The River Clyde catchment drains an area of approximately 3100km2 stretching from the Lowther Hills in the Scottish Borders to the Clyde estuary on the west coast of Scotland (Fig. 1). The catchment is predominantly rural, comprising moorland hill terrain in its upper headwaters and agricultural land in the middle reaches. In contrast, the lower Clyde is urbanised with one of the UK's foremost historical industrial centres, the city of Glasgow situated close to the tidal limit. Glasgow grew rapidly from a small ecclesiastical centre in the 1600s to become a major industrial powerhouse by the early 20th Century. Much of this development resulted from a combination of excellent transport/shipping links centred on the River Clyde and coal and iron mineral resources located in close proximity, which fuelled engineering and metal-processing activities during the Industrial Revolution. During the latter half of the 20th Century, heavy industry and coal-fired power generation declined (Browne et al. Reference Browne, Forsyth and McMillan1986). Over more recent decades, care has been taken to minimise chemical and metal pollution from industries along the Clyde corridor. However, several studies focussed on the Clyde estuary have observed persistent organic pollutants (POPs) in sediments and ascribed these to sources such as, but not limited to, landfill sites, legal sewage discharge and shipping and road run-off (Vane et al. Reference Vane, Ma, Chen and Mai2010). Additionally, persistent organic pollution from past industrial activity that is retained in surface and near-surface sediments from former industries such as power stations and oil refineries, which were essential for the development of Glasgow and were located in close proximity to the River Clyde, can still be a long-term environmental threat due, in part, to sediment remobilisation, reworking and chemical recalcitrance (Hursthouse et al. Reference Hursthouse, Adamczyk, Adamczyk, Smith and Iqbal1994; Edgar et al. Reference Edgar, Hursthouse, Matthews, Davies and Hillier2006; Vane et al. Reference Vane, Harrison and Kim2007a, Reference Vane, Ma, Chen and Mai2010, Reference Vane, Chenery, Harrison, Kim, Moss-Hayes and Jones2011).
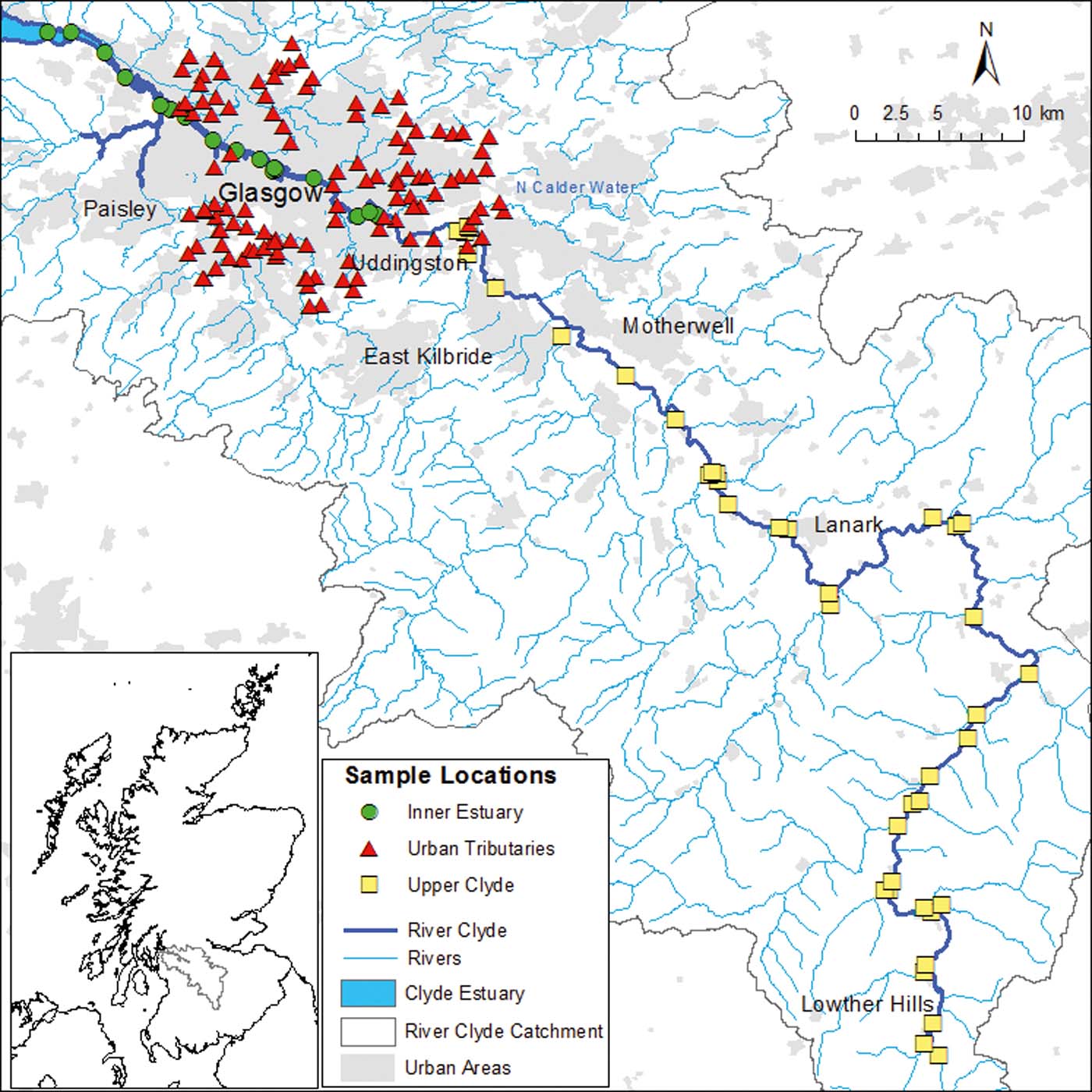
Figure 1 Location of the River Clyde sediment samples from the upper Clyde (yellow squares), urban tributaries (red triangles) and inner estuary (green circles). Geoscience data, BGS © NERC. Contains Ordnance Survey data © Crown Copyright and database rights 2016.
POPs such as polyaromatic hydrocarbons (PAH), total petroleum hydrocarbons (TPH) and polychlorinated biphenyls (PCB) are associated with a variety of industrial activities such as coal burning, gas works, shipping and power generation as well as incineration, demolition and made-ground including road-surface dusts (Yang et al. Reference Yang, Connell, Hawker and Kayal1991; Vane et al. Reference Vane, Kim, Beriro, Cave, Knights, Moss-Hayes and Nathanail2014).
The main PAH in soils and near-surface sediments may be ascribed to either petrogenic sources (from petroleum source) or from pyrolytic origin (from incomplete combustion of carbon-containing materials as wood, coal and petroleum), or a combination of the two. Previous studies have measured PAH concentration, predominately in Clyde estuary sediments, and concluded that they are useful indicators of current and former industrial activity in this system (Hursthouse et al. Reference Hursthouse, Adamczyk, Adamczyk, Smith and Iqbal1994; Rogers Reference Rogers2002; Vane et al. Reference Vane, Harrison and Kim2007a, Reference Vane, Chenery, Harrison, Kim, Moss-Hayes and Jones2011). Vane et al. (Reference Vane, Harrison and Kim2007a) found that the PAH concentrations of 11 samples from the inner Clyde estuary were comparable to other European estuaries whose environments are highly industrialised. Based on isomeric ratios of the different PAH, they estimated that the majority of the PAH had a pyrolytic source. This was in agreement with results reported by other studies from the Clyde estuary (Rogers Reference Rogers2002) and two Clyde tributaries (Wilson et al. Reference Wilson, Clarke, D'Arcy, Heal and Wright2005). By contrast, analysis of sediment cores from sites in the outer estuary revealed that most of the PAH had a petroleum and petroleum combustion origin (Vane et al. Reference Vane, Chenery, Harrison, Kim, Moss-Hayes and Jones2011). This was explained by the closer proximity of inner estuary samples to former domestic and industrial coal-burning sources, while the samples located further out in the estuary were more influenced by unburnt fuel emissions from shipping and traffic together with petroleum product spills and historic discharges from local industries (Vane et al. Reference Vane, Harrison and Kim2007a, Reference Vane, Chenery, Harrison, Kim, Moss-Hayes and Jones2011).
TPH are a ubiquitous compound class observed in low concentrations in pristine riverine/estuarine sediments and at much higher concentrations in urbanised areas as a consequence of industrial discharges, spills and shipping activities (Guo et al. Reference Guo, He, Yang, Lin and Quan2011; Vane et al. Reference Vane, Chenery, Harrison, Kim, Moss-Hayes and Jones2011). When analysed together, TPH and PAH concentrations facilitate the identification of changes in the use of different fuel sources (e.g., coal versus oil). Analysis of sediment cores from the River Clyde estuary revealed that the peak of TPH concentrations occurred at shallower depths than the total PAH, reflecting the earlier use of coal and later use of oil as a fuel source (Vane et al. Reference Vane, Chenery, Harrison, Kim, Moss-Hayes and Jones2011). Low TPH concentrations were measured during pre-industrial and in modern non-polluted sediments. The onset of environmentally significant concentrations of TPH did not occur until after ca.1915 and started to decrease from 1980s to present. This latest decrease in TPH concentrations is commensurate with both a decrease in industrial activities and an increase in environmental legislation, awareness and enforcement.
