CROATIAN HEALTHCARE SYSTEM AND FINANCING
Croatia is a small central European country (with approximately 4.3 million inhabitants); it became the 28th Member State of the European Union (EU) on July 1, 2013. In 2015, the gross domestic product (GDP) per capita was € 10459.
The Croatian healthcare system is based on the principles of social health insurance and is primarily determined by three key acts: the Health Care Act, the Mandatory Health Insurance Act, and Act on the Protection of Patients’ Rights. The basic legal framework of the healthcare system for drugs and medical devices and the main decision makers in Croatia are summarized in Table 1.
Table 1. Basic Legal Framework of the Healthcare System, for Drugs and Medical Devices and the Main Decision Makers in Croatia

Note. Sources: https://zdravlje.gov.hr/pristup-informacijama/zakoni-i-ostali-propisi/zakoni/zdravstveni-zakoni/1615, http://www.halmed.hr/en/O-HALMED-u/Zakoni-i-pravilnici/
Abbreviations: CHIF, Croatian Health Insurance Fund.
Healthcare Financing
Mandatory health insurance, implemented by the Croatian Health Insurance Fund (CHIF), is financed by the contributions paid by the insured, employers, contributions by other contribution payers, special contributions for using health care abroad, special contributions in case of a work-related injury, contributions from the state budget and income from interest, dividends, and other income. Supplementary and additional health insurance through CHIF and private health insurance are voluntary. Total spending on health care (1994–2013) ranged from 6.0 percent to 7.8 percent of the GDP; per capita spending on health care ranged from approximately €500 to € 1360 (Reference Džakula, Sagan, Pavić, Lončarek and Sekelj-Kauzlarić1;2).
The share of private expenditures for health care in the GDP in Croatia is around 1.18 percent of the GDP. In 2014, total expenditures from compulsory health insurance were ~ €2.8 billion; for total health care, ~ €2.5 billion; specifically, ~ €522 million (15.42 percent) for primary health care; ~ €1.05 billion (33.21 percent) for hospital health care and consultative specialist health care; ~ €435 million (16.98 percent) for prescription drugs; and ~ €86 million (3.56 percent) for orthopedic devices and prosthesis. From 2015, CHIF was withdrawn from the State Treasury system in an effort to improve the management of healthcare financial flows (3–5).
Healthcare Reforms
The Croatian healthcare sector has undergone major changes since the 1990s. In 2012, the Ministry of Health (MoH) was responsible for drafting and implementing the National Health Care Strategy 2012–20. The strategic developmental objectives of the healthcare system included strengthening the connections and continuity throughout the healthcare system, standardizing and improving the quality of health care by strengthening the HTA, increasing efficiency and effectiveness of the healthcare system, making health care more available and improving health indicators (6).
The aim of this article is to provide a brief, 7-year history of health technology assessment (HTA) implementation in Croatia through national and international activities.
METHODS
We used retrospective descriptive analysis of key documents related to the legal framework, process of decision making, and HTA. Analysis of plan for and experience of the Agency for Quality and Accreditation in Health Care and Social Welfare with the implementation of a transparent HTA process in Croatia was performed by analyzing seven of eight key components of the HTA implementation scorecard framework, a tool to assess the current status of HTA implementation: (i) HTA capacity building; (ii) HTA funding; (iii) legislation on HTA; (iv) scope of HTA implementation; (vi) quality and transparency of HTA implementation; (vii) use of local data; (viii) international collaboration (7). In-depth analysis of the appraisal process and decision making was not within the scope of this case-study (HTA implementation scorecard component v, Decision criteria). The main challenges and facilitating factors were also evaluated.
Relevant documents were identified by searching the Web sites of the main Croatian institutions (national healthcare decision makers) and the Agency for Quality and Accreditation in Health Care and Social Welfare, as the institution responsible for the HTA process at the national level. The Agency's internal documents were analyzed as well.
