Introduction
Parasites and pathogens cause harm to their host and have the potential to reduce host fitness, consequently hosts should be under strong selection to evolve adaptations to limit contact with parasites (avoidance), supress infections (resistance) and/or tolerate the detrimental effects of infection (Poulin, Reference Poulin2007; Schmid-Hempel, Reference Schmid-Hempel2011). Although resistance is often described in terms of anatomical, physiological, immunological defences, it can also include behavioural strategies that limit infection risk (Curtis, Reference Curtis2014; Buck et al., Reference Buck, Weinstein and Young2018). Parasite avoidance behaviours provide the first line of defence; these strategies include behaviours that reduce contact with infective stages/vectors, infected individuals or habitats with high infection risk (Curtis, Reference Curtis2014; Buck et al., Reference Buck, Weinstein and Young2018). Multiple factors can influence the effectiveness and/or expression of these traits: such as host age, sex, genetics, behaviours, body condition, infection history and current infection status (Wilson et al., Reference Wilson, Bjornstad, Dobson, Merler, Poglayen, Randolf, AF, Skorping, Hudson, Rizzoli, Grenfell, Heesterbeek and Dobson2002).
Prior to infection, habitat selection can influence exposure and susceptibility to infection when hosts choose habitats based on the local risk of infection (Parker et al., Reference Parker, Barribeau, Laughton, de Roode and Gerardo2011; Buck et al., Reference Buck, Weinstein and Young2018; Weinstein et al., Reference Weinstein, Moura, Mendez and Lafferty2018). Once infected, hosts may alter habitat use in order to reduce the costs of infection, clear the infection (e.g. behavioural fever) or reduce exposure to further infection (Binning et al., Reference Binning, Shaw and Roche2017). Barrile et al. (Reference Barrile, Chalfoun and Walters2021) showed that toads (Anaxyrus boreas boreas) infected with a pathogenic fungus (Batrachochytrium dendrobatidi) move into warmer, open habitats to help clear infection, indicating that habitat choice by toads is dependent on infection status. In general, host movement can be impacted across several scales (daily movement, migration and dispersal). Hosts can avoid parasitism by moving away from areas of high infection risk via dispersal (Boulinier et al., Reference Boulinier, McCoy, Sorci, Clobert, Danchin, Dhondt and Nichols2001; Binning et al., Reference Binning, Shaw and Roche2017; Baines et al., Reference Baines, Diab and McCauley2020). For instance, black-legged kittiwake (Rissa tridactyla) birds leave their natal colony in search of tick-free habitats to breed (Boulinier et al., Reference Boulinier, McCoy, Sorci, Clobert, Danchin, Dhondt and Nichols2001). Therefore, host propensity (motivation or likelihood) to disperse is likely influenced by current infection status as well as parasitism risk (Iritani and Iwasa, Reference Iritani and Iwasa2014; Baines et al., Reference Baines, Diab and McCauley2020; Zilio et al., Reference Zilio, Norgaard, Petrucci, Zeballos, Gougat-Barbera, Fronhofer and Kaltz2021). Changes in infection status can shift the cost-benefit ratio of remaining at a given habitat as well as the trade-off between philopatry and dispersal. Theoretical studies have shown that the evolution of dispersal propensity is dependent on host status, i.e. susceptible vs infected (Iritani and Iwasa, Reference Iritani and Iwasa2014; Iritani, Reference Iritani2015). The ability to disperse (i.e. morphological, physiological or behavioural traits for dispersal) can in turn be impacted by parasites that damage host tissues (e.g. wing muscles), drain resources and reduce mobility (McElroy and de Buron, Reference McElroy and de Buron2014; Binning et al., Reference Binning, Shaw and Roche2017). We hypothesize that host dispersal, specifically the propensity to disperse is influenced by the risk of parasitism, and that the response is mediated by current infection status.
Parasite infection status can directly impact subsequent risks of infection by altering host body condition, behaviour, immunity and energy reserves (Dobson and Hudson, Reference Dobson and Hudson1995; Poulin, Reference Poulin1996). How a host responds to an initial infection can affect host susceptibility to subsequent infection by the same parasite or secondary infection by another parasite (Mideo, Reference Mideo2009; Halliday et al., Reference Halliday, Penczykowski, Barres, Eck, Numminen and Laine2020). Such priority effects can potentially alter the structure of parasite assemblages (Hoverman et al., Reference Hoverman, Hoye and Johnson2013; Clay et al., Reference Clay, Duffy and Rudolf2020). This can generate a positive feedback loop whereby parasites increase their host's exposure or susceptibility to subsequent infections (Wilson et al., Reference Wilson, Bjornstad, Dobson, Merler, Poglayen, Randolf, AF, Skorping, Hudson, Rizzoli, Grenfell, Heesterbeek and Dobson2002). For example, fruit flies (Drosophila nigrospiracula) infected with a moderate number of mite (Macrocheles subbadius) were more likely to attract ectoparasites compared to uninfected flies (Luong et al., Reference Luong, Brophy, Stolz and Chan2017). In particular, hosts that rely on behavioural defences may be less able to mount a sustained defence against parasite attack if infection reduces their overall energy reserves (Horn and Luong, Reference Horn and Luong2019). In this study, we tested the hypothesis that current parasite infection levels negatively affect host resistance to further infection.