Other organic pollutants of anthropogenic origin commonly found in riverine/estuarine sediments are PCBs. They have been analysed in Clyde estuary sediments by a few studies that reported an association between the distribution of these compounds in the sediments and the presence of local industrial activities (Edgar et al. Reference Edgar, Davies, Hursthouse and Matthews1999, Reference Edgar, Hursthouse, Matthews and Davies2003; Vane et al. Reference Vane, Harrison and Kim2007a, Reference Vane, Chenery, Harrison, Kim, Moss-Hayes and Jones2011). Although all the sites analysed by these studies showed the presence of PCB, the total concentration of these compounds in most of the samples did not reach the threshold for probable effect concentrations (PEC; 676μgkg–1) defined by MacDonald et al. (Reference MacDonald, Ingersoll and Berger2000). This suggested that the PCB contamination in the sediments was not likely to adversely affect sediment-dwelling organisms.
As stated above, previous studies focused mainly on the analyses of POPs from the Clyde estuary. The objective of this study is to gain a better understanding of the distribution, source and fate of POPs in the entire River Clyde system by examining sediment PAH, TPH and PCB concentrations along its length, including the upper reaches of the Clyde, the Glasgow urban area and the estuary. The results are used to evaluate the potential toxicological impacts of the POPs on the local environment.
1. Method
1.1. Sample collection and preparation
The data presented here are based upon two existing sediment datasets for (i) the Clyde inner estuary (Vane et al. Reference Vane, Harrison and Kim2007a) and (ii) urban tributaries draining into the River Clyde from the Glasgow area (Fordyce et al. Reference Fordyce, Ó Dochartaigh, Lister, Cooper, Kim, Harrison, Vane and Brown2004). In addition, a new dataset of sediment samples from the upper Clyde was collected for this study to provide information on POPs concentrations in the largely rural environment upstream of Glasgow. The upper Clyde and inner estuary samples are river sediment samples (160km river-inner estuary transect), while the majority of the urban tributary dataset were collected from small streams (<3m across). The sample collection methods for these three areas are described in the following sections.
1.1.1. Inner estuary and urban tributary sediment samples
Inner estuary surface sediment samples (0–20cm) were collected between October and February 2003 at 17 sites in the Clyde inner estuary (Fig. 1) using either a stainless steel Van Veen or Day grab. After each deployment–collection cycle, the grab was cleaned with bottled tap water to avoid contamination from the previous site. Samples were wrapped in aluminium foil and sealed in polyethylene bags for transport to shore where they were immediately stored in a freezer (Jones et al. Reference Jones, Lister, Strutt, Entwisle, Harrison, Kim, Ridgway and Vane2004; Vane et al. Reference Vane, Harrison and Kim2007a). Urban tributary sediment samples (Fig. 1) were collected every km of stream length on all tributaries draining into the River Clyde within the Glasgow City Council area in June 2003. A total of 116 sediment samples were retrieved from the active water channel using a stainless steel trowel. Samples were stored in pre-cleaned 500mL glass jars fitted with screw caps lined with aluminium foil. They were kept in cool boxes following collection and frozen on return to the field base (Fordyce et al. Reference Fordyce, Ó Dochartaigh, Lister, Cooper, Kim, Harrison, Vane and Brown2004).
1.1.2. Upper Clyde sediment samples
In October 2010, 40 sediment samples were collected from the upper reaches of the River Clyde, upstream of Glasgow to its headwaters (Fig. 1). The material was taken from the active water channel using a steel trowel, removing any bulky organic matter and large stones before collection into a Rilsan sampling bag. Collected sediments were kept in cool boxes (4°C) and were frozen on return to the field base. Upon return to the laboratory, each sample was freeze-dried, disaggregated, passed through a brass sieve with an aperture of 2mm and milled to approximately <40μm. This method was chosen as a statistically robust method for high precision and accuracy PAH quantification (Bearcock et al. Reference Bearcock, Scheib and Nice2011; Beriro et al. Reference Beriro, Vane, Cave and Nathanail2014).
1.2. Sample analysis
All samples were analysed for total organic carbon (TOC; n=173) and a smaller subset comprising the urban tributary and upper Clyde sediments were analysed for TPH (n=153). Some samples of the inner estuary, urban estuary and upper Clyde were evaluated for PAH (n=109) and a smaller subset selected for PCB (n=72).
1.2.1. Total organic carbon (TOC)
TOC content (% wt/wt) was measured using an identical method to that previously published (Vane et al. Reference Vane, Harrison, Kim, Moss-Hayes, Vickers and Hong2009). Briefly, TOC was measured on 300mg of sediment using an Elementar VarioMax C, N analyser after acidification with hydrochloric acid (HCl). The limits of quantification were 0.18% (wt/wt).
1.2.2. Polyaromatic hydrocarbon (PAH) analysis
PAH analysis was carried out on all the inner estuary and upper Clyde sediment samples. A subset of 52 urban tributary samples were selected for PAH analysis on the basis of higher TOC results and on the locations of likely sources of contamination (Fordyce et al. Reference Fordyce, Ó Dochartaigh, Lister, Cooper, Kim, Harrison, Vane and Brown2004). Samples were spiked with internal standards: naphthalene-d8, biphenyl-d10, phenanthrene-d10, pyrene-d10, benzo[a]athracene-d10, benzo[a]pyrene-d12 and benzo[g,h,i]perylene-d12. They were extracted using an accelerated solvent extraction (ASE) system with dichloromethane/acetone 1:1v/v at 100°C and 2000psi and after being reduced in volume, the extract was transferred to a solid phase extraction (SPE) cartridge (Varian, Bond Elute TPH w.500mg Na2SO4, 1g sorbent, 3mL reservoir volume). The first fraction was eluted with pentane. The second fraction, which contained the PAH, was eluted with hexane/iso-propanol (97:3v/v), spiked with an extraction efficiency standard: methylnaphthalene-d10 and chrysene-d12 and reduced in volume.
Concentrations of PAH were measured using a Varian 3800 gas chromatograph (GC) coupled to a Varian 1200L triple quadrupole mass spectrometer (MS) operating in full scan mode (ionisation energy 70eV, mass range 47–500amu). The GC was fitted with an Agilent capillary column (DB-35ms Ultra Inert, 30m length, 0.25mm internal diameter (i.d.), 0.25μm film thickness). The oven temperature program was 60°C (isothermal for 1min) to 320°C (isothermal for 10min) at 6°Cmin–1. 1μL was injected at 280°C in splitless mode for 0.7min, split 1:20 thereafter. Helium carrier gas was 1mLmin–1. The PAH limits of detection (calculated as five times the background peak height of a pure standard normalised to a typical 1g of sample) ranged from 1.45 to 50.07μgkg–1. Quality control was achieved by determining PAHs in the CRM, NRCHS-5 Harbour Sediment and in a candidate soil from an inter-laboratory proficiency study, Contest 58.3c (LGC, Bury, UK) every nine samples. A procedural blank was analysed every 17 samples to ensure batch-to-batch quality.
1.2.3. Total petroleum hydrocarbons (TPH) analysis
Each sediment sample was extracted using the ASE 200, Dionex. The ASE conditions were: dichloromethane/acetone (1:1v/v) at 100°C and 2000psi. Extracts were dried using a TurboVap at 30°C and reconstituted in 1mL of toluene. An aliquot was spotted on to silica rod (Chromarods-S III) and the rods developed for 21min using n-hexane and 8min with toluene. The concentration of saturated and aromatic hydrocarbons was determined using an Iatroscan Mk6s instrument (Vane et al. Reference Vane, Harrison, Kim, Moss-Hayes, Vickers and Horton2008). This was calibrated for saturate hydrocarbons using pristine and aromatic hydrocarbons using triphenylene. The limit of quantification (LoQ) for total non-volatile hydrocarbons was 3mgkg–1.
1.2.4. Polychlorinated biphenyl (PCB) analysis
Sediments were spiked with PCB 19, 34, 62, 119, 131, 147 and 173 and extracted with hexane/acetone 1:1v/v followed by the addition of concentrated H2SO4 (Vane et al. Reference Vane, Harrison and Kim2007b, Reference Vane, Chenery, Harrison, Kim, Moss-Hayes and Jones2011). The hexane extract was reduced and cleaned using the same SPE method as for PAH and spiked with PCB 29 and 157 and reduced. Concentrations of PCB were measured using a Varian 1200L triple quadrupole mass spectrometer (GC/MS). The MS was operated in selected ion mode (m/z 71, 220, 256, 258, 292, 326, 328, 360, 362, 394, 396) with a scan time of 0.5s. Congener profile distributions for tri to hepta-chlorinated biphenyls were determined using five separate individual relative response factors for each congener group. A standard consisting of 2×5 individual PCB based on first and last eluting congener was used to define the ‘retention time window' for each group. The sum of these five groups is ∑tri-hepta PCB and ∑7 PCB comprised congeners 28, 52, 101, 118, 153, 138, 180. The individual congener limit of detection was 0.5ngg–1.