The Agency's plan for a transparent national HTA process implementation included six major aims: (1) The Croatian HTA Guideline should be made public; (2) international collaboration should be established as well as (3) collaboration at the national level, education, and HTA promotion; (4) HTA reports (national and in international collaboration) should be provided as well as (5) scientific publications and (6) a transparent Web page for HTA.
RESULTS
Descriptive analysis using the HTA implementation scorecard framework related to key components: (ii) HTA funding, (iii) Legislation on HTA, and (iv) Scope of HTA implementation (7)
Institutionalization of the Agency for Quality and Accreditation in Health Care and Social Welfare (in the Field of HTA)
The Agency for Quality and Accreditation in Health Care and Social Welfare (Agency) was established in 2007, based on the Croatian “Strategy of the development of the Croatian Health Care System 2006–2011” (6), as a legal, public, independent, governmentally funded, nonprofit institution under the Act on Quality of Health Care (Official Gazette No. 107/2007). The activities of the Agency commenced in 2009. The Agency consists of three departments responsible for healthcare quality, accreditation, and HTA: the Department for Quality and Education, the Department for Accreditation in Health and the Department for Development, Research, and HTA. According to the current Act on Quality of Health Care and Social Welfare (Official Gazette No. 124/2011), the Agency should provide the procedure for HTA and the database on HTA at the national level, propose the Ordinance on HTA to the MoH and provide continuous education in the field of HTA as well as national and international collaboration. The Croatian Ordinance on HTA passed the public consultation process in October 2016. The reasons why this Ordinance is still not in place despite the requirements from 2011 are not known. The legal compliance of the Agency is supervised by the MoH.
The aim of Croatian HTA process and reporting is to produce credible and standardized information that is relevant and useful to the main decision makers (MoH, CHIF, and hospital management teams) in the Croatian publicly-funded healthcare system and to meet their needs for reliable, consistent, timely, and relevant HTA information.
Key Documents Related to the Legal Framework, the Process of Decision Making, and HTA
The key documents related to the legal framework, the process of decision making, and HTA are presented in Table 2. The current status of the process of decision making and the HTA processes on drugs, medical devices, and procedures in Croatia is presented in Figure 1. In brief, the main Croatian decision makers (MoH, CHIF, and hospital managements) may request the Agency to perform HTA on the whole range of health technology from different life cycles, either as a single technology assessment (STA) or a multiple technology assessment (MTA). However, there is still no formal topic selection and prioritization process, but the MoH can ask for priority assessment. The Agency produces the requested HTAs with recommendations, created according to the Croatian HTA Guideline (8). National HTA reports are still in the Croatian language only; joint international reports (in form of rapid relative effectiveness [REA] assessment on pharmaceuticals, medical devices and other technologies as well as full [comprehensive] Core HTAs) are produced in the English language through EUnetHTA are further adapted to the national level and translated to the Croatian language. The same is true for HTA reports produced by other HTA institutions; if the topic and scope are relevant, these reports are further updated and adapted to the Croatian healthcare system.
Table 2. Key Documents Related to the Legal Framework, Process of Decision Making and HTA

Abbreviations: HTA, health technology assessment; EU, European Union; ISPOR, International Society for Pharmacoeconomics and Outcomes Research; CHIF, Croatian Health Insurance Fund.