We investigated the facultative ectoparasitic mite M. subbadius, which naturally infects the fruit fly D. nigrospiracula. Mite infection has deleterious effects on hosts including decreased longevity, fecundity and male copulatory success (Polak, Reference Polak1996; Polak et al., Reference Polak, Luong and Starmer2007). Both currently and previously infected flies also exhibit lower flight endurance, which may negatively impact dispersal capacity (Luong et al., Reference Luong, Penoni, Horn and Polak2015). The primary line of defence against infection is a behavioural defence (e.g. tarsal flicking, grooming), which has been shown to be energetically costly (Polak, Reference Polak2003; Horn and Luong, Reference Horn and Luong2019). If parasitism diminishes the host's capacity to mount an adequate behavioural defence against future mite attack, we expect previously infected flies to exhibit increased susceptibility to subsequent infections with increasing mite load. In general, dispersal has been shown to co-vary with specific behaviours such as locomotor activity (Hanski et al., Reference Hanski, Saastamoinen and Ovaskainen2006; Tung et al., Reference Tung, Mishra, Gogna, Sadiq, Shreenidhi, Sruti, Dorai and Dey2018). We, therefore, predict host activity, a proxy measure of dispersal propensity, will increase when exposed to parasites, but the response will depend on current infection status.
Materials and methods
Study system
Macrocheles subbadius (Acari: Macrochelidae) is a cosmopolitan facultative ectoparasite of numerous fly species, including D. nigrospiracula (Diptera: Drosophilidae) (Cicolani et al., Reference Cicolani, Passariello and Petrelli1978; Beresford and Sutcliffe, Reference Beresford and Sutcliffe2009). Natural infection levels vary according to the age of necrosis in the cactus with intensities as high as eight mites per fly observed in the field (Polak and Markow, Reference Polak and Markow1995). Laboratory cultures of D. nigrospiracula were initiated from adult flies collected from necrotic saguaro cacti in Arizona, USA, 2015. Flies were cultured in media containing instant potato flakes, Drosophila medium (Formula 4–24 Instant Drosophila Medium, Carolina Biological Supply Company, Burlington, NC, USA), active yeast and a small amount of autoclaved necrotic saguaro cactus. Flies were maintained in separate sex agar vials upon emergence in an incubator (12 h light, 25 °C: 12 h dark, 24 °C, 70% RH).
Mites were originally recovered from wild-caught infected flies and cultured in media under standard laboratory conditions (12:12 L:D light cycle, 25°C, 70% RH). Infected flies collected from the field were killed by crushing the thorax and the entire carcasses were placed directly into media. This method ensured that the mites were not damaged by attempts to remove them from the host. Mite media consisted of moist wheat bran, wood shavings and bacteriophagic nematodes as food. Adult female mites are the infectious stage and were collected from the mass culture the day of the experiment using Berlese funnels.
Activity and propensity to disperse
Tung et al. (Reference Tung, Mishra, Gogna, Sadiq, Shreenidhi, Sruti, Dorai and Dey2018) showed that among lines of Drosophila melanogaster selected for increased dispersal, locomotor activity and cellular respiration co-varied positively with dispersal. Here, we used fly activity level as a proxy for dispersal propensity in infected and uninfected D. nigrospiracula subject to parasitism risk. Unmated male (n = 97) and female flies (n = 99) were collected from the mass culture and aged for 10–20 days post-eclosion. Experimental flies were individually exposed to 0–3 mites for an hour within infection chambers to establish an initial infection, after which the number of mites and position of mite attachment were recorded. Infection chambers were constructed from a 200 μL pipette tip cut in half with both ends stoppered with cotton, which immobilized the host and prevented behavioural resistance. Following mite attachment, each fly was placed into separate chambers in a Multiple Animal Versatile Energetics (MAVEn) Flow-Through system (Sable Systems, Las Vegas, NV) to measure activity levels. The MAVEn system consists of 16 glass tubes, each flooded with a diffuse cone of infrared light that registers movement as changes in reflected light. A single female mite was introduced into each tube along with the fly to stimulate ectoparasite avoidance behaviours in the flies. Activity levels were monitored and analysed for the first 10 min. To account for the secondary mite's activity, we also measured the activity of a single mite (n = 46) in a separate chamber. We subtracted the mean value (of all single mite assays) from the activity of each fly exposed to a mite.