1.2.5. Data presentation
Correlations between TOC and the organic pollutants were assessed using linear regression plots. Likely sources of POPs were determined on the basis of isometric ratio plots (Vane et al. Reference Vane, Harrison, Kim, Moss-Hayes, Vickers and Horton2008, Reference Vane, Chenery, Harrison, Kim, Moss-Hayes and Jones2011). Maps showing the distribution of POPs in the samples (Annex I) in the River Clyde catchment were generated using the graduated symbol function in a geographic information system (ArcMap10.1, Environmental Systems Research Institute, ESRI®).
2. Results and discussion
The main groups of POPs (TPH, PAH and PCB) were quantified in 173 sediment samples from the River Clyde system. The samples were collected from three domains: the upper-freshwater Clyde, the urban tributaries of Glasgow and the inner estuary (Fig. 1).
2.1. PAH concentrations and distribution
The sum of 15 USEPA PAH concentrations was measured in a total of 109 sites (Figs 2–4). Sediments from the upper Clyde had the lowest PAH concentrations of the three fluvial domains, ranging from 0.1 to 3.3mgkg–1. However, one sample collected in the town of Lanark, adjacent to a sewage works, contained 42mgkg–1 PAH (Fig. 2). The near ten-fold increase in PAH concentration (relative to other upper Clyde sites) and close spatial proximity to a sewerage treatment works (STW) suggests that this may either be due to sorption of pre-existing PAH to organic-rich STW effluent or, alternatively, could be due to the STW effluent itself containing elevated amounts of PAH. A similar anthropogenic organic carbon overprinting within portions of the tidal Thames influenced by London's main STW situated at Crossness and Beckton, has been recently reported using changes in proportions of glycerol dialkyl glycerol tetraethers compounds (Lopes dos Santos & Vane Reference Lopes dos Santos and Vane2016). On balance, the site at Lanark was anomalous relative to the rest of the upper Clyde sediments and was excluded from the correlations on Fig. 3a.
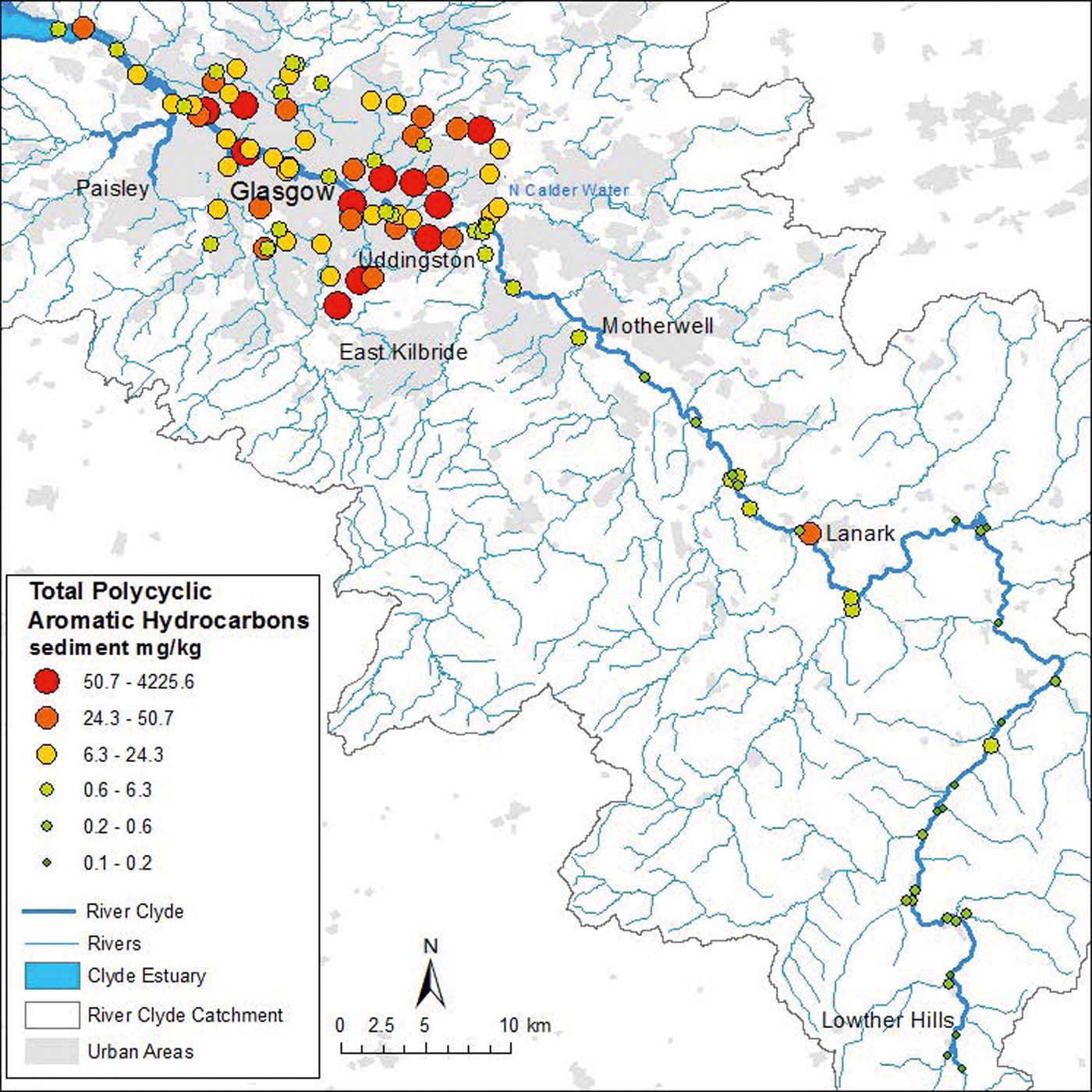
Figure 2 Total PAH concentrations in sediments of the River Clyde, UK. Geoscience data, BGS © NERC. Contains Ordnance Survey data © Crown Copyright and database rights 2016.

Figure 3 Correlation between TOC, PAH, TPH and PCB concentrations in the upper Clyde (yellow squares), urban tributaries (red triangles) and inner estuary (green circles). Blue lines represent the ecological sediment-quality guideline threshold effect concentration. Red lines represent the probable effect concentration. (a) TOC versus PAH Upper Clyde; (b) TOC versus PAH Urban tributaries; (c) TOC versus PAH Inner estuary; (d) TOC versus TPH Upper Clyde; (e) TOC versus TPH Urban tributaries; (f) TOC versus PCB Upper Clyde; (g) TOC versus PCB Urban tributaries; (h)TOC versus PCB Inner estuary; (i) PAH versus TPH Upper Clyde; (j) PAH versus TPH Urban tributaries. Abbreviations: TEC = threshold effect concentration; PEC = probable effect concentration; TOC = total organic carbon; TPH = total petroleum hydrocarbons; PAH = polyaromatic hydrocarbons; PCB = polychlorinated biphenyls.

Figure 4 Box and whisker plots of (a) TOC, (b) TPH, (c) PAH and (d) PCB concentrations in sediments from the three geographic domains across the Clyde River estuary. The boundary of the box indicates the 25th and 75th percentiles, the line within the box marks the median and error bars indicate the 10th and 90th percentiles. Dots indicate outlying points.
Sediments from the urban tributary domain had the highest PAH concentrations, ranging from 2.3 to 4226mgkg–1 (Fig. 4c). The latter site (proximal to a known bus oil spill site) was anomalous relative to the rest of the urban tributaries dataset (maximum 296mgkg–1) and was also removed from Fig. 2b as it would distort the correlation. The inner estuary sediment PAH values ranged from 0.6 to 30mgkg–1 (Figs 2, 3c, 4c). As expected, the lowest concentrations were associated with the rural upper reaches of the Clyde, many km upstream of Glasgow urban centre and associated satellite towns (Fig. 2).
Inspection of the concentration of benzo[a]pyrene (BaP), one of the most toxic of the parent PAH from the three fluvial domains of the Clyde, also confirms the total PAH concentrations, following a pattern of urban tributary>inner estuary > upper Clyde (Fig. 5). The higher concentrations of total PAH observed in the urban tributaries probably reflects: (1) close proximity to multiple industrial/traffic/urban pollution source(s); (2) less sediment dilution and mixing in these systems; and (3) general anoxic burial conditions, which enhances preservation and limits enzymatic microbial degradation of many organic compounds and biopolymers.

Figure 5 Benzo[a]pyrene concentrations along a 160-km land-to-sea transect spanning upper freshwater–rural Clyde, Glasgow's urban tributaries and the inner Clyde estuary. Outliers (solid dots), highest and lowest non-outliers (upper and lower whisker limits) and upper and lower quartiles (box limits).