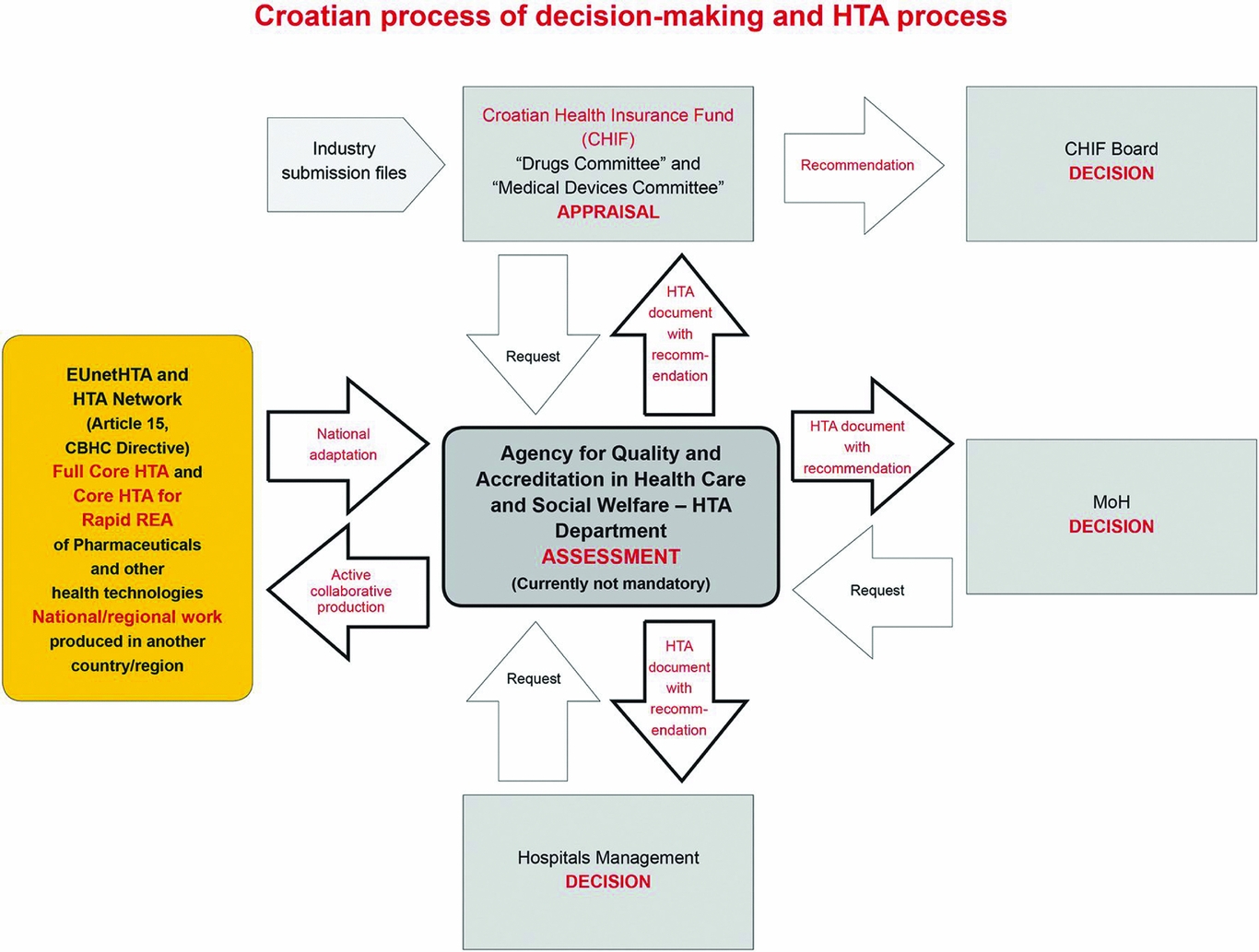
Figure 1. The Croatian process of decision making and the HTA process on drugs, medical devices, and procedures.
Abbreviations: HTA, health technology assessment; REA, relative effectiveness assessment; MoH, Ministry of Health.
Descriptive analysis using the HTA implementation scorecard framework related to key components: (i) HTA capacity building; (vi) Quality and transparency of HTA implementation; (vii) Use of local data; (viii) International collaboration (7)
Plan for a Transparent National HTA Process Implementation and Agency Experience
The formal activities of the Agency in the area of HTA began more than 7 years ago, in October 2009, at the Department for Development, Research, and HTA. The Agency's plan for a transparent national HTA process implementation included six major aims: (1) The Croatian HTA Guideline should be made public; (2) international collaboration should be established as well as (3) collaboration at the national level, education, and HTA promotion; (4) HTA reports (national and in international collaboration) should be provided as well as (5) scientific publications and (6) a transparent Web page for HTA.
