Psychopathy is a multifaceted clinical condition characterized by interpersonal/affective deficits (e.g. superficial charm, manipulative tendencies, lack of remorse or empathy) and antisocial deviance (e.g. impulsivity and aggression) (Cleckley, Reference Cleckley1941, 1976; Hare, Reference Hare2003). This devastating condition is related to a range of negative and dysfunctional outcomes including substance abuse, violence, criminal behavior, psychopathology (e.g. borderline personality disorder, internalizing disorders) (Douglas et al., Reference Douglas, Vincent, Edens and Patrick2006; Neumann and Hare, Reference Neumann and Hare2008; Hicks et al., Reference Hicks, Vaidyanathan and Patrick2010; Hemphälä and Hodgins, Reference Hemphälä and Hodgins2014; Hunt et al., Reference Hunt, Bornovalova and Patrick2015), and social maladjustment, including lower educational performance, unemployment, insufficient parenting, and poor social relationships (Ullrich et al., Reference Ullrich, Farrington and Coid2008; DeLisi et al., Reference Delisi, Vaughn, Beaver, Wexler, Barth and Fletcher2011; Beaver et al., Reference Beaver, Da Silva Costa, Poersch, Freddi, Stelmach, Connolly and Schwartz2014; Hunt et al., Reference Hunt, Bornovalova and Patrick2015). The recidivism rate in convicted criminals with psychopathy is higher than for other offenders (Hare, Reference Hare2003; Blair et al., Reference Blair, Mitchell and Blair2007; Neumann and Hare, Reference Neumann and Hare2008).
There are two historic conceptualizations of psychopathy that have influenced contemporary theory and research on this topic to the greatest extent. One is Cleckley's characterization of psychopathy as a ‘masked’ psychiatric illness in which qualities of intact cognitive function, social charm, and absence of nervousness or anxious-depressive symptoms conceal a severe pathology involving reckless unrestrained behavior and a lack of regard for the feelings and welfare of others. The other conceptualization, advanced by criminologically-oriented researchers (e.g. McCord and McCord, Reference Mccord and Mccord1964, Robins, Reference Robins1966), is of psychopathy as a predatory, aggressive form of criminal deviancy marked by emotional coldness, exploitativeness, and brutality toward others. Instruments for assessing psychopathy reflect these two conceptualizations to varying degrees. The best-established measure for use with adult offender samples, Hare (Reference Hare2003) interview-based Psychopathy Checklist-Revised, includes representation of some of Cleckley's mask features [i.e. through items pertaining to superficial charm and exaggerated self-confidence (grandiosity)] but not others (e.g. the absence of nervousness). The self-report measure that has been most widely used in contemporary research, the Psychopathic Personality Inventory (PPI; Lilienfeld and Andrews, Reference Lilienfeld and Andrews1996, Lilienfeld and Widows, Reference Lilienfeld and Widows2005), is designed for use in non-offender (community) samples and includes broader coverage of Cleckley's ‘mask’ features through subscales assessing social assertiveness and persuasiveness, emotional stability, and fearlessness. Other well-known psychopathy inventories, such as the informant-rated Antisocial Process Screening Device (APSD; Frick and Hare, Reference Frick and Hare2001) and the Self-Report Psychopathy Scale (Levenson et al., Reference Levenson, Kiehl and Fitzpatrick1995), emphasize the predatory-exploitative and reckless-unrestrained features of psychopathy, with limited coverage of the ‘mask’ features.
As a basis for reconciling alternative historic conceptualizations and integrating findings from studies using different assessment measures Patrick et al., (Reference Patrick, Fowles and Krueger2009) formulated the triarchic model of psychopathy. The model proposes that all historic characterizations of psychopathy, and different inventories that exist for assessing it, include representation of three dispositional attributes, termed disinhibition, boldness, and meanness. Although interrelated, these three dispositions have distinct symptomatic (phenotypic) expressions and are theorized to have differing neurobiological bases (Patrick et al., Reference Patrick, Fowles and Krueger2009; Patrick and Drislane, Reference Patrick and Drislane2015). Disinhibition involves deficient impulse control and weak self-regulation abilities, expressed in terms of difficulties in delaying gratification, tolerating frustration, and controlling negative emotions; boldness entails tendencies toward fearlessness, tolerance for novelty and risk, resilience to life stress, interpersonal dominance, and high self-confidence; and meanness encompasses tendencies toward callousness, shallow emotional attachment, exploitativeness, instrumental (predatory) aggression, deliberate cruelty, and excitement seeking through destructiveness (Patrick and Drislane, Reference Patrick and Drislane2015).
As evidence for the proposal that the triarchic dispositional constructs are represented to varying degrees in alternative conceptualizations and measures of psychopathy, a sizable body of research has shown that measures of the triarchic dispositions account for substantial portions of variance in different psychopathy inventories (e.g. Drislane et al., Reference Drislane, Patrick and Arsal2014, Sellbom et al., Reference Sellbom, Wygant and Drislane2015), making it possible to create effective scale measures of these dispositions using items from existing inventories that provide coverage of all three (e.g. Hall et al., Reference Hall, Drislane, Patrick, Morano, Lilienfeld and Poythress2014, Drislane et al., Reference Drislane, Brislin, Kendler, Andershed, Larsson and Patrick2015). The triarchic model constructs are also helpful in accounting for different variants (subtypes of psychopathy) that have been described in historic and contemporary literature. For example, ‘primary’ and ‘secondary’ variants of criminal psychopathy as described classically (Karpman, Reference Karpman1941) can be viewed as involving greater strength of mean/bold tendencies as compared with disinhibitory proclivities, respectively (Hicks and Drislane, Reference Hicks, Drislane and Patrick2018). Another typology, that of ‘successful’ v. ‘unsuccessful’ psychopathy, has been characterized in triarchic model terms as involving high levels of boldness v. disinhibition, respectively (Benning et al., Reference Benning, Venables, Hall and Patrick2018).
