Elevated pulmonary vascular resistance historically has been viewed as a contraindication to orthotopic heart transplantation in children,Reference Fricker, Addonizio and Bernstein 1 , Reference Huang, Trinkaus, Huddleston, Mendeloff, Spray and Canter 2 with a traditional cut-off used for pulmonary vascular resistance index >6 Wood Units×m2.Reference Mehra, Kobashigawa and Starling 3 , Reference Gazit and Canter 4 A prior investigation by Chiu et alReference Chiu, Russo, Davies, Addonizio, Richmond and Chen 5 questioned this cut-off by using the Columbia University transplant registry to examine the effect of pulmonary vascular resistance index on mortality. They found that, although pulmonary vascular resistance index did predict mortality when used as a continuous variable, dichotomisation of pulmonary vascular resistance index to determine a data-driven cut-off revealed a threshold value of 9.3 Wood Units×m2. The Columbia study, however, reflected patients from a single institution and included transplants as far back as 1984. As the authors then suggested, improved management of pulmonary hypertension and right ventricular dysfunction over the 27-year study period may have changed the relationship between pulmonary vascular resistance and post-transplant mortality.
We undertook the present study to address these limitations by using a comprehensive, national transplant database and by limiting the analysis to the modern era (10-year study period). This re-examination of the effect of pulmonary vascular resistance index on post-transplant mortality may help guide clinicians in risk stratification and decision making regarding the options of mechanical circulatory support, heart transplant, and heart–lung transplant.
Materials and methods
The Stanford University Institutional Review Board granted an exemption from review because this analysis uses de-identified data. Transplantation and post-transplant survival data were obtained using comprehensive data sets from the Organ Procurement and Transplantation Network through the Scientific Registry of Transplant Recipients. The Scientific Registry of Transplant Recipients data system includes data on all donor, wait-listed candidates, and transplant recipients in the United States, submitted by members of the Organ Procurement and Transplantation Network under the oversight of the Health Resources and Services Administration, United States Department of Health and Human Services, and has been described elsewhere. Data sets from the Organ Procurement and Transplantation Network and Scientific Registry of Transplant Recipients were supplied by the Minneapolis Medical Research Foundation pursuant to Health Resources and Services Administration contract number HHSH250201000018C. The authors alone were responsible for reporting and interpreting these data; the views expressed herein are those of the authors and not necessarily those of the United States Government.
All heart transplant recipients between 1 January, 2002 and 1 September, 2012 who were under 18 years of age at the time of transplant were identified. Follow-up information was available through 2 September, 2012. We excluded heart–lung transplants, those without pre-transplant haemodynamic data recorded in the Scientific Registry of Transplant Recipients database, and those with a primary diagnosis of complex structural CHD – consistent with the methodology in previous analysesReference Chiu, Russo, Davies, Addonizio, Richmond and Chen 5 – because of the difficulty of accurately determining pulmonary vascular resistance index from retrospective databases in these patients.Reference Fricker, Addonizio and Bernstein 1
Pre-transplant haemodynamic variables at the time of transplant are recorded in the Scientific Registry of Transplant Recipients database; cardiac output, body surface area, mean pulmonary artery pressure, and pulmonary capillary wedge pressure were used to calculate pulmonary vascular resistance index as (mean pulmonary artery pressure−pulmonary capillary wedge pressure)×(body surface area)/(cardiac output). Baseline demographic and patient characteristic variables were evaluated. As excess morbidity attributable to right ventricular dysfunction related to elevated pulmonary vascular resistance should be most apparent early in the post-transplant course, the primary outcome for this analysis was a 30-day all-cause mortality, as determined by a recorded death in the Scientific Registry of Transplant Recipients registry within 30 days of transplant, calculated from date of transplant to date of death. The secondary outcome was overall survival throughout Organ Procurement and Transplantation Network follow-up.
Statistical analysis
Baseline categorical variables were compared using Fisher’s exact test or Pearson's χ2-test, as appropriate, and continuous variables were compared using the Student's t-test. Mean values are reported as mean±standard deviation. Receiver operating characteristic curve analysis was performed on calculated pulmonary vascular resistance index to assess the predictive effect of pulmonary vascular resistance index on short-term mortality. An optimal threshold value of pulmonary vascular resistance index based on the receiver operating characteristic curve was then used to perform analysis of pulmonary vascular resistance index as a dichotomised variable, that is, low-pulmonary vascular resistance index and high-pulmonary vascular resistance index groups. Survival analysis was performed on these groups as a secondary outcome, using Kaplan–Meier curves for death at any time during Organ Procurement and Transplantation Network follow-up, with right-censoring at loss to follow-up or retransplant, and the log-rank test for intergroup survival comparison.
