parental concerns about feeding. In round 1, 6 (100%) surveys were completed, and respondents strongly agreed that all key topics were important for parents and family caregivers of infants with complex CHD. Two subtopics (support of oral feeding using cup feeding; support of oral feeding using finger feeding) did not meet the criteria for inclusion and were removed from consideration.
Based on open-ended responses, 28 additional evidence-based statements were added to subsequent survey rounds. This resulted in a final total of 206 evidence-based statements for inclusion in round 2 of the modified Delphi survey. The complete results of the round 1 survey can be seen in Table 3.
Table 3. Round one results: validation of key topics and subtopics

a All topics and subtopics refer specifically to infants with complex congenital heart disease (CCHD)
b The importance of each topic and subtopic for parents and caregivers of infants with CCHD was rated from 1 (strongly agree/very important) to 6 (strongly disagree/not at all important)
c Items with a mean > 3.00 were eliminated from future rounds. These items are italicised
Round 2
In round 2, 25 (100%) surveys were completed, with 89 (43.2%) statements reaching consensus (Supplementary Table S2). The topic of parental concern about feeding contained the highest percentage of statements reaching consensus (78.6%), while the topic of growth failure included the lowest percentage of statements reaching consensus (25%). The 117 statements that did not reach consensus in round 2 moved on to the final round of the modified Delphi survey process.
Round 3
A total of 23 (92%) surveys were completed in round 3. Of the 117 statements, 21 (17.9%) moved to a state of consensus, resulting in a final total of 110 (53.4%) statements of consensus, and 96 (46.6%) statements of non-consensus. The results of the round 3 survey, including all statements reaching consensus, can be seen in Supplementary Table S2. Parental concern about feeding remained the topic with the highest percentage of statements reaching an agreement, followed by human milk, clinical feeding practice, developing oral feeding skills, and growth failure (Fig 3).

Figure 3. N (%) of statements reaching consensus in each topic.
The 10 statements with the highest level of consensus all received “strongly agree” responses and are listed in Table 4. The 10 statements with the lowest level of consensus determined by both mean and standard deviation are presented in Table 5. Qualitative findings for 10 themes are shown in Table 6, with illustrative quotations. These quotations provide insight into the variation in current thinking that is guiding practice.
Table 4. Statements with the highest level of consensus

CCHD = complex congenital heart disease; ICU = intensive care unit
Table 5. Statements with the lowest level of consensus
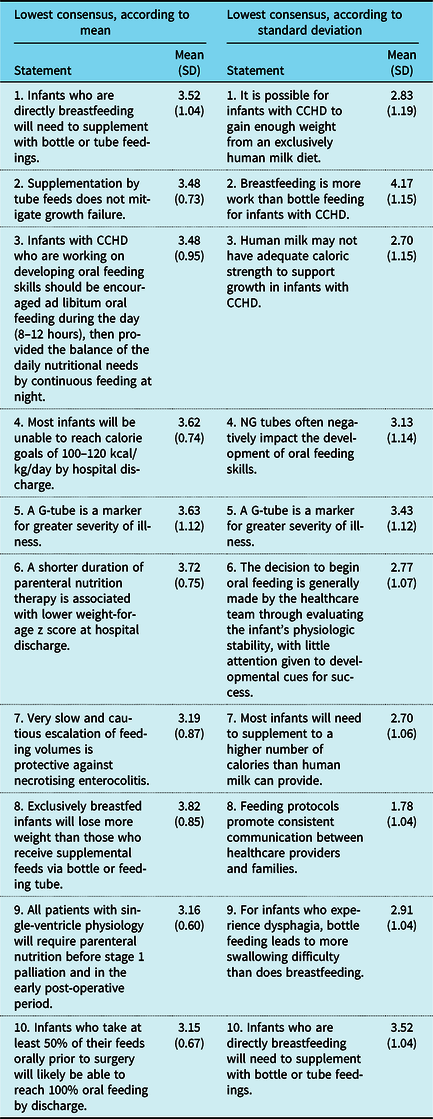
CCHD = complex congenital heart disease; G-tube = gastrostomy tube; NG = nasogastric
Table 6. Thematic areas of non-consensus with illustrative quotations

a T = topic; with T1, human milk; T2, developing oral feeding skills; T3, clinical feeding practice; T4, growth failure; T5, parental concern about feeding
b S = statement; followed by the number of the statement referred to by the qualitative response. Statement numbers can be found in Supplementary Table S2
CCHD = complex congenital heart disease; CHF = congenital heart failure; G-tube = gastrostomy tube; MOM = mother’s own milk; NEC = necrotising enterocolitis; NG = nasogastric; NICU = neonatal intensive care unit; PICU = pediatric intensive care unit; PO = by mouth; REE = resting energy expenditure
Discussion
In this modified Delphi survey, we identified areas of clinical consensus and non-consensus on evidence-based statements in regard to feeding infants with complex CHD. To our knowledge, this is the first study to examine clinical opinion on the provision of human milk for this population. Topics of non-consensus emerged through analysis of quantitative and qualitative data, and suggested potential gaps between research findings and practice. An improved understanding of these gaps is a critical first step towards future testing and refinement of feeding approaches for infants with complex CHD. Reference Anderson, Beekman and Kugler36 We first discuss the most notable area of consensus in this survey, and then turn our attention to five topics of non-consensus that spurred considerable discussion amongst the clinical expert patients. These areas of non-consensus are considered in the context of the available evidence, and suggestions for future directions are provided.