Of further note, the triarchic model dispositions are theorized to be associated with distinct developmental pathways and neurobiological processes (Patrick et al., Reference Patrick, Fowles and Krueger2009; Patrick and Drislane, Reference Patrick and Drislane2015). Risk factors such as poor emotion regulation, poor executive functions, lack of attachment, and harsh parenting are theorized to interact in complex ways leading to disinhibition, boldness, and meanness (Patrick et al., Reference Patrick, Fowles and Krueger2009). Disinhibition is hypothesized to reflect dysfunction in the prefrontal cortex and anterior cingulate cortex, brain structures that inhibit behaviors and regulate affect, as well as difficult temperament involving poor emotion regulation and weak executive control. Boldness is thought to reflect the phenotypic expression of genotypic fearlessness, associated with deficits in threat processing mediated by the amygdala. Meanness is thought to be based partly in fearlessness as well, but also in weak affiliative capacity (Patrick and Drislane, Reference Patrick and Drislane2015) arising from harsh, coercive parent–child interactions (Patrick et al., Reference Patrick, Fowles and Krueger2009).
As a specific means for assessing the dispositional domains of the triarchic model, Patrick (Reference Patrick2010; see also Drislane et al., Reference Drislane, Patrick and Arsal2014) developed a brief self-report inventory: the Triarchic Psychopathy Measure [TriPM; (Patrick et al., Reference Patrick, Fowles and Krueger2009)]. Unlike the Hare Psychopathy Checklist-Revised (PCL-R; Hare, Reference Hare2003), which was specifically designed to assess psychopathy in incarcerated individuals, the TriPM is designed to quantify dispositional subcomponents (facets) of psychopathy in the general population. A sizable body of literature supports the reliability and validity of the TriPM scales as indices of the three triarchic model constructs. They have good internal consistencies and high test-retest reliability, and show expected convergent and discriminant relations with other psychopathy measures, along with personality and clinical criterion measures, in both undergraduate and offender samples (Stanley et al., Reference Stanley, Wygant and Sellbom2013; Crego and Widiger, Reference Crego and Widiger2014; Drislane et al., Reference Drislane, Patrick and Arsal2014; Hall et al., Reference Hall, Drislane, Patrick, Morano, Lilienfeld and Poythress2014; Blagov et al., Reference Blagov, Patrick, Oost, Goodman and Pugh2015; Sellbom et al., Reference Sellbom, Wygant and Drislane2015).
A number of twin studies have examined the genetic and environmental etiology of psychopathic personality traits [for a recent review, see Waldman et al. (Reference Waldman, Rhee, Loparo, Park and Patrick2018)], in many cases using established psychopathy inventories such as the PPI (e.g., Blonigen et al., Reference Blonigen, Carlson, Krueger and Patrick2003), the ASPD (Ficks et al., Reference Ficks, Dong and Waldman2014), and the Youth Psychopathic Traits Inventory [YPI; e.g., (Larsson et al., Reference Larsson, Andershed and Lichtenstein2006; Larsson et al., Reference Larsson, Tuvblad, Rijsdijk, Andershed, Grann and Lichtenstein2007)] – but in some cases using study-specific psychopathy measures (e.g., Taylor et al., Reference Taylor, Loney, Bobadilla, Iacono and Mcgue2003; Viding et al., Reference Viding, Blair, Moffitt and Plomin2005).
These studies have found that heritable factors have a moderate to high influence on the phenotypic variance in psychopathic personality traits, whereas non-shared environmental factors have a small to moderate influence, and shared environmental factors have negligible influence. Another common finding is an absence of sex differences in the magnitude of the genetic and environmental variance components. Thus, the underlying genetic and environmental etiologies of psychopathic personality traits appear to be similar for both sexes despite the prevalence of psychopathic personality traits being higher among males than females (for reviews, see Tuvblad, Reference Tuvblad, Delisi and Vaughn2014, Tuvblad et al., Reference Tuvblad, Fanti, Andershed, Collins and Larsson2017, Waldman et al., Reference Waldman, Rhee, Loparo, Park and Patrick2018). This pattern of findings has been reported across the lifespan, among children (e.g., Viding et al., Reference Viding, Blair, Moffitt and Plomin2005, Reference Viding, Frick and Plomin2007; Fontaine et al., Reference Fontaine, Rijsdijk, Mccrory and Viding2010; Bezdjian et al., Reference Bezdjian, Tuvblad, Raine and Baker2011; Ficks et al., Reference Ficks, Dong and Waldman2014) and adolescents (e.g., Blonigen et al., Reference Blonigen, Hicks, Krueger, Patrick and Iacono2005; Larsson et al., Reference Larsson, Tuvblad, Rijsdijk, Andershed, Grann and Lichtenstein2007; Forsman et al., Reference Forsman, Lichtenstein, Andershed and Larsson2008; Tuvblad et al., Reference Tuvblad, Bezdjian, Raine and Baker2014, Reference Tuvblad, Wang, Bezdjian, Raine and Baker2015), as well as among adults (e.g., Blonigen et al., Reference Blonigen, Hicks, Krueger, Patrick and Iacono2006; Brook et al., Reference Brook, Panizzon, Kosson, Sullivan, Lyons, Franz, Eisen and Kremen2010; Beaver et al., Reference Beaver, Vaughn and Delisi2013; Hunt et al., Reference Hunt, Bornovalova and Patrick2015).