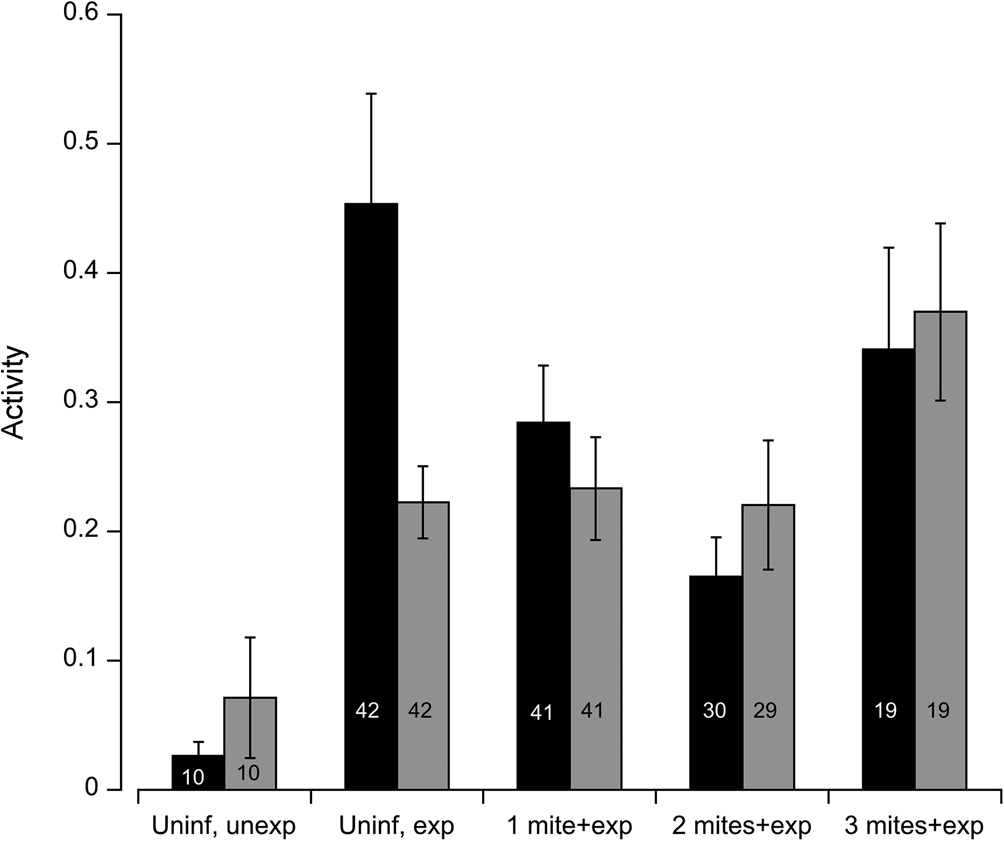
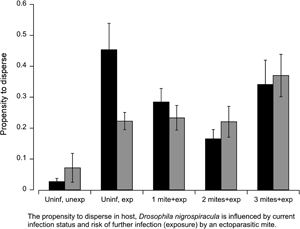
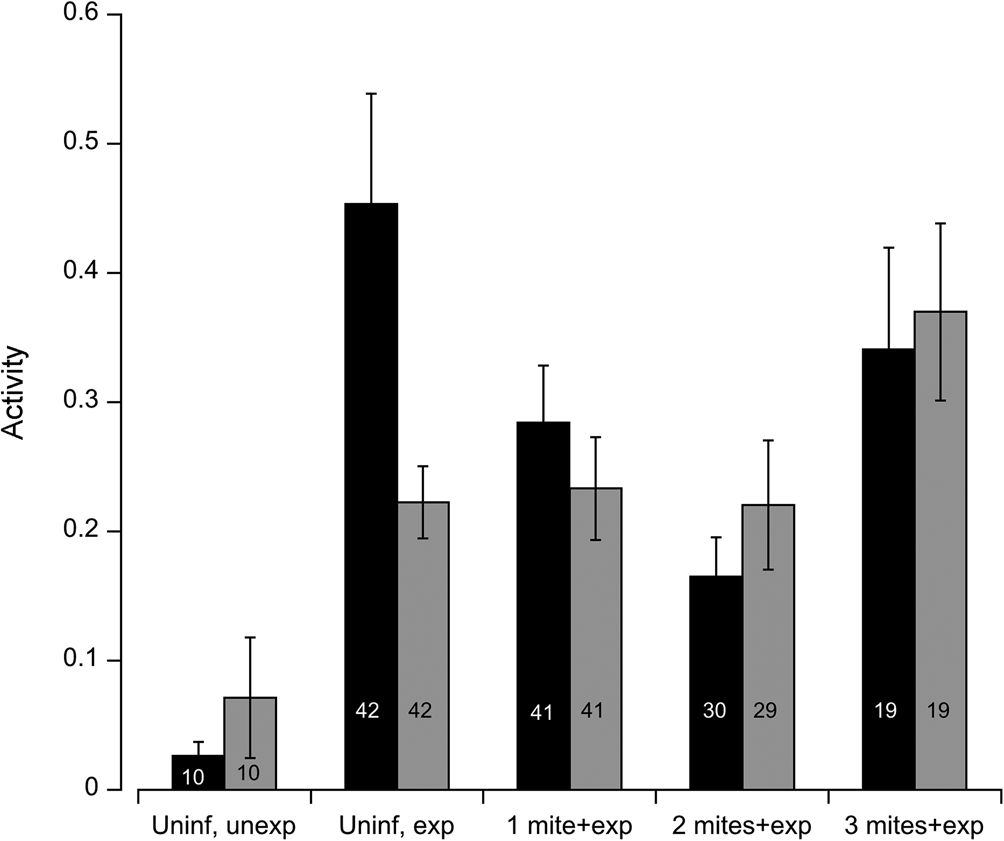
Fig. 1. Experimental flies were exposed to a single free-roaming mite in each of the MAVEn activity chambers. We measured the activity of individual flies in the following groups: uninfected and unexposed (Uninif, unexp), uninfected and exposed (Uninf, exp), infected with one mite and exposed (1 mite + exp), infected with two mites and exposed (2 mites + exp), and infected with three mites and then exposed (3 mites + exp). Black bars represent males; grey bars represent females. Numbers inside bars indicate sample size for each group. Error bars indicate ± 1 s.e.