Consensus
The results of this study highlight the critical role of human milk in feeding infants with complex CHD. The 10 statements reaching the highest level of consensus amongst clinical experts (Table 4) strongly support the provision of human milk as the first-line, preferred nutrition for infants with complex CHD, Reference Spatz24,Reference Slicker, Hehir and Horsley43 and indicate that human milk provision is a necessary medical and nursing intervention for this vulnerable population. Reference Davis and Spatz23 Study patients validated the existing body of research demonstrating that human milk is safe and effective for infants with complex CHD, Reference Edwards and Spatz15,Reference Davis and Spatz23,Reference Sahu, Singal and Menon44–Reference Zyblewski, Nietert, Graham, Taylor, Atz and Wagner46 with advantages such as a reduced risk of necrotising enterocolitis, Reference Mangili, Garzoli and Sadou6,Reference Cognata, Kataria-Hale and Griffiths29,Reference Lambert, Christensen and Henry47 infection prevention, Reference Spatz24,Reference Spatz25,Reference Sahu, Singal and Menon44,Reference Zyblewski, Nietert, Graham, Taylor, Atz and Wagner46,Reference El-Koofy, Mahmoud and Fattouh48 improved post-operative recovery, Reference Sahu, Singal and Menon44 a reduction in total number of parenteral nutrition days, Reference Spatz49 and strengthening of the caregiver/infant bond. Reference Froh, Deatrick, Curley and Spatz50 While the literature describes several interventions designed to improve rates of human milk feeding and breastfeeding for the general neonatal ICU population, Reference Meier, Johnson, Patel and Rossman51 only one model of care has demonstrated improved outcomes in infants with complex surgical anomalies, including those with complex CHD. Reference Edwards and Spatz15,Reference Spatz24 Future work is needed to ameliorate both the reported low rates of infants with complex CHD who receive human milk from birth, and the high risk for early weaning in this population. Reference Rendón-Macías, Castañeda-Muciño, Cruz, Mejía-Aranguré and Villasís-Keever52,Reference Tandberg, Ystrom, Vollrath and Holmstrom53
Non-consensus
Human milk
On the topic of human milk, there were two primary areas of non-consensus. First, the adequacy of human milk to support growth and the need for supplementation or fortification was an area of disagreement, with low consensus as to whether human milk can support the energy needs of infants with complex CHD. The literature is also inconclusive on this issue. Of the seven studies identified that compared human milk to formula and/or supplementation, two demonstrated greater weight loss in the formula/supplementation group, Reference Combs and Marino27,Reference El-Koofy, Mahmoud and Fattouh48 and one in the human milk group. Reference Boctor, Pillo-Blocka and McCrindle54 The remaining four studies, including the only randomised controlled trial on this topic, found similar weight gain between the two groups. Reference Sahu, Singal and Menon44,Reference Rosti, Vivaldo, Butera, Chessa, Carlucci and Giamberti45,Reference Fugate, Hernandez, Ashmeade, Miladinovic and Spatz55,Reference McCrary, Clabby and Mahle56 Taken together, the results from the literature indicate that it may be possible for many infants with complex CHD to achieve similar or improved growth on an unfortified, exclusive human milk diet, as compared to formula or other supplementation. To our knowledge, there have been no long-term studies examining the potential for an exclusive human milk diet in infants with complex CHD from birth into the first year of life, and none investigating techniques for targeted management of human milk (e.g., testing calorie content, fractionating milk for higher fat content Reference Spatz, Schmidt and Kinzler57,Reference Spatz58 ) in this population. In light of the inconclusive nature of the literature, we recommend that clinicians adopt an attitude of creative inquiry when managing growth and development, with a focus on structure and support for family caregivers to provide an exclusive human milk diet for their infant whenever possible. More research is needed to identify and validate best practices in managing exclusive human milk diets in infants with complex CHD.