The elevated PAH concentrations in the inner estuary demonstrates the impact that polluted inputs from the urban tributaries have on sediment quality in the Clyde relative to the rural upper reaches. However, PAH concentrations are lower in the inner estuary than in the urban tributaries, probably because the estuary is further from the main pollution inputs and also subject to dilution with cleaner sediment from other sources such as the outer Clyde. The total PAH concentrations in the River Clyde were comparable to those previously reported in other studies of UK estuaries. For instance, sediments from the Mersey, Tyne and Tees estuaries showed concentrations ranging 0.2–43mgkg–1 (Woodhead et al. Reference Woodhead, Law and Matthiessen1999; Rogers Reference Rogers2002; Vane et al. Reference Vane, Harrison and Kim2007b) that were similar to the concentrations in the inner estuary, upper Clyde and the majority of urban tributary samples. However, some PAH concentrations in the urban tributary sediments were much higher. Wilson et al. (Reference Wilson, Clarke, D'Arcy, Heal and Wright2005) analysed POPs in selected Scottish urban rivers and found values of total PAH concentration up to 52mgkg–1. Similarly, analysis of the distribution of PAHs in the River Seine, France, found maximum concentrations of 60mgkg–1 (Ollivon et al. Reference Ollivon, Garban and Chesterikoff1995). Ten of the urban tributary samples exceeded these values and were considerably more polluted than these urban rivers in Scotland and France. Of these, oil pollution was present at five of the sites and three of the samples were collected from industrial locations. Therefore, the higher concentrations reflect the fact that urban sediments and the surrounding soils act as sinks for historic and current industrial POPs pollution including oil/petroleum spills, slag materials from the former use of coal as an industrial fuel, degradation of tar-based road coverings and increased surface run-off containing traffic pollutants.
PAH are commonly associated with organic matter in sediments, and increased organic matter derived from the cross-contamination of sewerage networks can also have a detrimental impact on urban sediment quality (Selbig et al. Reference Selbig, Bannerman and Corsi2013). In this current work, a positive correlation exists between TOC content and PAH concentration for the samples of the upper Clyde and inner estuary (R 2=0.8 and 0.6, respectively; Fig. 3a, c). This possibly reflects the adsorption of PAH to the natural organic matter in the sediments in agreement with the results of other studies (Vane et al. Reference Vane, Harrison and Kim2007a, Reference Vane, Harrison and Kim2007b). On the other hand, urban tributary sediment PAH values show a weak correlation (R 2=0.1) with the TOC (Fig. 3b). This lack of correspondence between PAH and TOC is explained by some of the urban samples that were collected in semi-natural marshy/peat areas and have high natural TOC (e.g., 10–20%) and/or that soils and sediments receiving highly polluted wastes sometimes show weak correlation due to the consumption of organic coatings (active sorption sites) on mineral surfaces. Hence, overall, the greater variety of TOC and PAH sources in the urban area probably masks the correlation that might otherwise be observed between them.
The distribution of individual parent PAH compounds showed minor differences between the three areas (upper Clyde, urban tributaries and inner estuary; Fig. 6). Overall, the pattern corresponded to PAH assemblages that result from high-temperature combustion processes (McCready et al. Reference McCready, Slee, Birch and Taylor2000). This is characterised by an abundance of high molecular weight (HMW) PAH with 4–7 rings with predominance of fluoranthene, pyrene, benzo[b]fluoranthene and benzo[a]anthracene. Phenanthrene, the most thermodynamically stable of the three-ringed PAH, was also present in all sites but it is an indicator of a petrogenic origin when found in high concentrations (Budzinski et al. Reference Budzinski, Jones, Bellocq, Pierard and Garrigues1997). The urban tributary domain showed the largest variation in PAH distributions in comparison to the other sites; this suggested either a wide variety of PAH sources and associated mixing, or may also be attributable to less weathering/biodegradation in the urban domain as compared to fluvial-estuarine domains due to rapid burial and incorporation in the sediment. For instance, a few urban tributary samples contained a greater proportion of the low molecular weight (LMW) PAH (naphthalene, acenaphthene, fluorene, phenanthrene, anthracene) suggesting input of PAH from a petrogenic origin (Budzinski et al. Reference Budzinski, Jones, Bellocq, Pierard and Garrigues1997; Vane et al. Reference Vane, Harrison and Kim2007b).

Figure 6 Typical PAH profile of sediments from the upper Clyde, urban tributaries and inner estuary. PAH = polyaromatic hydrocarbons.
2.1.1. Use of PAH ratios to infer possible sources
To better assess the variations in the molecular weight of the PAH in the sediments, the ratio LMW/HMW (Brown & Peake Reference Brown and Peake2006) was applied to identify potential sources of the PAH. Almost all the samples in this study had a ratio <1, suggesting a combustion origin. Only six urban tributary sediments showed a LMW/HMW >1, suggesting a petrogenic origin. These sediments may be influenced by some oil input. In fact, in two of these samples, oil contamination was visible at the time of sampling.
A more common approach to identify the sources of PAH is the use of diagnostic ratios of selected parent PAH (Readman et al. Reference Readman, Fillmann, Tolosa, Bartocci, Villeneuve, Catinni and Mee2002; Yunker et al. Reference Yunker, Macdonald, Vingarzan, Mitchell, Goyette and Sylvestre2002; Vane et al. Reference Vane, Harrison and Kim2007a, Reference Vane, Harrison and Kimb; Tobiszewski & Namiesnik Reference Tobiszewski and Namiesnik2012). This approach is based on the analyses of PAH isomers with different degrees of thermodynamic stability, allowing the determination of the processes producing them. The application of these ratios can produce different results (Tobiszewski & Namiesnik Reference Tobiszewski and Namiesnik2012). Therefore, they are usually assessed graphically using a combination of two ratios to facilitate a more reliable estimate of sedimentary PAH source(s). Fig. 7 shows the application of four ratios used to estimate PAH sources in this study. The upper plot displays the ratios between phenanthrene and anthracene versus fluoranthene and pyrene. The results for most of the sediments confirm a pyrolytic PAH origin (shaded area). However, some of the upper Clyde and the urban tributary sediments have borderline petroleum values. Given the rural setting of the upper Clyde sediments, it is expected that PAH in this area largely derive from combustion, including burning of vegetation, as compared to direct spill of petroleum origin (Vane et al. Reference Vane, Rawlins, Kim, Moss-Hayes, Kendrick and Leng2013). Concentrations of anthracene for most of the samples of the upper Clyde were extremely low (at or below LoQ), which confounds use within isomeric bi-plots for source apportionment (Fig. 7). The middle plot (Fig. 7) shows the ratios between benzo[a]anthracene and chrysene as compared to fluoranthene and pyrene. It suggests a combustion origin for PAH for the majority of the samples and none of the samples showed an exclusively petroleum origin. Finally, the bottom plot (Fig. 7) showing the ratios between indeno[1,2,3-c,d]pyrene and benzo[g,h,i]perylene (BghiP) as compared to fluoranthene and pyrene, also signifies a pyrolytic origin for all the samples. Taken together, the isomeric and the LMW/HMW ratios indicate a pyrolytic PAH source in the Clyde sediments with a dominance of petroleum combustion in the upper Clyde and inner estuary, while most of the urban samples are on the border between petroleum and coal combustion as both will have been important in the urban domain.