Infection status and susceptibility to infection
We examined how an increasing mite load influences host susceptibility to further parasite attachment. Unmated male (n = 373) and female flies (n = 343) were collected from the mass culture and maintained in separate-sex vials for 10–20 days post-eclosion. In order to establish the initial infection, experimental flies were individually exposed to five female mites in an infection chamber (as described above). Control flies were placed in similar infection chambers without mites. After 1 h of exposure, the position and number of mite attached were recorded. Both infected and control flies were immediately exposed to secondary infection by a single mite in 1.5 mL microfuge tubes for 1 h. This larger tube allowed the flies to mount a behavioural defence against the mite. Infection status and position (neck, dorsal abdomen, side abdomen, ventral abdomen) of all mites were recorded. Mites were rarely located on the thorax or head of the fly (see results).
Part way through the experiments, we realized that we could not distinguish the mites if one either detached or changed positions on the fly. In this case, we could not determine if the new site of attachment was due to movement by the initial mite or attachment by the secondary mite. Henceforth, the mite introduced secondarily was marked on the idiosoma with archival ink covering roughly 25% of the dorsal side (Sakura Color Products Corporation, Osaka). The ink had no effect on the mite's attachment or survival throughout the experiment. Flies from the unmarked trials were excluded from the analysis if a mite detached and/or changed attachments sites during the assay. Trials were also omitted from analysis if the initial mite load decreased during the secondary exposure period (e.g. mites detached).
Statistical analyses
In the activity experiment, the mean activity level (arbitrary units) for a single mite was subtracted from the activity level for each fly, which was modelled with the independent variables exposure level, fly age, sex and block (trials conducted over several days). We performed two separate analyses to determine: (1) the effect of exposure on uninfected flies only and (2) the effect of exposure on flies with varying mite loads (0–3 mites).
In the secondary mite attachment experiment, we analysed the effect of initial mite load on the proportion of flies parasitized in the secondary infection; independent variables included the initial mite load, fly sex and age. Mite attachment site was analysed as the proportion of mites found at a given location on the fly, out of the total number of mites recovered at a given mite load.
All data were analysed using Generalized Linear Models in RStudio (R Development Core Team, 2016). The activity data were analysed with a Gamma error distribution (glm; R Stats Package) (R Development Core Team, 2016). The infection and attachment site data were analysed using a binomial error distribution. Backwards model selection was implemented to arrive at the minimal model; non-significant variables (ANOVA function, test = χ 2, P > 0.05) were removed from subsequent models. The change in deviance (henceforth referred to simply as ‘deviance’) resulting from the removal of a factor is reported along with the P value.
Results
Activity and propensity to disperse
Using locomotor activity as a proxy for dispersal propensity, we measure the overall activity level of uninfected and infected flies in the presence or absence of a questing mite, i.e. parasitism risk. Among uninfected flies, mite exposure (deviance = −19.8, P < 0.001) and sex (deviance = −8.58, P = 0.01) were important predictors of fly activity; age (deviance = 0.41, P = 0.59) and block (deviance = −0.237, P = 0.125) were not significant. The interaction between exposure and sex was marginally significant (deviance = −4.30, P = 0.05). The mean activity level among uninfected females (0.071 ± 0.148 s.d.) increased by threefold upon exposure (0.223 ± 0.180 s.d.) to a mite, whereas uninfected males increased their activity level by 17-fold in the presence of a questing mite (Fig. 1). Hence, parasitism risk increased the propensity to disperse in uninfected flies, especially among males.
In order to test how infection status influenced dispersal propensity, we analysed the activity of flies harbouring 0, 1, 2 and 3 mites upon secondary exposure to a questing mite. Since the interaction between initial infection and sex (deviance = −7.35, P = 0.01) was significant, males and females were analysed separately. The influence of infection status on female activity was marginally significant (deviance = −2.76, P = 0.09), age (deviance = −1.23, P = 0.25) and block (deviance = 0.185, P = 0.07) were not significant. The mean locomotor activity appears to increase somewhat once flies accumulated three mites. The relationship between initial infection among males and activity fit a quadratic function (deviance = −5.47, P = 0.03); age (deviance = 0.24, P = 0.64) and block (deviance = 0.44, P = 0.08) were not significant. Males displayed an initial decline in activity when infected with one or two mites, but the mean activity level increased markedly once flies were infected with three mites (Fig. 1). Once infected, the response to parasitism risk among males and females were comparable for a given mite load.