Multivariate logistic regression analysis was performed on pulmonary vascular resistance index as a continuous and dichotomised variable, and additional variables with p<0.2 in univariate analyses and/or in a comparison of low- and high-pulmonary vascular resistance index groups were included in the multivariate models. Results are presented as odds ratios (95% confidence interval) with p-values. Unless otherwise specified, all tests were two-tailed with a predetermined α for statistical significance of 0.05. Analyses were performed using Microsoft Excel (version 2010, Microsoft Corporation, Redmond, Washington, United States of America) and SAS 9.3 (SAS Institute, Cary, North Carolina, United States of America).
Results
A total of 3523 transplants were performed on paediatric recipients during the study period. Exclusion of records for patients with complex CHD (n=1618) and those without complete pretransplant catheterisation data (n=1206) left 699 patients for the analysis. The study population mean age was 9.4±5.9 years, and the mean pulmonary vascular resistance index was 3.89±3.12 Wood Units×m2. The 30-day mortality included 10 patients (1.43%) who died on an average of 11.2±8.2 days after transplant. Descriptive variables for the patients who died within 30 days are shown in Table 1. Overall mortality was 19.1% at a median (interquartile range) follow-up of 1087 (369–1830) days after transplant.
Table 1 Characteristics of patients with short-term mortality.

M/F=male/female; PVRI=pulmonary vascular resistance index
Diagnosis and cause of death descriptors as recorded in Scientific Registry of Transplant Recipients database
A receiver operating characteristic curve yielded a cut-off value of 3.37 Wood Units×m2 as the optimal threshold of pulmonary vascular resistance index to predict short-term mortality (Fig 1). This receiver operating characteristic curve had an area under the curve of 0.690, and the Hosmer–Lemeshow test showed acceptable goodness-of-fit (p=0.886). Sensitivity was 81% and specificity 55%, with a positive predictive value of 25% and a negative predictive value of 99.5%. This cut-off was then used to divide the study population into groups by a dichotomised pulmonary vascular resistance index: those with pulmonary vascular resistance index ⩾3.37 (n=317) and those with pulmonary vascular resistance index <3.37 (n=382). A comparison of baseline variables between the two groups is shown in Table 2; the higher pulmonary vascular resistance index subgroup was older (p<0.001) and had a larger body surface area (p<0.001). Otherwise no baseline differences were observed between these groups.

Figure 1 Receiver operating characteristic curve to determine the optimal threshold value of pulmonary vascular resistance index that predicts short-term post-transplant mortality.
Table 2 Characteristics of low- and high-PVRI groups.

PVRI=pulmonary vascular resistance index
Values are number (percentage) or mean±standard deviation, as appropriate
Univariate analysis for the primary outcome (30-day mortality) did not demonstrate a significant predictive effect of pulmonary vascular resistance index treated as a continuous variable (p=0.12), but pulmonary vascular resistance index dichotomised as a cut-off of 3.37 Wood Units×m2 did prove to be significant (odds ratio 4.92, 95% confidence interval 1.04–23.33, p=0.045). No other candidate variables reached statistical significance on univariate analysis (Table 3). The Kaplan–Meier analysis did not reveal significant differences in overall survival between the low- and high-pulmonary vascular resistance index groups (Fig 2, log-rank p=0.16).

Figure 2 Kaplan–Meier post-transplant survival analysis stratified by pulmonary vascular resistance index above (blue line) and below (red line) the data-driven threshold value of 3.37 Wood Units×m2.
Table 3 Univariate analysis of variable associations with 30-day mortality.

BSA=body surface area; MCA=mechanical circulatory support; PVRI=pulmonary vascular resistance index
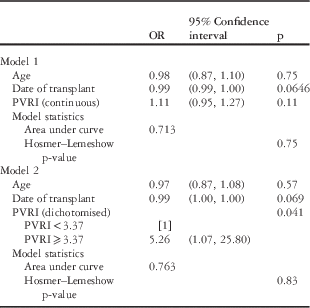
Two multivariate models were constructed for pulmonary vascular resistance index as a continuous variable and as a dichotomised variable (Table 4). Age and date of transplant were included in both models. Pulmonary vascular resistance index dichotomised remained a significant predictor (odds ratio 5.26, 95% confidence interval 1.07–25.80, p=0.041), but pulmonary vascular resistance index as a continuous variable remained non-significant (p=0.11), and age and date of transplant did not prove to be significant predictors in either model.