Further, a recent study used genealogical data from a sample of 178 socially housed chimpanzees to evaluate genetic and environmental contributions to the triarchic psychopathy dimensions. The three triarchic dimensions were assessed in this study using scales composed of construct relevant items from a caretaker-rating inventory of personality traits (Latzman et al., Reference Latzman, Patrick, Freeman, Schapiro and Hopkins2017). In the combined mother- and nursery-reared sample, heritability estimates were significant for both Boldness (h 2 = 0.43) and Meanness scales (h 2 = 0.32), with the heritability estimate for Disinhibition lower and non-significant (h 2 = 0.13). Heritabilities in the mother-reared subsample (n = 119) for Boldness, Meanness, and Disinhibition scales were 0.66, 0.65, and 0.36, respectively. The contribution of the shared environment was non-significant (Latzman et al., Reference Latzman, Patrick, Freeman, Schapiro and Hopkins2017). However, no study has been conducted yet with human participants to clarify the role of genetic and environmental etiologic influences in the triarchic model dispositions as indexed by the Disinhibition, Boldness, and Meanness scales of the TriPM in humans.
The present study used data from a large sample of young adult twins for the purposes of addressing the following research questions:
(1) What are the comparative contributions of genetic and environmental influences to Disinhibition, Meanness, and Boldness?
(2) Do the genetic and environmental etiologies of these three dispositions differ between males and females?
(3) To what degree do the three triarchic dispositions overlap in terms of their genetic and environmental etiologies?
Method
Participants and procedures
The current sample was drawn from participants tested in the University of Southern California Risk Factors for Antisocial Behavior (RFAB) twin study. RFAB is a prospective longitudinal study of the interplay of genetic, environmental, social, and biological factors on the development of antisocial and aggressive behaviors from childhood to emerging adulthood. Participating families were recruited from the Los Angeles urban community and the sample is representative of the ethnic and socio-economic diversity of the greater Los Angeles area (Baker et al., Reference Baker, Tuvblad, Wang, Gomez, Bezdjian, Niv and Raine2013). The TriPM was only administered during the fifth (and most recent) wave of data collection, during which n = 1 016 19–20-year-old twins (mean age = 19.86, s.d. = 1.34) provided data via an online survey. The racial composition of the sample was 33% Caucasian, 33% Hispanic, 12% African-American, 4% Asian, and 18% mixed. The twin-pair composition consisted of 20% monozygotic male, 22% monozygotic female, 14% dizygotic male, 18% dizygotic female, and 25% dizygotic opposite-sex [see Baker et al., (Reference Baker, Tuvblad, Wang, Gomez, Bezdjian, Niv and Raine2013) for full details on data collection and testing protocol for all five waves of the RFAB study].
Measures
Psychopathic personality domains
The TriPM (Patrick et al., Reference Patrick, Fowles and Krueger2009; Patrick, Reference Patrick2010), http://www.phenxtoolkit.org] is a 58-item self-report questionnaire specifically developed to assess the constructs of disinhibition, boldness, and meanness. Items from the Disinhibition (20 items), and Meanness (19 items) scales are taken from the Externalizing Spectrum Inventory (ESI; Krueger et al., Reference Krueger, Markon, Patrick, Benning and Kramer2007); specifically, they comprise sets of items selected to index the general disinhibitory and callous-aggression factors, respectively, of the ESI (see Patrick et al., Reference Patrick, Kramer, Krueger and Markon2013). The third TriPM scale, Boldness, consists of 19 items from an inventory (Patrick et al., Reference Patrick, Kramer, Vaidyanathan, Benning, Hicks and Lilienfeld2017) developed to formalize a measurement model of the ‘fearless dominance’ construct represented in the PPI (Lilienfeld and Widows, Reference Lilienfeld, Widows and Cutler2008).
The TriPM items are answered using a four-point Likert scale, and scored from 0 to 3 (where 0 = False, 1 = Somewhat False, 2 = Somewhat True, 3 = True;) scores for each scale were computed in the current study as the mean of constituent items. Because two of the three scales were showed appreciable positive skewness (Disinhibition = 1.20, Meanness = 1.20), scores on these scales–along with the Boldness scale, which showed modest negative skewness (−0.28), were ranked and normalized using the statistical software SAS (Blom, Reference Blom1958) to approximate a normal distribution. After transformation, skewness values indicated that the distributions for the three scales became closer to symmetric (Disinhibition = 0.02, Meanness = 0.04, Boldness = 0.00). The Kolmogorov–Smirnov Test for normality indicated that transformed Boldness (K-s.d. = 0.02, p > 0.15) and Disinhibition (K-s.d. = 0.03, p = 0.06) followed a normal distribution, with Meanness only mildly skewed (K-s.d. = 0.03, p < 0.05).