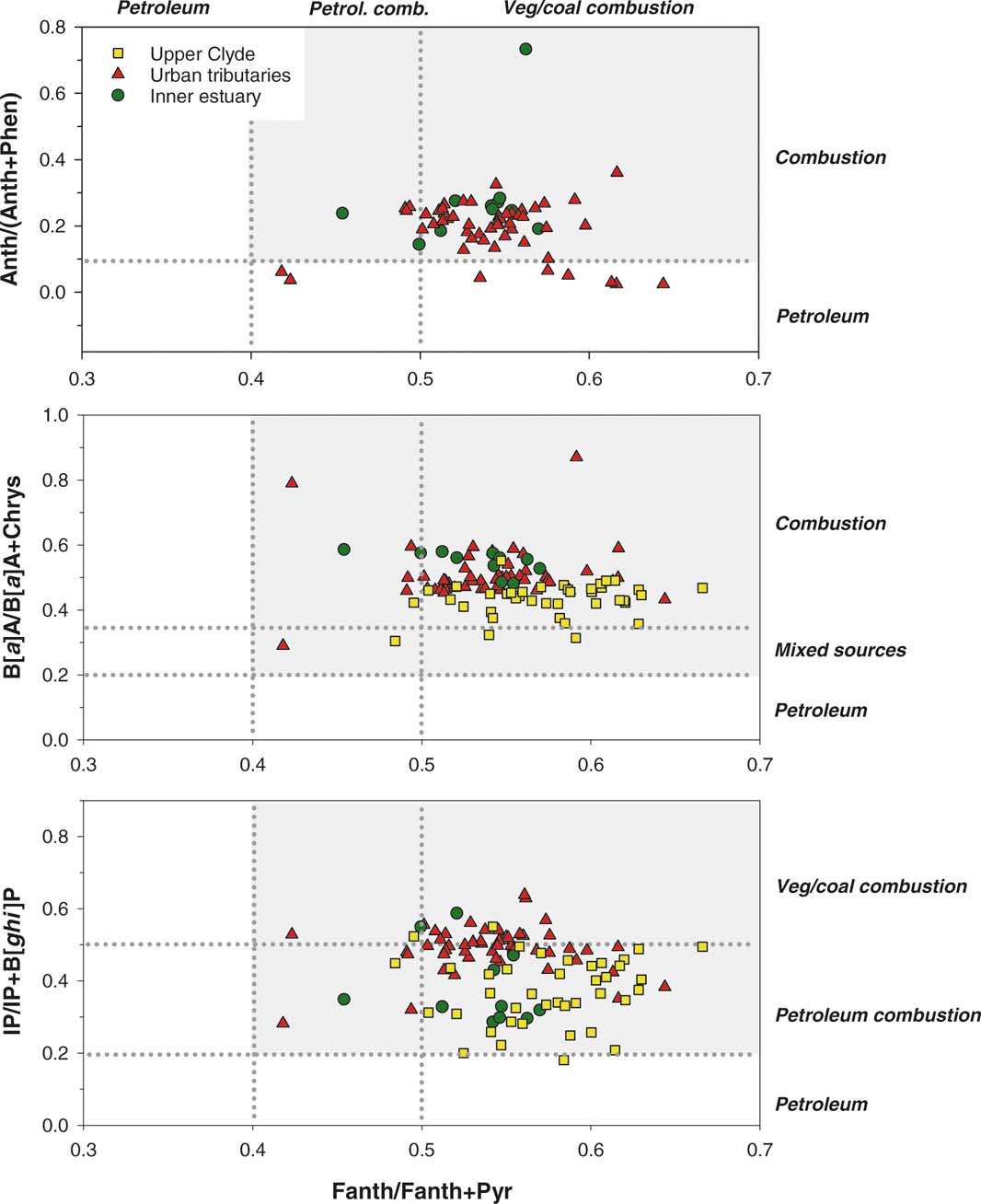
Figure 7 PAH isomeric ratio plot for samples from the upper Clyde (yellow squares), urban tributaries (red triangles) and inner estuary (green circles).
Previous studies have used the non-isomeric ratio of BaP/BghiP to infer vehicular emission PAH (Katsoyiannis et al. Reference Katsoyiannis, Terzi and Cai2007; Tobiszewski & Namiesnik Reference Tobiszewski and Namiesnik2012) where values >0.6 infer traffic exhaust emissions and <0.6 to infer non-traffic PAH source (Mi et al. Reference Mi, Lee, Chen, Yang and Wu2000; Lim et al. Reference Lim, Ayoko, Morawska, Ristovski and Jayaratne2007). Inspection of Fig. 8 shows that the majority of sites have BaP/BghiP ratios >0.6, which suggests all three domains – namely, upper, urban tributary and estuarine – are, to a lesser or greater extent, receiving PAH generated from traffic emissions, either from transport of diesel and/or unleaded vehicle exhaust particulates transported either in water as road run-off or by aerial deposition, or both. The incorporation of PAH derived from petrol and diesel vehicular exhaust into surface sediments is not entirely surprising in the urban (Glasgow) and estuarine reaches of the River Clyde since these domains are flanked by numerous major motorways such as the M82, M74 and M8. However, the exceedance of the BaP/BghiP >0.6 traffic exhaust source criteria in the upper Clyde rural-reaches probably signifies that either minor roads located alongside or in close proximity to the Clyde contribute to fluvial sedimentary PAH pollution or that there is a diffuse (atmospheric) traffic emission source. Alternatively, it is possible that some other factor confounds the diagnostic use of the BaP/BghiP ratio in the upper Clyde. For example, the managed burning of moorland vegetation in Yorkshire, UK (e.g., heather and associated species), reported PAH concentrations which yield BaP/BghiP of up to 1.6 (Vane et al. Reference Vane, Rawlins, Kim, Moss-Hayes, Kendrick and Leng2013). Therefore, caution does need to be exercised when applying BaP/BghiP as a marker for vehicular emissions in moorland settings because even small amounts of burnt heather could potentially increase the BaP/BghiP values to such an extent that they exceed the 0.6 value and are then easily misinterpreted.
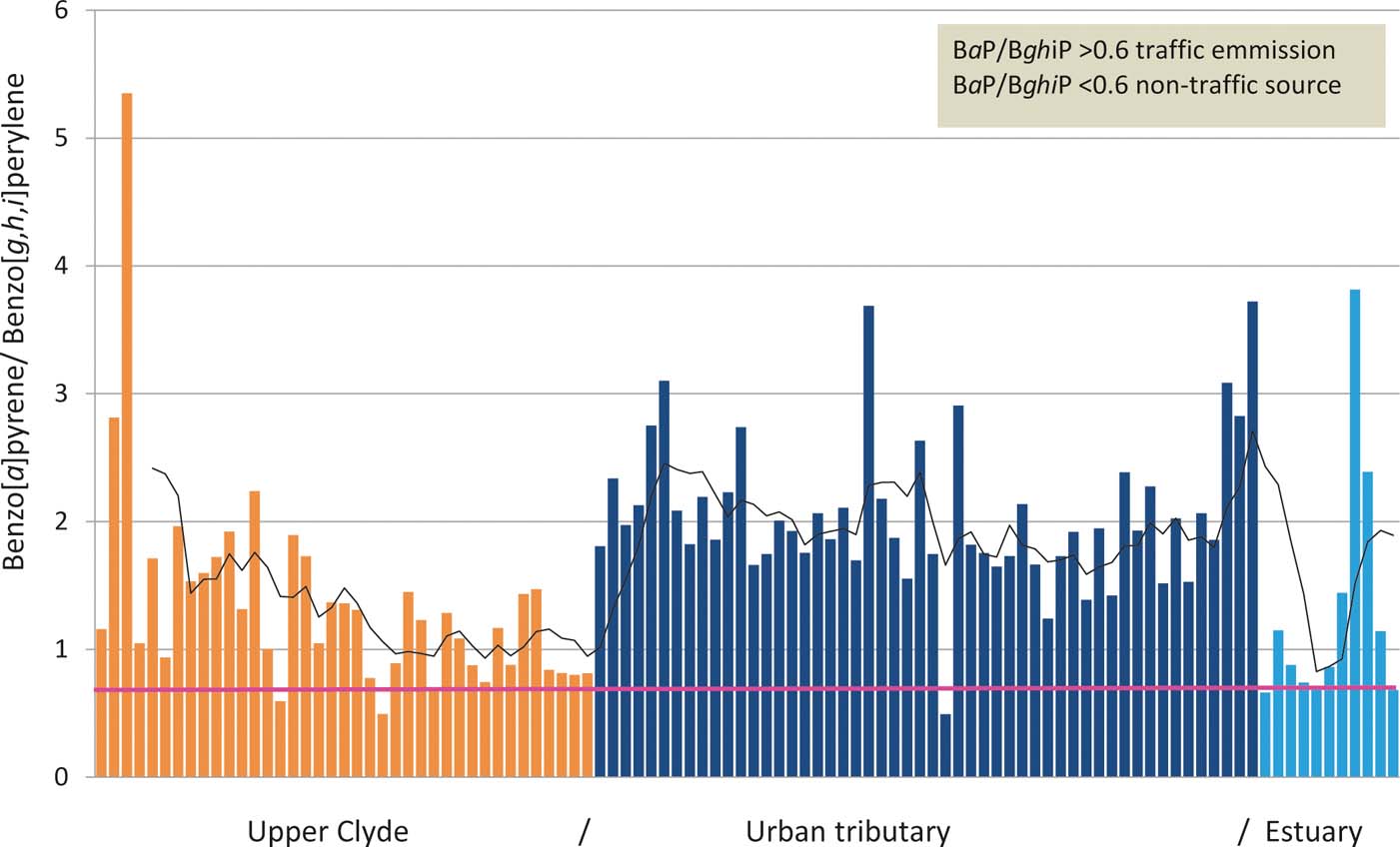
Figure 8 Benzo[a]pyrene/Benzo[g,h,i]perylene along a 160-km land-to-sea transect of the River Clyde.
2.2. TPH concentrations and distribution
Sedimentary TPH concentrations in the upper Clyde ranged from <3mg to 260mgkg–1 with a mean of 68mgkg–1 (Figs 4b, 9). These values are similar to those reported from undisturbed sediments from mangroves of southern China (mean 58mgkg–1) and barrier island river inlets of New Jersey, USA (mean 231mgkg–1) (Vane et al. Reference Vane, Harrison, Kim, Moss-Hayes, Vickers and Horton2008, Reference Vane, Harrison, Kim, Moss-Hayes, Vickers and Hong2009 and references therein). This comparison confirms the general TPH criteria of <100mgkg–1, which is taken to indicate natural sources of TPH (e.g., algae, plant waxes) as compared to 100 to <500mgkg–1 indicating low anthropogenic petroleum hydrocarbon input and >500mgkg–1 for sites receiving appreciable anthropogenic petroleum pollution.