The second area of non-consensus related to the topic of human milk involved the safety and feasibility of breastfeeding for infants with complex CHD. Patients held a range of opinions on whether breastfeeding is more work than bottle feeding for these infants, but offered a limited explanation for their responses. An often-cited study on this topic demonstrated that breastfeeding causes less cardiorespiratory stress in infants with complex CHD, Reference Marino, O’Brien and LoRe26 with similar results reported in other at-risk populations. Reference Meier and Anderson59,Reference Chen, Wang, Chang and Chi60 Yet, nearly one-quarter of the clinical experts in this study agreed that breastfeeding is more work for infants with complex CHD. This discrepancy between research findings and clinician perspective suggests that the traditionally held idea that breastfeeding is too difficult for infants with complex CHD Reference Tregay, Wray and Crowe14,Reference Lambert and Watters38 may still exist today. This is not surprising, given the dearth of research on this topic. In reviewing the literature since 1990, 12 publications specifically focused on breastfeeding infants with CHD were identified, Reference Davis and Spatz23,Reference Marino, O’Brien and LoRe26,Reference Combs and Marino27,Reference Lambert and Watters38,Reference Spence, Swinsburg, Griggs and Johnston61–Reference Gregory68 including only 6 research studies (excluding case studies). Future research is needed to more fully understand the safety and feasibility of breastfeeding in the context of complex CHD, and best practices to support caregivers in achieving direct breastfeeding with these vulnerable infants should be identified and widely implemented.
Feeding tubes
Statements on tube feeding were subject to non-consensus throughout the study. Best practices in the use of nasogastric tubes to supplement oral intake and the potential impact of nasogastric feeding on the development of oral feeding skills emerged as key areas of discussion. Clinicians did not agree on a strategy for organising and timing nasogastric feeds (e.g., restricted oral feeds; continuous feeds at night), and most of the discussion centred around optimising opportunities for cue-based oral feeding. Few studies identified in the literature focus specifically on nasogastric feeding for infants with complex CHD, and their small sample sizes preclude generalisation. Reference Premji and Chessell69,Reference Furlong-Dillard, Neary and Marietta70 Thus, clinicians and centres are left to craft guidelines that may be largely based on tradition and centre-specific experience, with resultant variation in clinical practice. Reference Medoff-Cooper, Naim, Torowicz and Mott64 Especially considering that the timing and organisation of nasogastric tube feeding and the progression to oral feeding can be a major source of stress and frustration for family caregivers, Reference Hartman and Medoff-Cooper12,Reference Tregay, Wray and Crowe14,Reference Taylor, Cloherty, Alexander, Holloway, Galvin and Inch71 future work to delineate evidence-based, infant-centred, holistic best practices is crucial for improving the standard of care in this area.
Patients did not agree about the ability of supplemental tube feeding to mitigate weight loss or facilitate catch-up growth after discharge. While it seems logical that tube feeding would lead to at least some catch-up growth, particularly in very sick infants who are between stages of surgical palliation (interstage), the literature does not support this theory. Eight of the 12 available studies on this topic found that tube-fed infants with complex CHD experienced reduced weight gain during the interstage period, compared to those who were orally fed. Reference Hehir, Rudd and Slicker19,Reference Lambert, Pike and Medoff-Cooper33,Reference Di Maria, Glatz and Ravishankar72–Reference Uzark, Wang and Rudd77 Only one study has demonstrated superior growth in infants who were tube fed. Reference Ciotti, Holzer, Pozzi and Dalzell78 It is probable that feeding tubes allow critically ill infants to maintain a higher rate of growth than they would be able to achieve with oral feeding alone. However, to our knowledge, there is no conclusive evidence that supports this theory in infants with complex CHD, and there is considerable variation in institutional and provider criteria determining the necessity of feeding tubes, especially gastrostomy tubes, in this population. Reference Slicker, Sables-Baus and Lambert30,Reference Lambert, Pike and Medoff-Cooper33,Reference Hill, Hehir and Bartz79 Future research is needed to more clearly understand how tube feeding affects growth in infants with complex CHD, and to determine best practices in supporting growth and development for infants who are tube fed.