Statistical analyses
Twin design and twin correlations
The classical twin design estimates the relative contribution of genetic and environmental influences to the attribute of interest based on the different levels of phenotypic and genetic similarity between monozygotic (MZ; identical) and dizygotic (DZ; fraternal) twins. The variance of a measured trait can be parsed into subcomponents reflecting additive genetic influences (A; also referred to as narrow-sense heritability), dominant genetic influences (D) or shared environmental influences (C), and non-shared environmental influences (E). Genetic effects can be defined in terms of an additive genetic value (i.e. alleles at a single locus add up to affect a trait) and a dominant deviation [the deviation from purely additive effects, non-additive genetic influences which represent interactions between alleles at the same locus (dominance, D) or on different loci (epistasis)]. Shared environmental factors refer to non-genetic influences that contribute to similarity within pairs of twins. Because dominant genetic and shared environmental influences are negatively confounded, these two factors cannot be estimated simultaneously in a sample of MZ and DZ twins reared together. Data for twins reared together did not contain sufficient information to permit evaluation of the contrasting effects of dominant genetic and shared environmental influences. To estimate dominant genetic and shared environmental influences simultaneously would, for example, require an adoptive sibling design (Rijsdijk and Sham, Reference Rijsdijk and Sham2002). Finally, non-shared environmental factors include experiences that make siblings dissimilar from one another, along with statistical error (Neale and Cardon, Reference Neale and Cardon1992).
Twin correlations (degree of similarity of twins their co-twins) can provide an initial indication of the underlying sources of variance in Disinhibition, Boldness, and Meanness. For example, twin correlations for MZ pairs that are higher than twin correlations for DZ pairs indicate the likely presence of genetic effects. Conversely, MZ twin correlations that are more comparable with DZ twin correlations indicate the likely presence of both genetic and shared environmental effects. If the MZ twin correlation is more than twice the DZ twin correlation, this could indicate the presence of dominant genetic effects (Neale and Cardon, Reference Neale and Cardon1992).
Univariate twin modeling
To estimate the relative contributions of additive genetic (A), shared environmental (C) or dominant genetic (D), and non-shared environmental influences (E, plus error) to each TriPM scale, univariate models were fit separately for Disinhibition, Boldness, and Meanness scores. To assess whether the magnitude of genetic effects differ between males and females (i.e. whether quantitative sex differences are present), only data from same-sexed twin pairs are required. However, to determine whether or not the same set of genetic influences contributes to a trait in males and females (i.e. whether qualitative differences are present), data from opposite-sexed twin pairs are also needed. If different genetic influences contribute, then the DZ opposite-sex twins will be less genetically similar for the trait than DZ same-sex twins, and their genetic correlation will be less than 0.5. This can be tested by allowing the genetic correlation for opposite-sex twin pairs (r Gmf) to be freely estimated in the model, rather than being fixed to 0.5.
For each domain, we started with a full model to evaluate whether additive genetic influences (A) or non-additive genetic effects (D) were important (Models 1a, 1b). Then we tested whether the means (Model 1c) and variances (Model 1d) of the phenotypic scores could be equated across males and females. Subsequent models (Models 2a, 2b, 2c and 2d) tested for sex differences (including genetic correlations and variance components A, C/D or E) sequentially by: (1) fixing r Gmf to be 0.5; (2) equating the contribution of A; (3) equating the contribution of C; and (4) equating the contribution of E. Finally, if any significant decrease in model fit was evident for the full sex-limitation model (Model 2d), we turned to the scalar sex-limitation model (Model 3a), which is less restrictive than the full sex-limitation model (Model 2d) in that it allows for sex-differing variances at the phenotypic level but equal means and proportion of each variance component (A, D/C or E) across males and females.
Bivariate twin modeling
Should there be any significant correlations among Disinhibition, Boldness, and Meanness, a bivariate genetic analysis with a correlated factors solution would be employed to investigate the genetic and environmental architecture of the phenotypic correlations. This correlated factors solution decomposes the variance of each phenotype as well as the co-variances between two measures into genetic, shared environmental, and non-shared environmental factors. It is also possible to calculate the proportion of each phenotypic correlation that is due to genetic factors (i.e. bivariate heritability), shared environmental factors (i.e. bivariate shared environment), and non-shared environmental factors (i.e. bivariate non-shared environment). These estimates are proportions that range from 0.0 to 1.0, and illustrate the extent to which a phenotypic correlation between two traits is mediated by genetic and/or environmental factors.
The proportion of variance explained by additive genetic factors is denoted by a 2, the proportion of variance explained by shared environmental factors is denoted by c 2, and the proportion of variance explained by non-shared environmental factors is denoted e 2. r g is the correlation between additive genetic factors of two distinct phenotypes, r c is the correlation between shared environmental factors of two distinct phenotypes, and r e is the correlation between non-shared environmental factors of two distinct phenotypes. The bivariate heritability is calculated as [✓(a 2 variable 1) × r g × ✓(a 2 variable 2)]/r variable 1, variable 2; bivariate shared environment is calculated as [✓(c 2 variable 1) × r c × ✓ (c2 variable 2)]/r variable 1, variable 2; and the bivariate non-shared environment is calculated as [✓(e 2 variable 1) × r e × ✓(e 2 variable 2)]/r variable 1, variable 2.