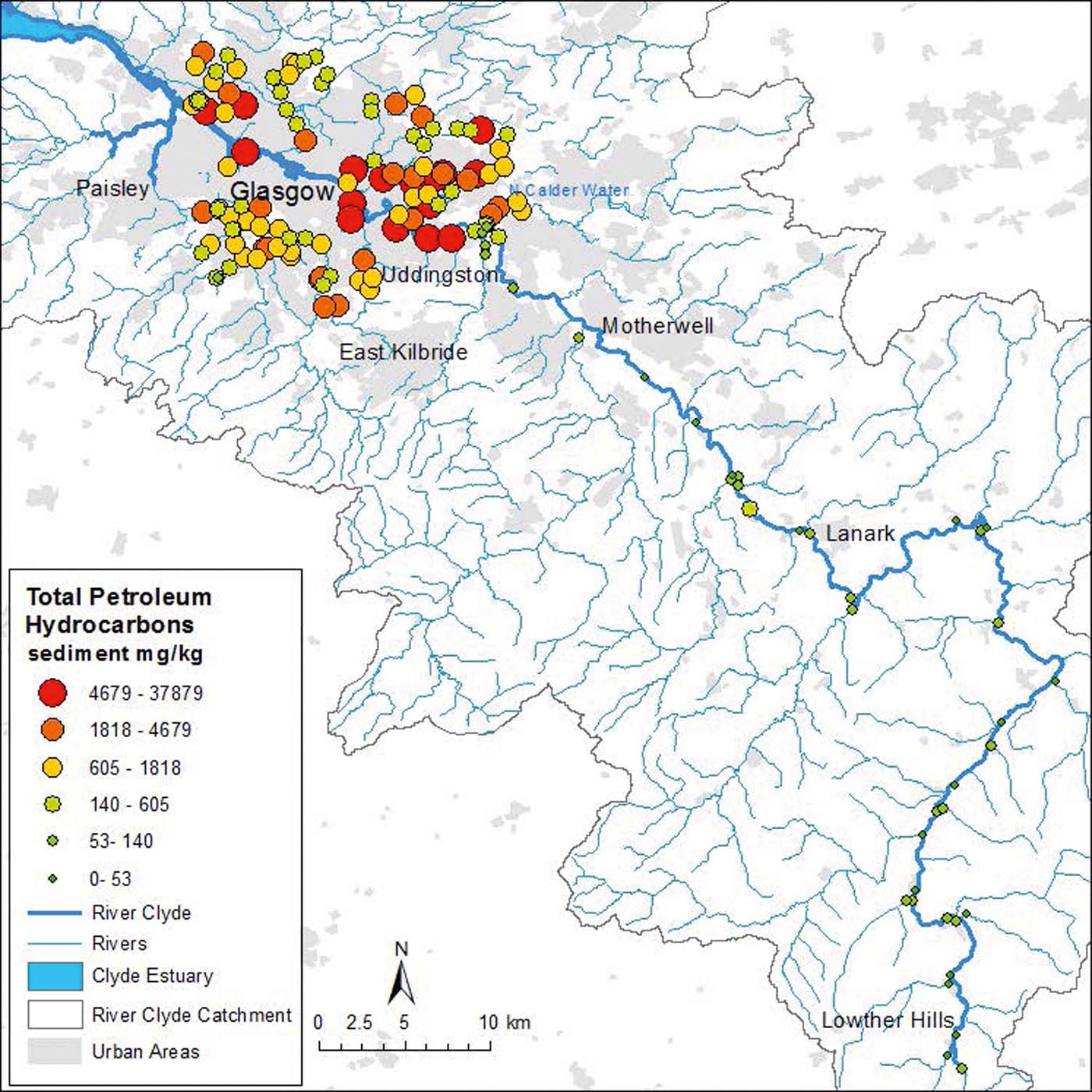
Figure 9 Total (non-volatile) petroleum hydrocarbon concentrations in sediments of the River Clyde, UK. Geoscience data, BGS © NERC. Contains Ordnance Survey data © Crown Copyright and database rights 2016.
In contrast, tributaries TPH from the urban domain (Glasgow) ranged from 72 to 37879mgkg–1 with a mean 2779mgkg–1. Of these, 86 sites were above >500mgkg–1 criteria (significant petroleum inputs) and 26 were between the 100 to 500mgkg–1 banding, suggesting low but discernible anthropogenic petroleum hydrocarbon pollution, with the exception of one site, which was <100mgkg–1, suggesting mainly natural background. The concentrations encountered in the urban tributaries of Glasgow are higher than that reported from historical sediment cores of the Clyde estuary (maximum 4386mgkg–1; Vane et al. Reference Vane, Chenery, Harrison, Kim, Moss-Hayes and Jones2011) and sites from Southampton estuary (maximum 3100mgkg–1; Knap et al. Reference Knap, Williams and Lysiak1982). This probably reflects closer proximity to pollution sources in the urban domain and less associated dilution by mineral matter. The latter hypothesis being, in part, confirmed by the highest TPH concentration recorded in the urban samples (37879mgkg–1) due to oil contamination at the sample site. Interestingly, a parallel onshore study (this issue) of TPH from urban Glasgow soils (5–10cm depth interval; n=82) had lower concentrations from 40 to 2505mgkg–1 (mean 353mgkg–1) (Kim et al. Reference Kim, Vane, Moss-Hayes, Beriro and Fordyce2018). Therefore, on balance, it may be inferred that Glasgow's urban tributaries have a higher amount of TPH than soils because they receive multiple direct discharges of waste and road run-off containing TPH, and that this loading is preserved and accumulates in the surface sediment, whereas the soils are subject to generally lower PAH loadings and have decreased PAH preservation potential due to factors such as weathering, microbial decomposition and photo-degradation. From a global perspective, the TPH values shown here for urban tributary samples are fairly high and are comparable to those reported in soils subject to oil contamination (e.g., Liu et al. Reference Liu, Chang, Whang, Kao, Pan and Cheng2011).
Examination of the TPH and TOC bi-plots for upper Clyde and urban tributary samples showed a stronger correlation of R 2=0.5 compared to R 2=0.2, respectively (Fig. 3e), and, similarly, the correlation between TPH and PAH in the upper Clyde sediments (R 2=0.6, Fig. 3i) was stronger than in the urban tributary sediments (R 2=0.3, Fig. 3j). This weak correlation between TPH and PAH in the urban tributaries suggests that the TPH may have other sources besides petroleum; for instance, urban samples that have high TPH but low PAH due to industrial (e.g., paper mills and processing) and sewage waste effluent that could account for higher organic material but not necessarily higher PAH.
2.3. PCB concentrations and distribution
Total PCB were analysed in 72 sediments collected from three domains (freshwater/urban/inner estuary) within the River Clyde catchment (Fig. 10). Total concentrations (e.g., summed tri-, tetra-, penta-, hexa- and hepta-chlorinated congeners) ranged from 2 to 61μgkg–1 (mean of 12μgkg–1) in sediments from the upper Clyde (Figs 3f, 4d). However, one sample from the North Calder Water tributary junction in Uddingston contained 147μgkg–1 PCB. This site showed much higher concentrations relative to the rest of the upper Clyde and was excluded from the correlations presented in Fig. 3f. In sediments from the inner estuary, PCB concentrations ranged from 5 to 130μgkg–1 and a mean of 46μgkg–1 (Figs 3h, 4d). Sediments from the urban tributaries were the most polluted, with PCB concentrations ranging from 3 to 310μgkg–1 and a mean of 137μgkg–1 (Figs 3g, 4d). Again, one sample from a site with a known history of industrial and oil pollution contained unusually high concentrations of 809μgkg–1 and was, therefore, excluded from the correlations of Fig. 3g. Similar to the TOC, TPH and PAH concentrations (Fig. 4a–c), sediments from the upper Clyde had lower amounts of PCB with higher values in the inner estuary and highest contents in the urban tributaries. The range of PCB concentrations from the three domains are in broad agreement with previous studies on sediments from the Mersey and Clyde estuaries (30–1409μgkg–1; Edgar et al. Reference Edgar, Davies, Hursthouse and Matthews1999, Reference Edgar, Hursthouse, Matthews and Davies2003; Vane et al. Reference Vane, Harrison and Kim2007a, Reference Vane, Harrison and Kimb, Reference Vane, Chenery, Harrison, Kim, Moss-Hayes and Jones2011). The mean PCB value for the entire freshwater-estuarine Clyde system is 52μgkg–1, which is lower than the values for Mersey estuary (123μgkg–1; Vane et al. Reference Vane, Harrison and Kim2007b) but higher than those for the inner Thames estuary (14μgkg–1; Scrimshaw & Lester Reference Scrimshaw and Lester1995).