Growth failure
While the topic of growth failure included the lowest percentage of statements reaching consensus, patients offered little explanation for their disparate answer choices. Many statements related to growth failure focused on specific details of a nutritional plan (e.g., volume, calories, protein, use of parenteral nutrition). The lack of consensus may reflect the persistent variation in clinical practice for infants with complex CHD. Reference Slicker, Sables-Baus and Lambert30,Reference Tume, Balmaks and da Cruz31,Reference Lambert, Pike and Medoff-Cooper33,Reference Alten, Rhodes and Tabbutt34,Reference Anderson, Beekman and Kugler36,Reference Simsic, Carpenito and Kirchner80 While there have been recent efforts to create evidence-based feeding protocols for this population, Reference Karpen21,Reference Ehrmann, Mulvahill and Harendt22,Reference Slicker, Hehir and Horsley43,Reference Furlong-Dillard, Neary and Marietta70,Reference del Castillo, McCulley and Khemani81–Reference Braudis, Curley and Beaupre84 provider- and centre-specific feeding practice continues to be the norm. A particularly striking example is found in Slicker et al., (2016), in which only 3 out of the 46 (7%) United States of America centres participating in the National Pediatric Cardiology Quality Improvement Collaborative (NPC-QIC) adopted the NPC-QIC’s published guidelines for feeding readiness evaluation after stage 1 palliation. Reference Slicker, Sables-Baus and Lambert30 While there is certainly a need for flexibility in tailoring feeding care based on an infant’s severity of illness and clinical course, there are considerable benefits associated with feeding protocol implementation. Reference Hehir, Rudd and Slicker19,Reference Ehrmann, Harendt and Church35–Reference Furlong-Dillard, Miller and Sward37,Reference Furlong-Dillard, Neary and Marietta70 Future research should focus on the development of comprehensive, infant-centred, tailorable feeding protocols for infants with complex CHD, and should examine barriers to protocol adoption.
Implications
The findings from this study can inform practice and provide direction for future research. In regard to practice, clinical interventions focused on supporting human milk and breastfeeding for infants with complex CHD are needed. A model for care that has been tested specifically for infants with complex surgical anomalies Reference Edwards and Spatz15,Reference Spatz24 may be particularly useful to guide practice and increase rates of human milk provision and breastfeeding in this population. Moreover, by linking evidence on feeding infants with complex CHD to practice, findings from this study have the potential to sensitise healthcare providers to areas of miscommunication and uncertainty experienced by their patients. This expanded understanding could allow providers to mitigate stress for family caregivers of infants with complex CHD. Our study findings also highlight key areas where gaps may exist, and suggest five foci for future research: (1) the adequacy of human milk to support growth and the need for supplementation or fortification; (2) the safety and feasibility of breastfeeding for infants with complex CHD; (3) best practices in the use of nasogastric tubes and their potential impact on the development of oral feeding skills; (4) the ability of supplemental tube feeding to mitigate weight loss or facilitate catch-up growth; and (5) prevention and treatment of growth failure.
Strengths and limitations
The use of the modified Delphi method in this study elicited an understanding of consensus and non-consensus through three survey rounds in a relatively short amount of time, without geographical constraints. This method is non-confrontational, which, when compared to face-to-face group discussion, substantially reduces biasing of response and the risk of a single dominant opinion skewing results. Qualitative data were particularly useful for interpreting areas of non-consensus. The major limitation of this study is the relatively small sample size of clinical experts. While the number is adequate for achieving representative consensus, Reference Akins, Tolson and Cole40 clinical expert responses may not be generalisable to all situations and settings. Additionally, patients were asked to evaluate short statements that were designed to reflect current research findings. It was beyond the scope of this study to offer explanatory context for these statements, which may have helped facilitate greater consensus. Between the second and third rounds of the survey, the coronavirus disease 2019 pandemic caused significant changes to the healthcare system, which may have impacted patient responses in the final round. To account for any historical change, we asked patients if they thought their answers had been affected by the current pandemic (answer choices: “yes,” “no,” “not sure”). No respondents chose “yes;” therefore, it is unlikely that this survey was impacted by coronavirus disease 2019.
Conclusion
This study identified areas of consensus and non-consensus in regard to evidence-based statements about feeding infants with complex CHD. The results demonstrate strong clinical consensus as to the importance of human milk, but reveal a need for further identification and validation of best practices in managing a human milk diet for these infants. Areas of non-consensus may be particularly prone to variation in practice, and need further development of evidence-based feeding management strategies. Healthcare providers should be sensitive to the potential for miscommunication or uncertainty experienced by families in regard to these topics, and work with a coordinated, interdisciplinary approach to mitigate any psychological distress that could affect these caregivers.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951120004370.
Acknowledgements
The authors wish to acknowledge Liz Weinfurter, MLIS, B.A., Liaison and Instruction Librarian in the Health Sciences Library at the University of Minnesota, for her assistance with the literature search.
Conflicts of interest
None.
Ethical standards
Not applicable.
Financial support
This project was supported by the National Institutes of Health’s National Center for Advancing Translational Sciences, grant UL1TR002494. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health’s National Center for Advancing Translational Sciences. Additional support was provided by the Pediatric Device Innovation Consortium at the University of Minnesota.