Adequacy of model fit was evaluated using the Comparative Fit Index [CFI; Bentler, Reference Bentler1990); 0.0 = worst fit, 1.0 = best fit)], the Root Mean Squared Error of Approximation index (RMSEA; McDonald, Reference Mcdonald1989) 0.05–0.10 = fair fit, e.g. (Kline, Reference Kline1998; Hu and Bentler, Reference Hu and Bentler1999; Hooper et al., Reference Hooper, Coughlan and Mullen2008), the Bayesian Information Criterion (BIC), and the Akaike information criterion (AIC; Akaike, Reference Akaike1987); for both the AIC and BIC, smaller values indicating better fit (Raftery, Reference Raftery1995). The goodness of fit was also compared with the difference in the chi-square statistic (χ2). Model fit was assessed using the χ2 difference statistic when models were nested. Otherwise, BIC would be the primary index to compare. All models were fit to the data using version 5.2 of the Mplus statistical software package (Muthén and Muthén, Reference Muthén and Muthén1998–2007).
Results
Reliability, descriptive statistics, and phenotypic and twin correlations
In the present study, Cronbach's Alpha (α) values were α = 0.84 for TriPM Disinhibition; α = 0.84 for Meanness; and α = 0.81 for Boldness. For all three scales, male twins had significantly higher scores than female twins, Table 1. As shown in Table 1, the patterns of twin correlations (higher among MZ twins than DZ twins) indicate that genetic influences are likely important for all three domains; there also appear to be sex differences in the genetic and environmental variance components, given that same-sex DZ twins are more similar to one another than DZ opposite-sex twins. Further, higher MZ than DZ cross-twin cross-trait correlation indicates a genetic overlap across different domains, Table A1.
Table 1. Descriptive statistics and twin correlations for Disinhibition, Boldness and Meanness at ages 19–20 years

MZ, monozygotic; DZ, dizygotic.
a Raw data.
b Transformed data.
*p < 0.05, independent sample t tests, twin correlations were based on transformed data.
The patterns of the phenotypic correlations across the three domains differed slightly based on sex. Overall, TriPM Meanness and Disinhibition were moderately positively correlated in both males and females. Boldness and Disinhibition were modestly negatively correlated in both sexes, but this negative relationship was only significant for male twins. Finally, Meanness and Boldness were correlated at a small, significant level in the sample as a whole, though their association was nonsignificant within each gender subgroup (see Table 2 for full results).
Table 2. Phenotypic correlations for Disinhibition, Boldness and Meanness at ages 19–20 years

Note. Males are presented above the diagonal and females are presented below the diagonal.
*p < 0.05, correlations are based on transformed data.
Univariate genetic analyses
Model-fitting results for each of the three TriPM psychopathy domains are displayed in Table 3. For each domain, constraining the value of R Gmf to 0.5 in opposite-sex twin pairs (as compared with freely estimating its value) did not produce a significant decrease in model fit, indicating no qualitative sex differences in variance components.
Table 3. Univariate genetic results and parameter estimates for Disinhibition, Boldness and Meanness at ages 19–20 years.

AIC, Akaike information criterion; BIC, Bayesian information criterion; RMSEA, root mean square error of approximation; CFI, Comparative Fit Index; CI, confidence interval Models were ordered to reflect increasing parsimony.
Model fittings were assessed through χ2 difference and its significance values when models were nested. Otherwise, BIC would be the primary index to compare. Scalar sex limitation model was conducted for disinhibition only as the E components cannot be equated across different sexes. Best-fitting model for each factor was marked in bold. A2, C2, D2, and E2 estimates represent additive genetic, shared environmental, non-additive genetic, and non-shared environmental variance components, respectively. r Gmf: genetic correlation for opposite-sex twin pairs. The insignificant estimates were italicized and underscored.
For TriPM Disinhibition, a full ACE model demonstrated a better fit than an ADE model. Subsequent sex-equating models (i.e. models 2a–2d) and scalar sex-limitation models (i.e. 3a, as described above) indicated that although the phenotypic means and relative contributions of each variance component could be equated across males and females, the phenotypic variances differed significantly across sexes. According to the best-fitting model [χ2 (20) = 19.48, p = 0.49], genetic factors accounted for 49% (95% confidence interval (CI) 25–89%) of the total variance, shared environmental factors accounted for 5% (95% CI 0–72%), and non-shared environmental factors accounted for the remaining 46% (95% CI 38–55%) of the variance.
For TriPM Boldness, model comparisons suggested that males and females exhibited no significant differences in either phenotypic variances or in any of the etiologic variance components – though, as noted above, mean values were significantly different between males and females. A full sex-limitation model indicated that an ADE model provided the best fit (Model 2d). According to this best-fitting model (χ2 (20) = 20.83, p = 0.41), 30% (95% CI 1–65%) of the variance was due to additive genetic factors and 19% was due to dominant genetic influences (95% CI 0–56%), with the remaining 51% (95% CI 43–63%) attributable to non-shared environmental factors.
For TriPM Meanness, an ACE model fits the data better than an ADE model. Similar to Boldness, males and females could be equated on the phenotypic variances and each etiologic variance component [though differing in phenotypic means, χ2 (20) = 29.15, p = 0.08]. Genetic influences accounted for 24% (95% CI 4–63%) of the phenotypic variance in Meanness scores, shared environmental influences accounted for 18% (95% CI 2–50%), and non-shared environmental factors accounted for the remaining 58% (95% CI 48–69%) of the variance.