Table 4 Multivariate logistic regression models for 30-day mortality.
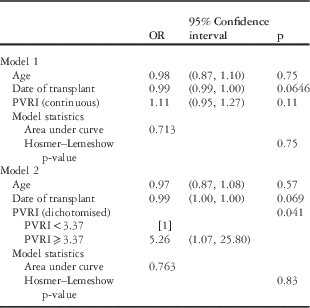
PVRI=pulmonary vascular resistance index
Discussion
These results provide less convincing evidence than the previous analysesReference Huang, Trinkaus, Huddleston, Mendeloff, Spray and Canter 2 , Reference Chiu, Russo, Davies, Addonizio, Richmond and Chen 5 that elevated pulmonary vascular resistance remains a dose-dependent risk factor for early mortality after paediatric heart transplant. Compared with the Columbia study,Reference Chiu, Russo, Davies, Addonizio, Richmond and Chen 5 our analysis found a lower optimal threshold for pulmonary vascular resistance index to separate low- and high-risk patients (3.37 Wood Units×m2 versus 9.29 Wood Units×m2), and unlike in their analysis pulmonary vascular resistance index treated as a continuous variable was not a significant predictor of 30-day mortality. The receiver operating characteristic curve for pulmonary vascular resistance index in this data set demonstrates a smaller area under the curve (0.690) than that in the Columbia study (0.863); moreover, whereas the sensitivity (81% versus 80%), positive predictive value (25% versus 24%), and negative predictive value (99.5% versus 99.3%) were similar, the specificity was lower (55% versus 91.5%). On univariate and multivariate analyses, pulmonary vascular resistance index treated as a dichotomised variable around the receiver operating characteristic-determined cut-off value remained significant, but the confidence intervals were wide and came close to crossing 1.0 in both cases.
These observations suggest that, while pulmonary vascular resistance index treated as a dichotomised variable did demonstrate a statistically significant effect on outcome, its clinical significance may be quite modest compared with that suggested by previous analyses. The outcome difference observed at this lower threshold value may simply reflect the separation between the lowest risk patients and those with even modestly elevated risk. Our analysis does not suggest to us that the traditional use of a pulmonary vascular resistance index above 6 Wood Units×m2 or the use of the threshold from the Columbia study (9.29 Wood Units×m2) as contraindications to transplant should be replaced with aggressive use of a threshold of 3.37 Wood Units×m2; rather, it suggests to us that the use of any threshold value may have diminished clinical utility.
Individual analysis of the 10 patients who died within 30 days of transplant is limited by the information present in the registry, but it does not demonstrate a pattern that would suggest a dominant contribution of right ventricular dysfunction to post-transplant mortality in patients with elevated pulmonary vascular resistance index (Table 1). Of five patients with a pulmonary vascular resistance index over 5 Wood Units×m2, one (pulmonary vascular resistance index=9.36 Wood Units×m2) was attributed to acute rejection, and one (pulmonary vascular resistance index=5.10 Wood Units×m2) to a cerebrovascular accident. The remaining three deaths (in patients with pulmonary vascular resistance index=9.49, 8.33, and 6.67 Wood Units×m2) experienced death on postoperative day 13, 5, and 9, respectively. Their causes of death, such as “multiple organ failure”, “cardiac arrest”, and “ventricular failure” are too vague to make conclusive determinations, but no specific role for elevated pulmonary vascular resistance index can be inferred, and timing of these deaths makes it seem less likely that acute right ventricular failure was the aetiology.
We would suggest that the conclusions drawn by the Columbia group, that is, that a pulmonary vascular resistance index cut-off of 6 might be too restrictive and a pulmonary vascular resistance index of 9 might be a more predictive cut-off, might be revised in light of these results. Instead, we conclude that there may be diminishing evidence that pulmonary vascular resistance index should be used as an exclusion criterion for paediatric heart transplantation at all. Although we did find a data-driven cut-off value for pulmonary vascular resistance index that remained significant on multivariate analysis, it was lower than even historically accepted cut-off values (6 Wood Units×m2). The lack of a significant effect of pulmonary vascular resistance index as a continuous variable, the comparatively poor area under the curve and specificity of the receiver operating characteristic curve, and the marginal significance – wide confidence intervals with p-values close to 0.05 – of the estimates for the risk conferred by pulmonary vascular resistance index treated as a dichotomised variable collectively suggest that pulmonary vascular resistance index is less predictive than it has been in past analyses, and make the finding of any meaningful threshold value less compelling.