Infection status and susceptibility to infection
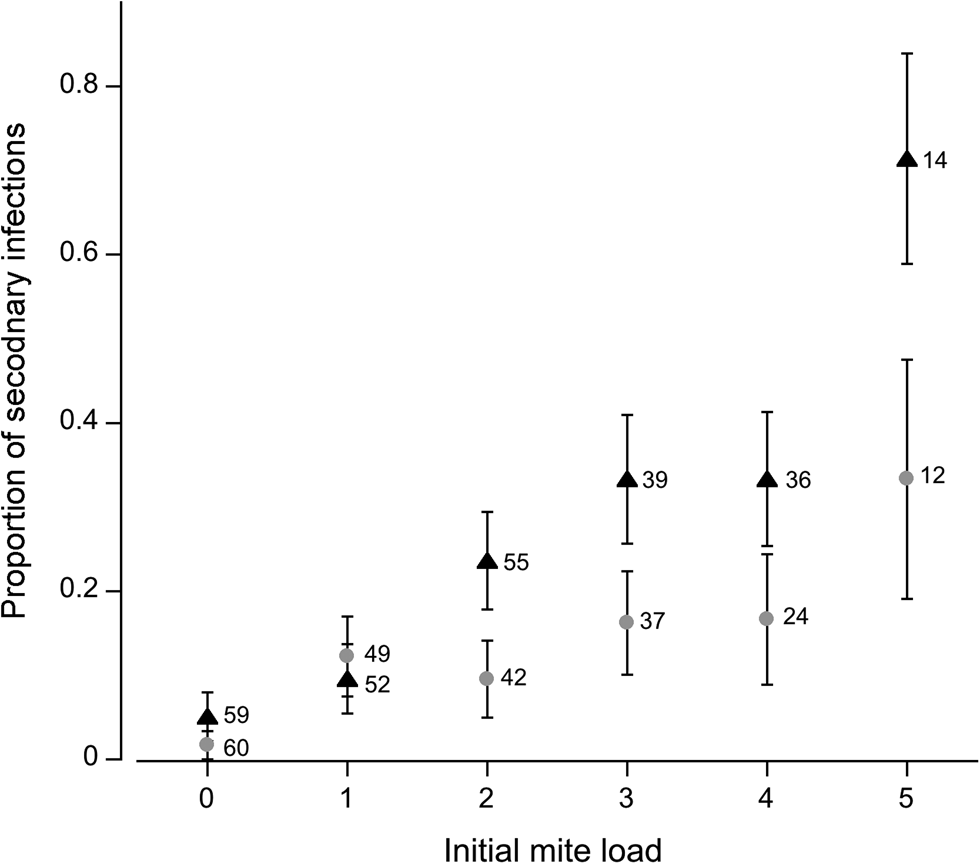
The probability of a secondary infection increased with initial infection level. Initial mite load (deviance = −33.4, P < 0.001) and sex (deviance = −9.75, P = 0.002) were significant factors in determining secondary mite attachment. No interactions were statistically significant (P > 0.05). Susceptibility to further infection increased with mite load (Fig. 2). The females experienced a gradual rise in susceptibility as mite load increased. By comparison, susceptibility among male flies increased noticeably once infection levels reached two mites, and once again when five mites were attached. This disparity is likely due to a smaller body size on average among male flies (see discussion).
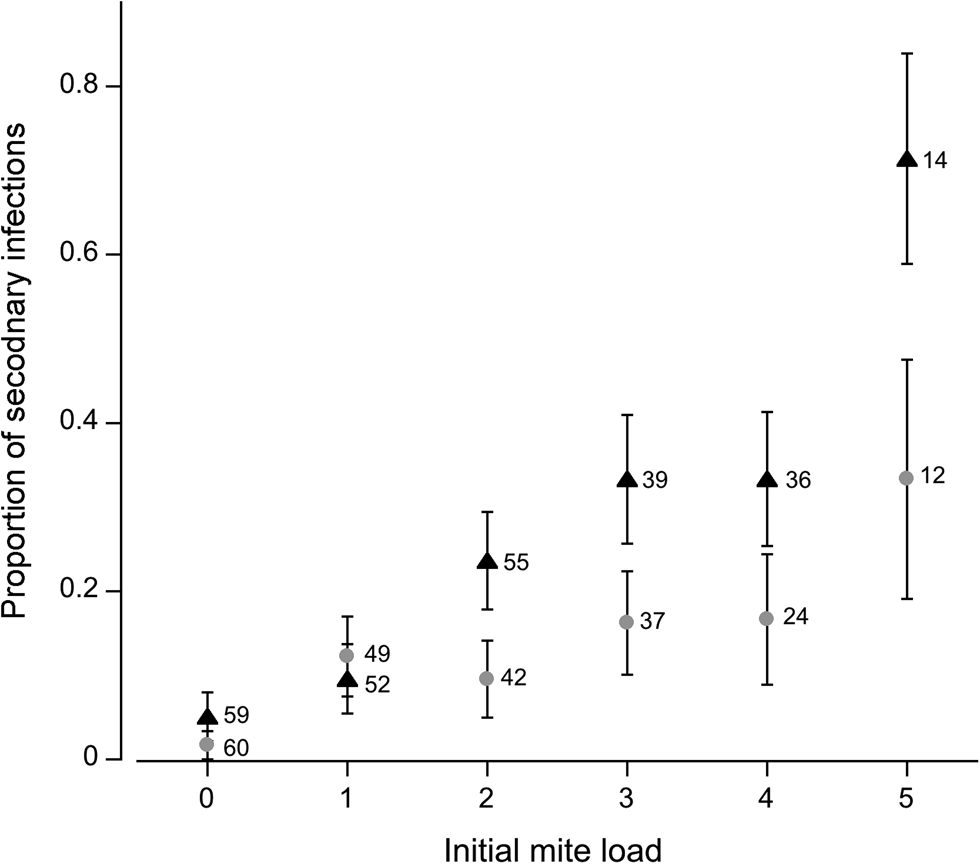
Fig. 2. Proportion of flies infected with a secondary mite. Flies of varying mite loads were exposed to a single mite in the secondary infection. Grey circles represent female flies; black triangles represent males. Numbers adjacent to data points represent sample sizes; error bars indicate ± 1 s.e.