Bivariate genetic analysis
Next, a bivariate genetic model with correlated factors was fit to Meanness and Disinhibition, the two TriPM scales that were significantly related to each other in both males and females. Table 4 summarizes the model fitting results, in descending order of model complexity. Consistent with the univariate genetic results, the best-fitting bivariate model also supported scalar sex differences in variance for the Disinhibition scale, as well as significant sex differences in average phenotypic scores for the Meanness scale. Males and females exhibited equivalence of etiologic variance components (A, C, and E) for both Meanness and Disinhibition.
Table 4. Bivariate genetic results and parameter estimates for Meanness and Disinhibition

AIC, Akaike information criterion; BIC, Bayesian information criterion; RMSEA, root mean square error of approximation; CFI, Comparative Fit Index.
Models were ordered to reflect increasing parsimony. Model fittings were assessed through χ2 difference and its significance values when models were nested. Otherwise, BIC would be the primary index to compare. Best-fitting model for each factor was marked in bold. A2, C2, D2, and E2 estimates represent additive genetic, shared environmental, non-additive genetic, and non-shared environmental variance components, respectively.
Figure 1 presents this bivariate genetic model with a correlated factor solution for these two scales, i.e. a joint biometric model incorporating both Disinhibition scores and Meanness scores, along with parameter estimates and 95% CIs. Based on the formulas detailed in the Methods section, genetic overlap explained 26% of the total covariance between Meanness and Disinhibition (r g = 0.53, 95% CI 0.06–1) and shared environmental overlap explained 39% (r c = 1, 95% CI −1–1), with the remaining 35% attributable to non-shared environmental overlap (r e = 0.34, 95% CI 0.20–0.44).
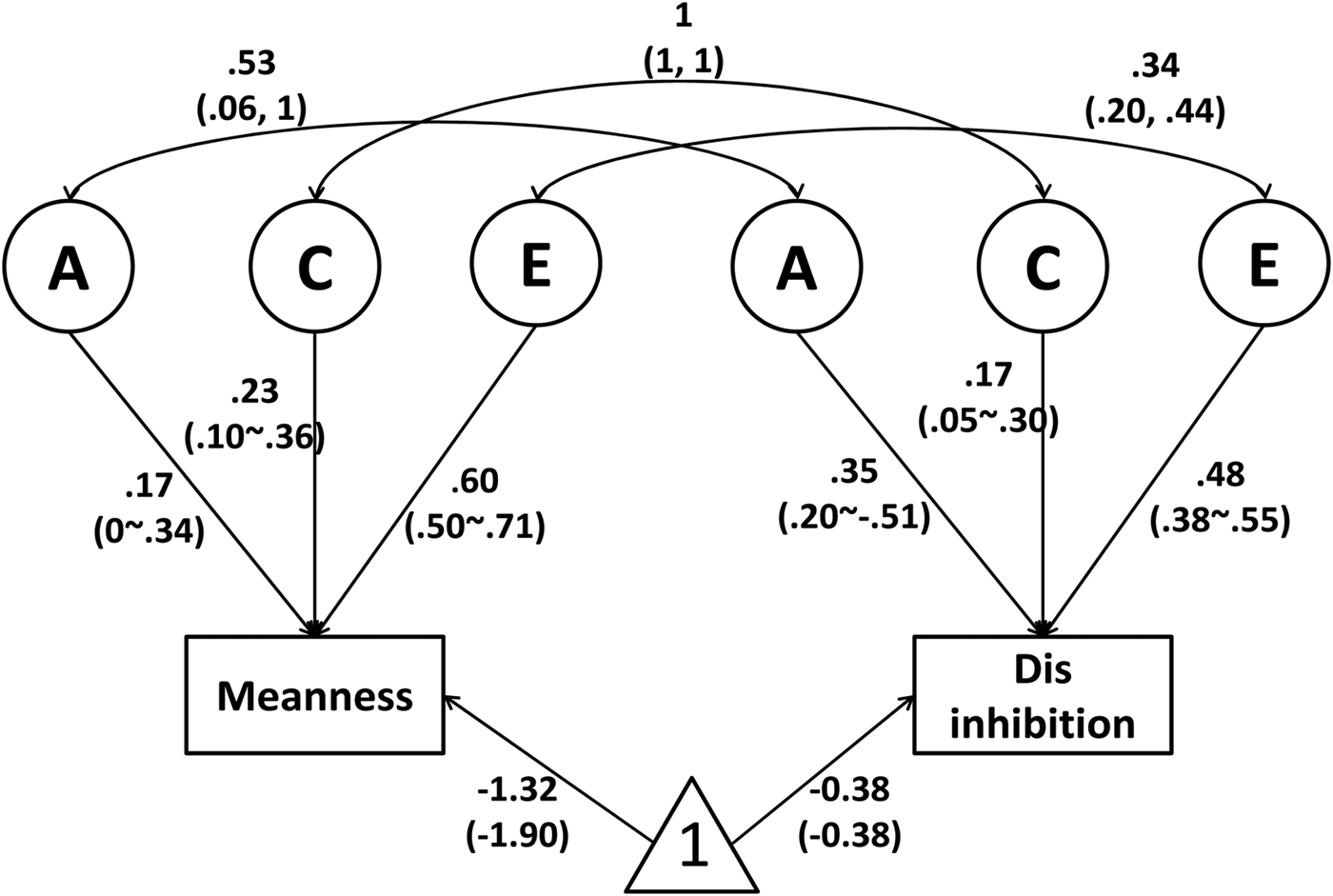
Fig. 1. Correlated factor solution, i.e. a joint biometric model incorporating both Disinhibition scores and Meanness scores, with 95% CIs. The number 1 in the triangle indicates a constant, and the loadings to each observed variable denotes the intercept/mean value of that variable, male and (female). A = additive genetic variance, C = shared environmental variance, E = non-shared environmental variance.