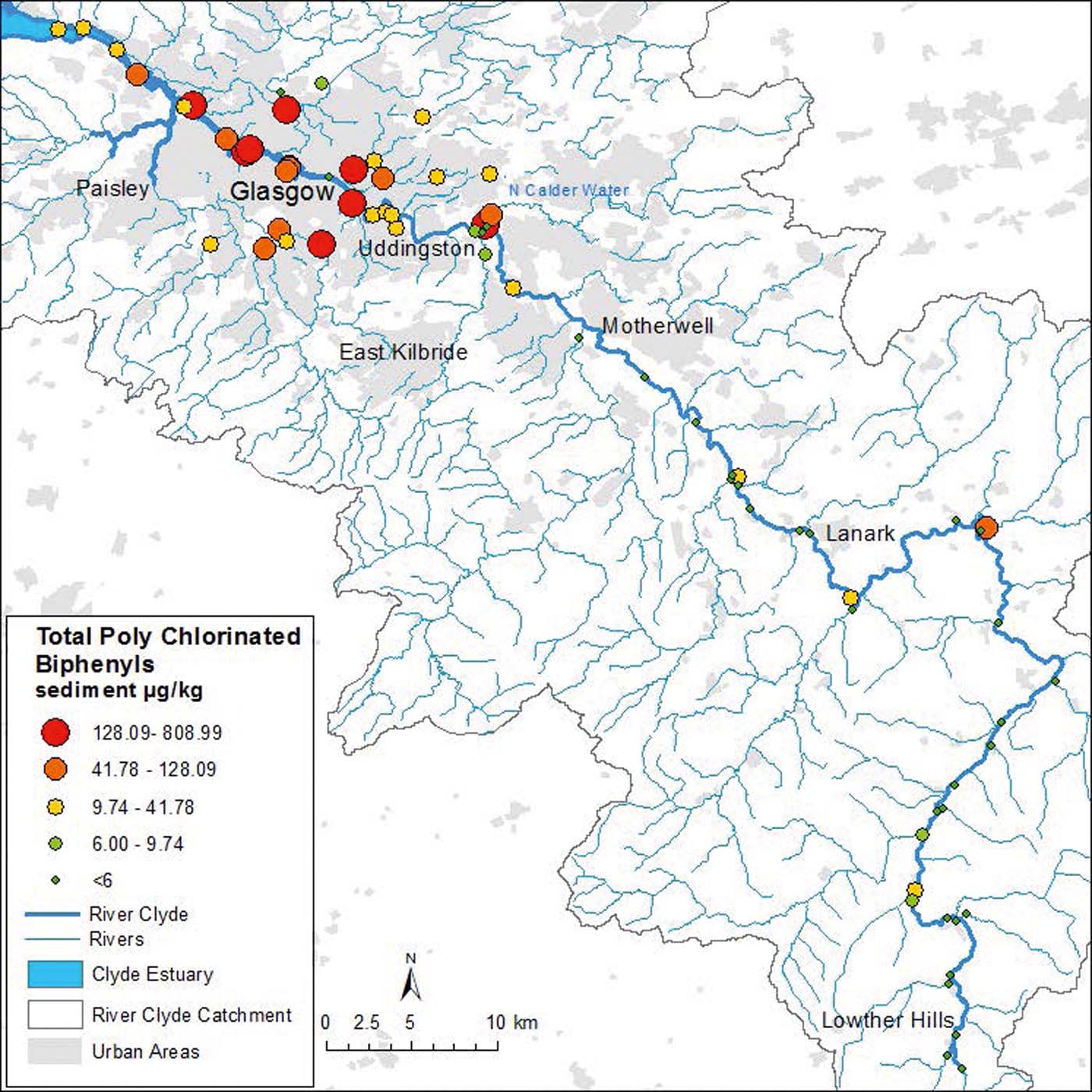
Figure 10 Total PCB concentrations in sediments of the River Clyde, UK. Geoscience data, BGS © NERC. Contains Ordnance Survey data © Crown Copyright and database rights 2016.
Total PCB concentration showed a weak correlation with the TOC in sediments from the upper Clyde and urban tributaries (R 2=0.0008 and 0.02; Fig. 3f, g) and a moderate positive correlation for the inner estuary (R 2=0.5; Fig. 3h). The low correspondence between PCB and TOC in the upper Clyde samples is probably due to variable amounts of natural organic matter (e.g., humic and structural biopolymers), with high TOC entering and being deposited in the river from the rural catchment as well as a low PCB input. In contrast, the weak PCB-to-TOC correlation observed in the urban tributaries may be due, in part, to the overloading of active sites for sorption, particularly in urban samples with high PCB contents and not particularly high TOC located in sites with oil and industrial contamination. A similar lack of correlation between TOC and PCB was also found for estuarine sediments in the Mersey river (Vane et al. Reference Vane, Harrison and Kim2007b), where the authors explained this as a consequence of local PCB input from industrial point sources.
Changes in the distribution of 3- to 7-chlorine (Cl) PCB homologue groups from the different environmental domains can potentially indicate shifting PCB sources and/or environmental processes (Vane et al. Reference Vane, Kim, Beriro, Cave, Knights, Moss-Hayes and Nathanail2014, Reference Vane, Chenery, Harrison, Kim, Moss-Hayes and Jones2011). Figure 11 shows the typical homologue group profile for the rural upper, urban tributary and inner estuary. Differences between the upper/urban/estuary domains are clear (Fig. 11). For example, the upper rural Clyde sediments mainly comprise 5-Cl PCB and lower amounts of 4-Cl and 3-Cl PCB. As the upper rural Clyde has far fewer pollution sources, the PCB homologue group distribution is more homogeneous. The urban tributary sites' homologue group profiles were more variable, with some dominated almost entirely by 5-Cl PCB and others comprising mainly 3-Cl homologues and lower proportions of 4-Cl as well as minor amounts of 5- and 6-Cl (Fig. 11). The greater range of chlorination in the urban tributary sediments may be due to the wider diversity of sources (e.g., construction materials released from demolition sites (paint, caulking), electrical transformer fluids, incinerators, leaking land-fill sites) common to the urban environment of Glasgow. In contrast, sediments from the inner estuary display a mixture spanning 3–7Cl groups in broadly similar proportions. The PCB congener profile in the inner estuary probably reflects multiple sources within the entire catchment.
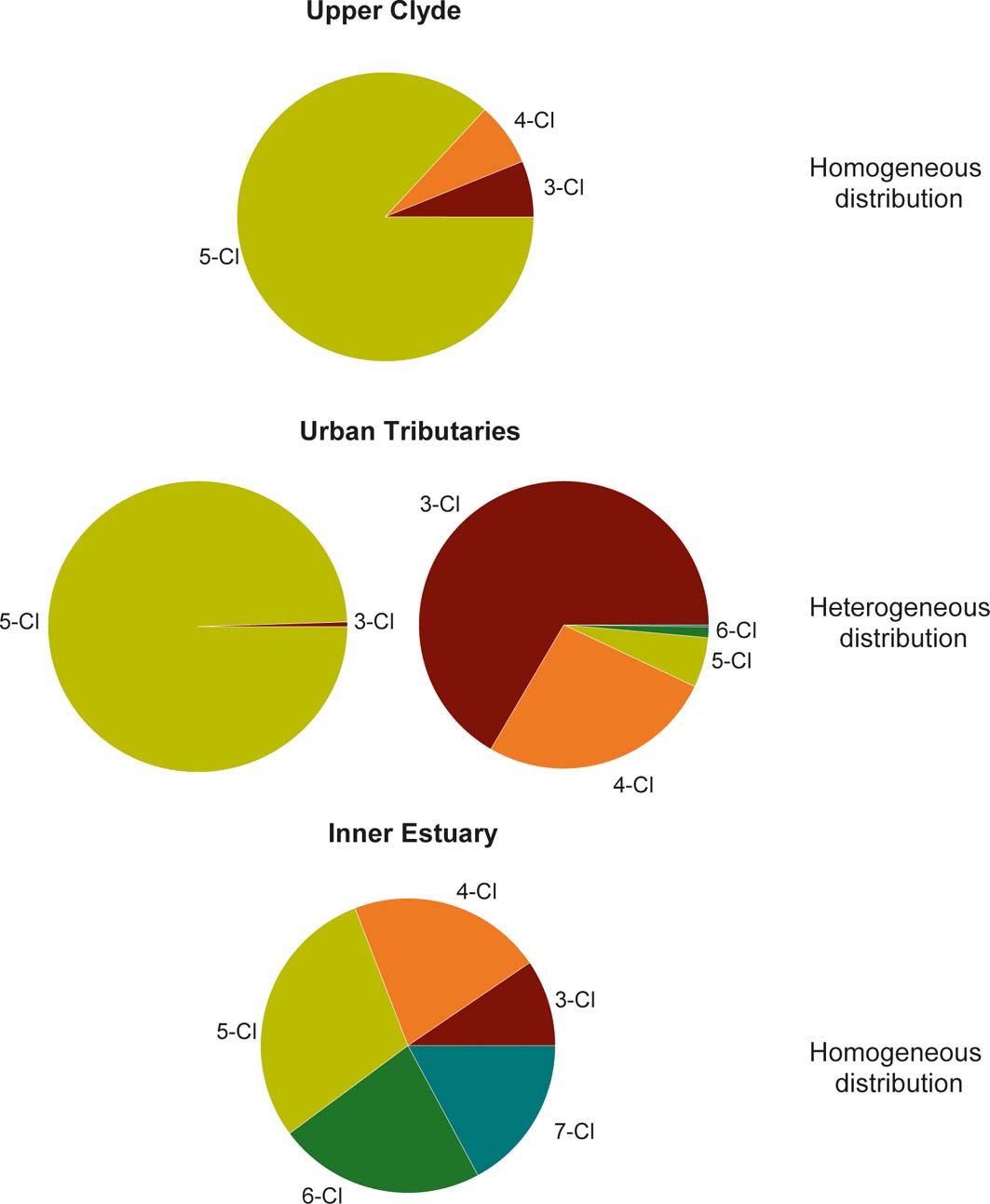
Figure 11 Example congener distributions for upper, urban (tributaries) and inner estuary of the Clyde. The degrees of chlorination (3–7-Cl atoms) shown are consistent for the majority of sediments within upper freshwater Clyde and inner estuarine domains, but are highly variable within the urban tributaries of Glasgow.