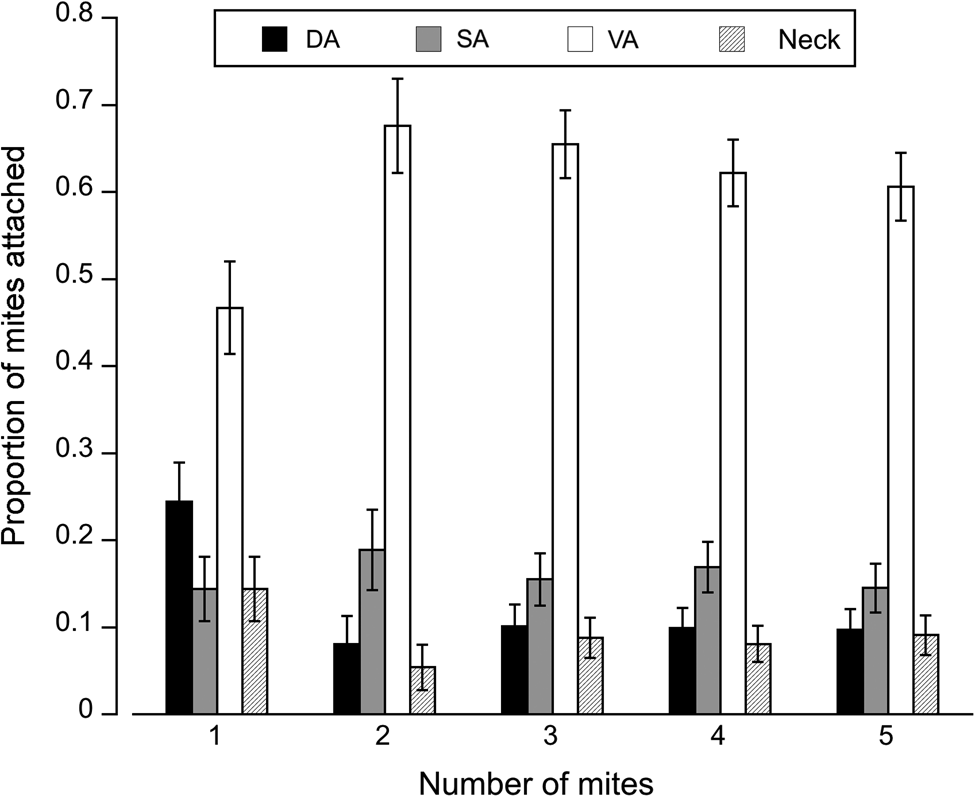
The number of mites attached varied by body region (deviance = 4.36, P < 0.001, Fig. 3); mite density and the interaction between these two factors were not significant (P > 0.05). Overall the most common site of attachment was the ventral abdomen; the second most common site was the dorsal abdomen, except when only a single mite was attached. Notably, the proportion of mites found on the dorsal abdomen and neck dropped precipitously with mite load, with mites favouring the ventral abdomen. The proportion of mites attached to the side of the abdomen remained stable across various levels of infection (Fig. 3).
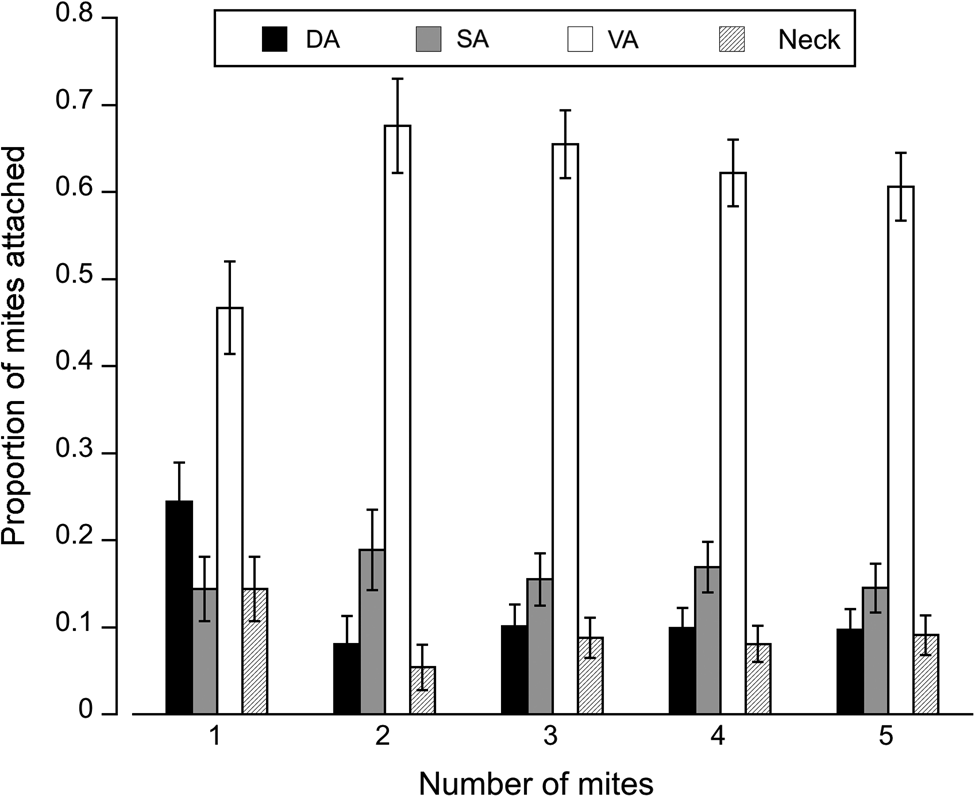
Fig. 3. Attachment site of mites on different regions of the fly: DA, dorsal abdomen; SA, side abdomen; VA, ventral abdomen, and neck. Total number of mites observed on flies infected with: 1 mite = 90, 2 mites = 74, 3 mites = 148, 4 mites = 167 and 5 mites = 155. Error bars represent ± 1 s.e. of proportions.