Discussion
This study utilized data from a mixed-sex twin sample to examine the contributions of genetic and environmental influences to dispositional facets of psychopathy represented in the triarchic model, as assessed by subscales of the TriPM. In line with other studies (Stanley et al., Reference Stanley, Wygant and Sellbom2013; Crego and Widiger, Reference Crego and Widiger2014; Drislane et al., Reference Drislane, Patrick and Arsal2014; Hall et al., Reference Hall, Drislane, Patrick, Morano, Lilienfeld and Poythress2014; Patrick and Drislane, Reference Patrick and Drislane2015), we found that the three subscales of the TriPM demonstrated good internal consistencies and overlapped to varying degrees with one another. Within the sample as a whole, Disinhibition and Meanness correlated to a moderate positive degree, whereas Boldness and Meanness showed a modest positive association and Boldness and Disinhibition showed a slight negative correlation. Additionally, for each TriPM scale, we found the mean score to be higher for males than females, indicating that psychopathic proclivities of each type are somewhat more prevalent among males than females. Of note, the prevalence of psychopathy has also been found to be higher among males than females across incarcerated and community samples (Nicholls et al., Reference Nicholls, Ogloff, Brink and Spidel2005; Verona and Vitale, Reference Verona, Vitale and Patrick2006).
For TriPM Disinhibition and Boldness, genetic and environmental variance components could be constrained to be equal, indicating that genetic and environmental influences contributed to these psychopathy facets to the same extent in men and women. In participants as a whole, genetic and non-shared environmental influences, but not shared environment, contributed to observed variance in these TriPM scales. Similarly, for Meanness, there were no sex differences in the etiologic components of variance, but for this psychopathy facet, shared environmental influences contributed along with genetic and non-shared environmental influences to the observed phenotypic variance. Though our analyses (as noted above) did reveal higher mean scores for each of the three TriPM scales in males than in females, no differences in the magnitude of genetic and environmental variance components were found across male and female participants. This finding is consistent with the bulk of results from other studies that have investigated sex differences in the genetic and environmental etiology of psychopathic personality (Waldman and Rhee, Reference Waldman, Rhee and Patrick2006; Tuvblad, Reference Tuvblad, Delisi and Vaughn2014; Tuvblad et al., Reference Tuvblad, Fanti, Andershed, Collins and Larsson2017). Despite the consistent sex difference in mean levels of psychopathic personality, however, the underlying etiologies of psychopathic personality appear to be similar for both sexes. There may still be biological and social differences between males and females that could explain the greater mean levels of the psychopathic personality seen in males. Further systematic research is needed to evaluate this possibility and determine whether distinct circumstances or experiences contribute to the heightened expression of psychopathic personality features in males.
Vis-a-vis other symptom-oriented measures of psychopathy (e.g. PCL-R, APSD, YPI), the TriPM Disinhibition construct taps into the antisocial deviance features of psychopathy, whereas the TriPM Boldness taps into the interpersonal features. Thus, our findings for Disinhibition and Boldness are well in line with the majority of prior research showing that genetic and non-shared environmental influences account primarily for the variance in psychopathic personality traits (Waldman and Rhee, Reference Waldman, Rhee and Patrick2006; Tuvblad, Reference Tuvblad, Delisi and Vaughn2014; Tuvblad et al., Reference Tuvblad, Fanti, Andershed, Collins and Larsson2017), as well as for variance in related phenotypes of antisocial behavior and externalizing problems generally (Krueger et al., Reference Krueger, Hicks, Patrick, Carlson, Iacono and Mcgue2002; Rhee and Waldman, Reference Rhee and Waldman2002; Tackett et al., Reference Tackett, Lahey, Van Hulle, Waldman, Krueger and Rathouz2013). By contrast, our finding of a contribution of the shared environment along with genetic influences to Meanness indicates that this disposition arises from heritable determinants together with environmental factors common to siblings within a family, whereas Disinhibition and Boldness appear to arise from heritable factors without the involvement of shared family environment.
Our etiological results for Meanness contrast with those of some prior twin studies examining the genetic and environmental etiology of psychopathic personality traits (Waldman and Rhee, Reference Waldman, Rhee and Patrick2006; Tuvblad, Reference Tuvblad, Delisi and Vaughn2014), but are consistent with certain others. Vis-a-vis other measures of psychopathy, the TriPM Meanness scale taps into the affective features of psychopathy. Two other studies that have reported a contribution of the shared environment to this facet of psychopathy in younger-aged samples are ones conducted by Fontaine et al., (Reference Fontaine, Rijsdijk, Mccrory and Viding2010) and Viding et al., (Reference Viding, Frick and Plomin2007). Another study by Ficks, Dong, and Waldman (Reference Ficks, Dong and Waldman2014) that used data for a sample of 885 twin pairs (age range: 4.4–17.8 years), in which the APSD (Frick and Hare, Reference Frick and Hare2001) was used to assess psychopathic personality symptoms, found contributions of shared environmental influences (along with genes and non-shared environment) to scores on two of three ASPD symptom dimensions – i.e. Callous-Unemotionality and Impulsivity/Conduct Problems (19% of variance in each case), but not Narcissism (for which contributions were evident only for genes and non-shared environment). A further study by Tuvblad et al. (Reference Tuvblad, Fanti, Andershed, Collins and Larsson2017) used data for a sample of 1189 5-year-old twins from the Preschool Twins in Sweden Study in which psychopathic personality was rated by teachers, and found contributions of both genetic and shared environmental factors to psychopathic personality traits. Specifically, genetic influences accounted for 57, 25, and 74% of the variance, respectively, in arrogant–deceitful interpersonal traits, callous–unemotional affective traits, and impulsive–irresponsible behavioral traits, while shared environment accounted for 17, 48, and 9% of the variance in these three trait domains.