Croatian HTA Guideline
To ensure a transparent, scientific, evidence-based HTA process, in February 2011, the Agency issued first the Croatian Guideline for HTA Process and Reporting, in the English language, with a Code of Practice for declaring and dealing with conflicts of interest in the HTA process, a Form for disclosure of potential conflict of interest in the HTA process and an Authorship statement. This first edition (8) was based on HTA Guidelines from the National Institute for Health and Clinical Excellence, the Canadian Agency for Drugs and Technologies in Health, the Belgian Health Care Knowledge Centre, the Danish Centre for Health Technology Assessment, and EUnetHTA Core Models, adapted to the Croatian healthcare setting. Such an approach was possible due to common scientific methodology and some processes in HTA.
It was envisaged from the beginning that, if already published Core HTA and/or HTAs from other countries exist, they will be critically appraised for quality and further updated and adapted to the Croatian healthcare setting. Despite the fact that full economic analyses are still not mandatory (only budget impact analysis is mandatory for the reimbursement process of drugs and medical devices), the part of this guideline called “Guide for the Economic evaluation of health technologies: Croatia,” was written to be in place when full economic analyses become a mandatory part of the HTA process.
Other documents important for the assessment process were created as well, such as the “Topic proposal form” and “Template for patient group input in the HTA process.”
International Collaboration, Activities, and Memberships
The activities in EU projects by the Department for Development, Research, and HTA include active involvement in five projects: the EUnetHTA Joint Action 1 Project (2010–12); EUnetHTA Joint Action 2 Project (2012–15); EUnetHTA Joint Action 3 Project (2016–20); FP7 Project European-Study on Quantifying Utility of Investment in Protection from Tobacco (EQUIPT) (2013–16); and the H2020 project SELFIE: Sustainable intEgrated care modeLs for multi-morbidity: delivery, Financing, and performance. This was recognized to be of utmost importance for HTA capacity-building in Croatia and recognition on the national, European, and global level.
The Agency is, by means of the responsible person for HTA, a member of scientific HTA societies such as ISPOR, ISPOR HTA Roundtable Europe, ISPOR HTA Council, HTAi, European HTA Network, and acts as the WHO National Contact Point on HTA. The Agency representative acted as the Workshop Review Committee Co-chair for the ISPOR 17th Annual European Congress in 2014 and as the Associate Editor on IJTAHC Theme: EUnetHTA (November 2014). She was also the former Chair of the EUnetHTA Plenary Assembly (2012–14) and Co-chair of ISPOR HTA Roundtable Europe (2013–16); she participated in the development of the ISPOR HTA Training program, acted as a co-organizer of the pilot program held in June 2015, in Zagreb, Croatia, where she served as a Faculty member as well. The EUnetHTA Plenary Assembly and one EUnetHTA work package meeting were also held in Croatia.
Collaboration at the National Level, Education, and HTA Promotion
National collaboration, education, and HTA promotion include collaboration with different stakeholders, meetings, different HTA publications in the Croatian language, and educational materials posted on the Web site. The meeting of international experts with main HTA users in Croatia was held in January 2010; HTA symposiums were held during the Croatian congresses on pharmacoeconomics and outcome research, with international participation (2010–15); two National Conferences, in 2013 and 2015 (on Health Care Quality, Accreditation and HTA), as well as TAIEX Project Workshop were held as well.
The later took place in December 2010, organized by the Technical Assistance Information Exchange Instrument of the European Commission (TAIEX) in co-operation with the Agency HTA Department. The 2-day Workshop “Health Technology Assessment; Main Principles, HTA Process, and Report” attracted 110 participants from different stakeholders and eight EU experts. The Agency is currently organizing educational workshops for patient organizations representatives. The national proposal “Development of Health Technology Assessment” was sent to the Croatian MoH in January 2013 to be applied for different European funds if recognized as priority; currently it could be applied for EU Structural Funds.