Discussion
The results showed that the infection status and risk of subsequent parasitism significantly impacted host locomotor activity, which is positively correlated with dispersal (Hanski et al., Reference Hanski, Saastamoinen and Ovaskainen2006; Clobert et al., Reference Clobert, Le Galliard, Cote, Meylan and Massot2009; Cote et al., Reference Cote, Clobert, Brodin, Fogarty and Sih2010). Uninfected flies exhibited increased levels of locomotor activity when exposed to a mite (i.e. parasitism risk). This is consistent with previous observations that flies exposed to mites performed significantly more ambulatory events than unexposed flies (Horn and Luong, Reference Horn and Luong2019). The increase in locomotor activity was especially pronounced among male flies, which likely reflects the relatively higher cost of infection among males compared to females. Flies harbouring two to four mites had lower copulatory success, and this effect was greater among males; i.e. the magnitude of parasite-mediate sexual selection was stronger for males than females (Polak and Markow, Reference Polak and Markow1995). This disparity is corroborated by how flies respond physiologically to the presence of mites: male flies increase their metabolic rate by 31% relative to 15% among females (Horn et al., Reference Horn, Mierzejewski, Elahi and Luong2020). Also, female flies can discriminate between oviposition sites based on the presence of mites in the environment, suggesting they may disperse from suboptimal environments to deposit eggs (Mierzejewski et al., Reference Mierzejewski, Horn and Luong2019). Hence, the risk of infection increases the propensity or willingness to dispersal, which in turn serves as an important mechanism for escaping parasitism.
Once infected with one or two mites, the activity level of males exposed to another mite, while higher than unexposed males, was significantly lower compared to uninfected, exposed males. So while the risk of infection elicited an increase in activity relative to unexposed flies, the propensity or willingness to disperse was tempered by a parasite-mediated decrease in locomotor activity. Indeed, flies infected with mites experience decreased flight performance compared to uninfected flies (Luong et al., Reference Luong, Penoni, Horn and Polak2015). This effect persisted until the flies accumulated three mites, at which time the activity level rose sharply. The cost of infection is dependent on the number of mites and duration of infection; three mites pose a much higher cost than one or two mites, including a higher risk of mortality (Polak, Reference Polak1996; Polak et al., Reference Polak, Luong and Starmer2007). A possible explanation for the precipitous increase in activity is that at high levels of parasitism the cost of infection overwhelms the host's ability to tolerate otherwise low-mid levels of infections. Alternatively, male flies at risk of parasite-mediated mortality may increase their mating effort (Polak and Starmer, Reference Polak and Starmer1998). Thus, the increased activity among these highly infected males may represent increased mate seeking, not increased dispersal. Future experiments could observe infected males in the presence and absence of female conspecifics to test these hypotheses.
Female flies also exhibited higher levels of activity upon exposure to a mite, but they managed to sustain the increased activity even after becoming infected, suggesting that females are able to better tolerate the costs of infection than males. Larger flies may be more resistant to infection, and female D. nigrospiracula are larger than males (Horn and Luong Reference Horn and Luong2021). Females infected with three mites appear to increase their activity level somewhat, suggesting a slight increase in propensity or motivation to disperse. For a given level of infection (1–3 mites), both females and males responded similarly to the risk of further infection. Future studies should manipulate the relative level of risk by varying the density of mites and/or hosts in the environment to test whether it influences the propensity to disperse. Theory suggests that hosts can evolve context-dependent, plastic dispersal behaviour in response to parasite prevalence and risk (Deshpande et al., Reference Deshpande, Kaltz and Fronhofer2021; Zilio et al., Reference Zilio, Norgaard, Petrucci, Zeballos, Gougat-Barbera, Fronhofer and Kaltz2021).
As predicted, susceptibility to infection increased with the level of current infection. The probability of acquiring additional mites among males increased more rapidly with current mite load compared to female flies. The cost of infection is higher among males due to stronger parasite-mediated sexual selection and a smaller body size on average than females. Parasite-induced changes in energy demands or allocation (e.g. for parasite growth, tissue repair, immune activation) likely divert energy away from behavioural defence against ectoparasites. Furthermore, time spent grooming, an energetically demanding activity, can result in greater susceptibility to future mite infection (Horn and Luong, Reference Horn and Luong2019). Parasite-mediated reductions in behavioural defences are likely driving the increase in susceptibility to further infection.