Examples of shared environmental factors that may contribute to meanness (callous-unemotionality) and perhaps other psychopathy facets include family-related factors (e.g. neglect, prenatal stressors) and contextual factors in the surrounding community (Murray and Farrington, Reference Murray and Farrington2010; Tuvblad et al., Reference Tuvblad, Bezdjian, Raine and Baker2013). There is evidence that such factors exert stronger influences at younger ages, with a pattern of decreasing shared environment and a concomitant increase in heritability over the course of development typically found; this has been reported for several phenotypes including personality traits, cognitive abilities, and aggression (Plomin et al., Reference Plomin, Defries, Mcclearn and Mcguffin2008). These findings suggest reasons for inconsistencies in results across different studies. Participant age may be one factor accounting for variable results across different studies; another may be sample size – i.e. smaller-N twin studies may lack the power needed to detect shared environmental factors (Plomin et al., Reference Plomin, Defries, Mcclearn and Mcguffin2008). These findings, for meanness and the other psychopathy facets (disinhibition, boldness) also have interesting clinical implications. One is that earlier interventions that target family dynamics along with behavior patterns of children will be needed to curb longer-term antisocial outcomes. Another is that psychopathic proclivities of all three types are likely to increase in strength across time if left untreated.
A further finding of the current study was that the phenotypic correlation between TriPM Disinhibition and Meanness was explained by common genetic and non-shared environmental factors. Boldness and Meanness have been posited to include some common underlying etiologic element of low genotypic fear, which can be expressed in different phenotypic ways (Patrick et al., Reference Patrick, Fowles and Krueger2009). Meanness is theorized to involve a more malignant, maladaptive expression of fearlessness that arises when normal attachment and socialization processes are blocked or impeded (Kochanska, Reference Kochanska1997; Hall et al., Reference Hall, Drislane, Patrick, Morano, Lilienfeld and Poythress2014). Of note, however, Boldness and Meanness were only modestly correlated in the current sample, suggesting that any etiological factors in common between the two were likely quite small.
Limitations
There are some limitations that should be borne in mind when considering these results. First of all, our data were collected from a community sample of young adult twins; thus, the findings we report may not generalize to incarcerated samples. There is also some concern about how accurately individuals with psychopathic personality traits can report on their own behavioral proclivities (Lilienfeld and Fowler, Reference Lilienfeld, Fowler and Patrick2006). Our reliance on self-report assessment of psychopathic symptomatology in the current study (i.e. using the TriPM) may, therefore, constitute an additional limitation. There are also certain assumptions related to the twin research design itself (Plomin et al., Reference Plomin, Defries, Mcclearn and Mcguffin2008) that may not hold in particular samples; for example, twin-based heritability estimates are time and population specific. Also, the classical ACE twin model assumes that genes and environment do not interact and are uncorrelated (for more detailed discussions, see: Rijsdijk and Sham, Reference Rijsdijk and Sham2002, Plomin et al., Reference Plomin, Defries, Mcclearn and Mcguffin2008, Tuvblad and Baker, Reference Tuvblad, Baker, Huber, Brennan and Bannasch2011). Finally, the a path for Meanness was non-significant, yet the genetic correlation between Meanness and Disinhibition was significant in the bivariate genetic model (Fig. 1). This suggests that the genetic correlation should be viewed with caution. However, the additive genetic factor (heritability) was significant for Meanness in the univariate model (Table 3). It is possible that lack of power accounts for the non-significant a path despite the genetic correlation being significant, given that the sample size remained the same but the number of parameters in the bivariate model was twice that of the univariate model.
Conclusions
The present study investigated the genetic and environmental underpinnings of psychopathic personality dimensions measured by the TriPM, and tested for sex differences in these underpinnings. Men exhibited greater mean levels on all three subscales of the TriPM compared with women. In the genetic analyses, no sex differences were found in etiologic components of variance for Disinhibition, Boldness, or Meanness scales, suggesting that genetic and environmental effects influence these traits in a similar manner in young men and women. The results for the TriPM Disinhibition, Boldness, and Meanness scales both affirm and complement findings from other twin studies that have used symptom-oriented measures of psychopathy. Future research should examine the underlying physiological and neurobiological mechanisms of these distinct psychopathy dimensions to further clarify how and why they manifest to varying degrees in the general population.
Acknowledgements
This study was funded by NIMH (R01 MH58354). Adrian Raine was supported by NIMH (Independent Scientist Award K02 MH01114-08). We thank the Southern California Twin Project staff for their assistance in collecting data, and the twins and their families for their participation.
APPENDIX
Table A1. Intra-twin and inter-twin correlations between Meanness and Disinhibition across zygosity groups