Production of HTA Reports
In 2010, the Agency started the production of international and national HTA reports according to the Croatian HTA Guideline, in response to requests from the Croatian MoH, CHIF, and hospital managements. These HTA documents with recommendations were used in final evidence-based informed decision making. Since 2011 until December 2015, 31 HTA documents have been completed (20 national and 11 international) in the form of STAs (n = 4; 13 percent) or MTAs (n = 27; 87 percent). More than 65 percent were on medical devices/procedures. The first HTA was performed in an international collaboration with the Austrian Ludwig Boltzmann Institute for HTA on anti–vascular endothelial growth factor on diabetic macular edema (Reference Zechmeister and Huić9), and transcatheter aortic valve implantation was the topic of the first national HTA (Reference Huić10). National HTA reports are written in the Croatian language only and are fully visible on the Agency Web page.
Scientific Publications
During the 2010–15 period, sixteen scientific publications were published as the result of international collaboration or produced at the national level.
HTA Web Page
All documents or information related to HTA as well as all HTA reports, both those completed and in progress, are transparently published on the Agency's Web page.
Main Challenges And Facilitating Factors
The main challenges recognized by the Agency's HTA staff, which delay the implementation of optimal, sustainable HTA process, and mandatory HTA activities in Croatia are: (i) the legal framework is not fully in place (HTA is still not mandatory for reimbursement/investment or disinvestment processes); (ii) there are limited human resources (up to October 2013, there was only a single employee for HTA; there are currently two permanent full-time employees, four part time employees, and two temporary full-time employees); (iii) there is not sufficient funding; and (iv) stakeholder involvement is limited.
The facilitating factors are active international collaboration through EUnetHTA (joint production of HTA reports, EUnetHTA tools and processes) and ISPOR (although active involvement in ISPOR HTA Roundtable Europe, ISPOR HTA Council, ISPOR HTA Training Program, ISPOR Congresses), promotion of HTA, and production of national and international HTA reports.
DISCUSSION
HTA is still not fully implemented in Croatia. There are important barriers that need to be overcome.
As a latecomer in the HTA field, Croatia has been developing the HTA process at the national and international level during the past 7 years. With proper introduction of HTA as a priority, HTA implementation in Croatia was supported by Article 15 on the Cooperation on HTA, Cross-Border Health Care Directive; HTA Network Strategy paper; Commission Implementing Decision; HTA Network Reflection paper on Reuse of Joint Work in national HTA activities and the recent WHO Resolution – Health intervention and technology assessment in support of universal health coverage (11–15). Poland has already established public HTA organization with the recognition of HTA as a public priority. Despite the greater need for the formal role of HTA in the health policy decision-making process due the lower health status and more limited financial resources for healthcare system, insufficient human capacity and financial resources for HTA influence HTA development and the evidence-based decision-making process in the Central and Eastern European (CEE) countries in comparison to the Western European countries (Reference Kaló, Gheorghe, Huic, Csanádi and Kristensen7).
In comparison with the majority of other CEE countries (Reference Kaló, Gheorghe, Huic, Csanádi and Kristensen7), Croatia is on the final step to full implementation of a sustainable HTA process at the national level. Because of published data on HTA impact different jurisdictions (Reference Jacob and McGregor16–Reference Guthrie, Bienkowska-Gibbs, Manville, Pollitt, Kirtley and Wooding19), Croatia recognizes HTA as a tool for the rationalization of the healthcare system and as a tool against possible corruption because of its full transparency and unbiased assessment process. The Croatian national HTA report on Particle Beam Radiation Therapies for Cancer (Reference Huić20), requested by the Croatian MoH, could be used as example of how a HTA report could lead to rationalization of the healthcare system: in 2013 the Agency issued negative recommendations on building the Croatian Particle Beam Therapy Center, thus allowing the re-distribution of resources into other evidence-based health technologies. In 2015, different evidence-based, unbiased HTA reports produced by the Agency upon CHIF requests in the form of MTAs (on very expensive drugs for the treatment of chronic hepatitis C, prostate and lung cancer) were used by the CHIF Drugs Committee in the appraisal process and final reimbursement decisions (Reference Huić and Tandara Haček21–Reference Huić, Tandara Haček and Boban23).