However, mite preference may also be driving the probability of subsequent infections. Previous research has shown that mites preferentially infected hosts that are already harbouring mites (Luong et al., Reference Luong, Brophy, Stolz and Chan2017), creating a positive feedback loop. Aggregation of arthropods may involve host-derived semio-chemicals or other chemical cues (Egan et al., Reference Egan, Barth and Hanson1975; Wertheim et al., Reference Wertheim, van Baalen, Dicke and Vet2005; Koenraadt and Dicke, Reference Koenraadt and Dicke2010). For example, injury-induced bleeding and elevated levels of CO2 among parasitized Drosophila may act as attractants for mites (Luong et al., Reference Luong, Brophy, Stolz and Chan2017; Horn et al., Reference Horn, Mierzejewski and Luong2018; Brophy and Luong, Reference Brophy and Luong2021. In other words, mite-related cues (aggregation pheromones) and host-related cues (kairomones) may mediate mite attraction to certain hosts, increasing the probability of re-infection. Positive feedback (reducing host behavioural defence and increasing mite preference) could create a ‘snowball effect’, generating additional infection heterogeneity within the host population (Johnson and Hoverman, Reference Johnson and Hoverman2014).
Mite preference for specific attachment sites, tempered by host ability to defend against the mites, may also be driving the distribution of mites among host body regions. The overall proportion of mites attached to the dorsal abdomen or neck (less favourable regions) was highest when only a single mite was attached; i.e. probability of a mite being found on these regions decreased with mite load, shifting instead to the ventral abdomen. Since flies defend themselves primarily through grooming (e.g. leg and tarsal flicking), the ventral abdomen is likely well defended relative to other parts of the body (Horn and Luong, Reference Horn and Luong2019). However, as mite load increased, flies were less able to defend themselves and mites were able to establish on the ventral abdomen (preferred site). Moreover, aggregating on a particular region of the host could dilute an individual's risk of being dislodged or overwhelmed by host defences (Sonenshine, Reference Sonenshine1991; Poulin, Reference Poulin2007).
Mites may show preference for the ventral abdomen because it provides access to a large surface area for attachment, a large volume of haemolymph, and cuticle that is relatively less sclerotized than other parts of the body, all of which can potentially increase feeding success. Aggregating on a particular host region may also enhance the feeding efficiency of ectoparasites. Studies on ticks have found increased feeding performance at higher tick densities due to the shared bioactivities of salivary compounds (Davidar et al., Reference Davidar, Wilson and Ribeiro1989; Wang et al., Reference Wang, Hails, Cui and Nuttall2001; Ogden et al., Reference Ogden, Casey, French, Adams and Woldehiwet2002). Hence, the observed distribution of ectoparasites on a host may be the outcome of a dynamic interplay between host defence and parasite preference.
In general, parasites can influence host dispersal by impacting (1) the propensity to disperse depending on the risk of infection and (2) the ability to disperse via changes in morphological, physiological and behavioural traits associated with movement. By exploiting host resources, parasites can cause mortality, reduce host movement and/or overall levels of activity with consequences for dispersal (McElroy and de Buron, Reference McElroy and de Buron2014; Binning et al., Reference Binning, Shaw and Roche2017; Zilio et al., Reference Zilio, Norgaard, Petrucci, Zeballos, Gougat-Barbera, Fronhofer and Kaltz2021). On the other hand, parasite exposure and/or infection can also increase the tendency to disperse (Suhonen et al., Reference Suhonen, Honkavaara and Rantala2010; Zilio et al., Reference Zilio, Norgaard, Petrucci, Zeballos, Gougat-Barbera, Fronhofer and Kaltz2021). Individuals may disperse to avoid exposure to parasites and reduced infection risk (Curtis, Reference Curtis2014; Buck et al., Reference Buck, Weinstein and Young2018). Uninfected backswimmers (Notonecta undulate) exhibited increased dispersal propensity in the presence of an ectoparasite, but at the same time increasing mite load decreased host dispersal ability (Baines et al., Reference Baines, Diab and McCauley2020). The combined effects of parasite risk and parasite infection on dispersal will in turn depend on several factors, including the intensity and prevalence of infection (i.e. local level of risk), the density of conspecifics (i.e. competition, risk dilution), mating opportunities, availability of resources, and intrinsic host factors such as body condition, size, age, sex, developmental stage and infection status.
In conclusion, host infection status and parasitism risk can operate together to mediate host dispersal behaviour and susceptibility to further infection. Parasites can influence host dispersal in a state-dependent (e.g. parasite-mediated decrease in movement) and/or context-dependent (e.g. to escape infection risk) manner, with potentially significant implications for gene flow, as well as other population and ecosystem level processes.
Data
The data presented in this study can be accessed on TBD.
Acknowledgements
Thanks to C. Horn for helpful feedback on the manuscript and for maintaining the fly cultures.
Author contributions
T.B. designed the study and collected the data. T.B. and L.T.L. performed the statistical analyses and wrote the article.
Financial support
Funding was provided by the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (#435245).
Conflict of interest
None.
Ethical standards
Not applicable.