One plausible explanation for this discrepancy compared with past studies is change over time, that is, the hypothesis that our analysis of a more recent cohort of paediatric heart transplants (2002–2012) than the Columbia study (1984–2010) reflects improvement in the management of acute postoperative right ventricular dysfunction, including pharmacologic options, such as pulmonary vasodilators, as well as mechanical circulatory support options, for example, right ventricular assist devices. This idea is consistent with the observation that the overall 30-day mortality rate was lower in this analysis (1.43% versus 3.2%).
The hypothesis that improved management of post-transplant right ventricular dysfunction has mitigated the effect of elevated pulmonary vascular resistance index as a marker of risk cannot be proven from this analysis. The low event rate of early paediatric post-transplant mortality (<2/year nationwide), however, precludes single-institution approaches to this question, and we are not aware of a better national or international source of data to help answer this question.
There are several significant limitations to this analysis. First, although the Scientific Registry of Transplant Recipients database has the advantages of being inclusive of all transplants performed at all transplant centres nationwide, it remains subject to the limitations of a retrospective database analysis, such as coding error, missing data, and heterogeneity between centres in how haemodynamic variables are tested and reported. For instance, although it is probably possible to calculate a meaningful pulmonary vascular resistance index in most patients with complex CHD, with the sites of catheter measurement individualised to each patient’s specific anatomy, retrospective registry data are unreliable for this purpose. As such, this analysis cannot provide information about the role of pulmonary vascular resistance in predicting post-transplant mortality in that large fraction of paediatric transplant candidates. It also lacks information on how pulmonary vasodilator responsiveness might modulate the effect of pulmonary vascular resistance index on risk, as the registry structure does not allow for an unambiguous interpretation of whether vasoreactivity was tested, and if so, how pulmonary vascular resistance responded. Similarly, it lacks detailed cause of death information that would permit a nuanced understanding of whether elevated pulmonary vascular resistance contributed to the cause of death in any individual case.
Second, as a retrospective analysis of transplants that have been performed, this analysis – like the Columbia study and others – is subject to the limitation of reflecting risk only in patients who have been deemed transplant candidates by at least one centre. It cannot provide information on the relationship between pulmonary vascular resistance and post-transplant mortality in children who are currently not viewed as transplant candidates. This limitation should be tolerated, given that the ideal design to evaluate the effect of pulmonary vascular resistance on post-transplant mortality is unlikely to ever occur, that is, a trial that randomises high-pulmonary vascular resistance patients between heart transplant candidacy and an alternative therapy, such as heart–lung transplant, destination therapy, mechanical circulatory support,Reference Brancaccio, Filippelli and Michielon 6 or palliative care. We are limited to an analysis of existing transplant outcomes.
Third, this analysis excluded children with complex structural heart disease because of the unreliability of retrospectively assessing meaningful pulmonary vascular resistance index measurements, and therefore it does not have direct external validity to the population of transplant candidates who have failed palliation of complex congenital anomalies. The difficulties of investigating the effect of elevated pulmonary vascular resistance in that population – for example, the need for prospectively adjudicated pulmonary vascular resistance index measurements in a large enough congenital population that one could assess the relationship with post-transplant mortality – will likely persist. Our results may remain the best available related evidence to guide decision making.
The advantages of this analysis are that they provide results from a larger, comprehensive national population of paediatric heart transplant patients and find what we interpret to be a less significant effect of pulmonary vascular resistance than has been previously documented. This finding should inform further investigations into the question of whether there are high-pulmonary vascular resistance patients who carry unacceptably high risk of mortality after heart transplant and would be better served by alternate therapeutic pathways, and if so, how best to identify them. In the meantime, the significant downsides of those alternatives – for example, excessive waitlist times for paediatric heart–lung transplant and barriers to long-term durability of destination therapy mechanical circulatory support in the paediatric population – may prompt clinicians to reassess the degree to which elevated pulmonary vascular resistance index is thought of as a contraindication to transplant candidacy.
Acknowledgements
None.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of Interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the Stanford University Institutional Review Board.