Croatia has already recognized the importance of international collaboration and joint HTA work at the European level. For a small country like Croatia, European cooperation on HTA is essential due to limited human and financial resources. International collaboration is one of eight key components of the HTA implementation scorecard for CEE countries developed by Kaló et al. (Reference Kaló, Gheorghe, Huic, Csanádi and Kristensen7). International collaboration and joint HTA work at the European level could also help to overcome the so-called balanced drugs assessment system, which has been recommended by some authors for middle-income and CEE countries (Reference Dankó and Petrova24). Simply transferring the data and the results from other jurisdictions without local adaptation and adjustment is not an option due to the well-known limitations of such transferability (Reference Sculpher, Pang and Manca25).
During the past 7 years, Croatia has already had a very positive experience in the joint production of full Core HTA and rapid REA documents through EUnetHTA, now a scientific and technical cooperation mechanism of the European HTA Network (Reference Huić, Nachtnebel, Zechmeister, Pasternak and Wild26), as well as methodological guidelines, tools (such as the Core Model as flexible format and content, the POP Database, and a submission template for drugs and medical devices) and processes (such as the Scoping meetings with stakeholders).
The added value of the Agency HTA activities that have already been recognized are improved local competence and capacity in HTA, learning by doing, the ability to recognize barriers and facilitating further changes and improvements on the national and European level (Reference Kleijnen, Toenders and de Groot27), national awareness and political recognition of the concrete benefits of HTA, as well as effective communication and cooperation with relevant policy and decision makers. After joint assessment, less time is needed for the production of national HTA reports, increasing the number and quality of national reports. Furthermore, the international and scientific visibility of the Agency in the HTA field is increased through joint work and publishing of scientific papers.
Two examples are two joint assessments on innovative drugs (canagliflozin for the treatment of diabetes mellitus type 2 and Ramucirumab [Cyramza®] in combination with paclitaxel as second-line treatment for adult patients with advanced gastric or gastro-esophageal junction adenocarcinoma) (http://www.aaz.hr/hr/procjena-zdravstvenih-tehnologija/baza), which have been completed at the European level before manufacturers requested reimbursement at the national level. Both reports were used to demonstrate the importance of HTA to the main decision makers in Croatia (MoH and CHIF) as well as showing what challenges are need to be overcome at the national level.
It can be expected that the HTA process will become sustainable in the near future and a mandatory part of the regular reimbursement, investment, or disinvestment processes and the implementation of health technologies in health care in Croatia, that all key stakeholder group will become actively engaged in the HTA process, and that Croatia will participate in sustainable production of objective, reliable, timely, transparent, and transferable HTA information among Member States within the permanent European HTA network. The ultimate goal is to make a positive difference to the patients and improve the quality of health care (Reference Guegan, Huić and Teljeur28).
CONCLUSIONS
HTA is not yet sustainable and mandatory in the reimbursement/investment or disinvestment decision process in Croatia. There are still barriers to overcome.
To implement it fully, the support and commitment of government institutions (political decisions) with a full legal framework in place is needed. Capacity building (educated permanent, full- or part-time staff), appropriate stakeholders involvement, further sustainable national and international cooperation and collaboration (network), and appropriate funding are of utmost importance as well.
CONFLICTS OF INTEREST
All authors report having no potential conflicts of interest.