It should also be borne in mind that although PCBs are classified as persistent they are not entirely inert and that different congener distributions observed in the three domains could arise, in part, from the preferential aerobic dechlorination and/or anaerobic reductive processes, or a combination of the two. However, disentangling the specific PCB decay pathways is beyond the scope of this study because: (1) it is better addressed using a compound specific isotope approach; and (2) such studies are confounded by manifold sources and a paucity of information concerning the starting formulations used. Nevertheless, the distinct distributions (Fig. 11) confirm the general notion that industrial estuaries act as sinks for original and environmentally attenuated PCB inputs (river and its tributaries) and that the sediments, therefore, contain a full range of chlorine substitution, five homologues in approximately equal amounts, which arises because of multiple industrial inputs and also a range of microbial mediated decay processes.
2.4. Evaluation of the organic pollution potential toxicological effects on the local environment
There are currently no river sediment quality guidelines (SQGs) in the UK. However, to evaluate the possible toxicological effects of sediment POPs on aquatic ecosystems in the River Clyde, concentrations in the three geographic domains were compared to the consensus-based stream-bed SQGs (MacDonald et al. Reference MacDonald, Ingersoll and Berger2000). Most of the sediments from the upper Clyde were below the PAH threshold effect concentration (TEC; 1.6mgkg–1; Fig. 3a). A few sediments from this area were between the TEC and the PEC (22.8mgkg–1). In the inner estuary, most of the sediments were between the TEC and the PEC, with only three samples exceeding the PEC value (Fig. 3c). The urban tributary sediments were more polluted with the majority of samples between the TEC and the PEC, and several sediments exceeding the PEC value (Fig. 3b). This suggests that the local biota may be adversely affected by the sedimentary PAH concentration in the urban domain.
Unfortunately, no TPH threshold is stated in the consensus-based SQGs. However, as the concentrations of TPH, especially in the urban tributary sediments, are so high, it is reasonable to assume that these pollutants may be causing an adverse effect to the local biota. A study of the ecotoxicological effects of TPH on the intertidal benthic organisms off Goa reported an adverse effect to the macrobenthos exposed to a maximum concentration of 89mgkg–1 TPH (Ingole et al. Reference Ingole, Sivadas, Goltekar, Clemente, Nanajkar, Sawant and Ansari2006). The study also reported changes in the benthos community structure as the number of species significantly decreased at these concentrations (Ingole et al. Reference Ingole, Sivadas, Goltekar, Clemente, Nanajkar, Sawant and Ansari2006). Although the present study concerns river sediments spanning both freshwater and estuarine (partly saline) conditions and, therefore, the environmental conditions and the biota will differ from Ingole et al. (Reference Ingole, Sivadas, Goltekar, Clemente, Nanajkar, Sawant and Ansari2006), a stronger adverse effect of TPH on the biota is likely based on the much higher concentrations measured herein. We assert that pollution in the urban tributaries warrants further investigation, particularly if the sediment compartment is coupled to in-field biomonitoring of bioindicator species such as that previously conducted in Milford Haven waterway (Langston et al. Reference Langston, O'Hara, Pope, Davey, Shortridge, Imamura, Harino, Kim and Vane2012).
When compared to the consensus-based SQG (MacDonald et al. Reference MacDonald, Ingersoll and Berger2000) for total PCB pollution, the majority of samples from the upper Clyde and the inner estuary are below the TEC (59.8μgkg–1; Fig. 3f, h). Only a few samples from these areas are between the TEC and the PEC, and none of the samples exceed the PEC (676μgkg–1). For the urban tributaries, 11 of the 19 samples analysed for PCB were below the TEC and the rest were between the TEC and PEC (Fig. 2g). Therefore, according to these criteria and sites examined, it is unlikely that the sedimentary PCBs are adversely altering the biota of the Clyde.
2.5. Sediment quality guidelines (SQGs) for disposal at sea
Marine Scotland, a directorate of the Scottish Government responsible for the management of Scottish seas, have set non-statutory action levels for sediment PAH and PCB as part of a weight-of-evidence-based approach to the licensing for disposal of dredged material at sea. Assessment of POPs concentrations in River Clyde surface sediments using the Marine Scotland guideline (2011) showed that just five sites exceeded the total PCB Action Level 2 (AL2; 180mgkg–1), which may be defined as being possibly unsuitable for disposal at sea, whereas 23 sites fell between 20 and 180mgkg–1, suggesting further assessment required, and 67 sites fell below Action level 1 (AL1; 20mgkg–1), suggesting the PCBs hosted in the sediments are of no immediate concern. Of those sites that exceeded PCB AL2, all were located within the urban tributaries (Glasgow) away from the main Clyde channel (Fig. 1).
Evaluation against the PAH benchmark (0.1mgkg–1 for the majority of individual PAH) or approximately 1.5mgkg–1 (sum 15 PAH) showed that 35 sites were below AL1 (no concern) and 72 sites were above the benchmark. However, it is rather unclear what this exceedance means in terms of disposal management/site clean-up, partly because no values for the upper criterion (AL2) have been reported and also because coastal and river management typically asses a variety of factors other than chemical concentrations, including bioassays in concert with evaluation of microbenthic community structure.
The Marine Scotland disposal guidelines report a lower AL1 of 0.01mgkg–1 for dibenzo(a,h)pyrene, presumably due to its similarly high toxicity/carcinogenic activity, to BaP, a key PAH considered harmful to human health (Cave et al. Reference Cave, Wragg, Harrison, Vane, Van de Wiele, Nathanail, Ashmore, Thomas, Robinson and Daly2010). In this current study, eight upper Clyde, 11 estuary and 47 urban tributary sites exceeded the dibenzo(a,h)pyrene AL1 value, again suggesting at the very least that further assessment is required prior to disposal/movement.
3. Conclusions
3.1. Distribution of organic pollution
We show that surficial sediments from tributaries of Glasgow city, UK, have higher amounts of POPs (PAH, TPH, PCB) than either the freshwater reaches or inner estuarine Clyde. For example, the urban tributaries had mean sediment concentrations of 124mgkg–1 for PAH, 2279mgkg–1 for TPH and 137μgkg–1 for PCB, compared to lower mean concentrations of 2mgkg–1 for PAH, 68mgkg–1 for TPH and 12μgkg–1 for PCB in upper Clyde and 12mgkg–1 for PAH and 47μgkg–1 for PCB in the inner estuary. This study suggests that Glasgow city has an obvious anthropogenic organic geochemical footprint that is about 60 (PAH), 33 (TPH) and 11 (PCB) times higher, on average, than the rural upper Clyde domain. If PAH, PCB and TPH are given equal weighting in terms of environmental effect, then the overall pattern of organic pollution in the Clyde proceeds in the order urban tributaries > inner estuary > upper Clyde.
3.2. Environmental health effects
A comparison of POPs concentrations to published consensus-based SQGs suggests that sediment in the urban tributaries may have an adverse effect on sensitive biota. We also observed that the majority of sites from the urban tributaries exceed the concentrations recommended for disposal at sea by Marine Scotland. However, it should be borne in mind that these sediments are not from the main channel of the River Clyde and are, therefore, not subject to maintenance and capital dredging activities that typically generate large volumes of sediment containing legacy pollutants. Individual PAH levels, as exemplified by the toxic PAH dibenzo[a,h]pyrene, were found to exceed AL1 in both urban tributary and inner estuary domains.
3.3. Tracking sources of organic pollution
PAHs were observed at every site in the Clyde, confirming their widespread occurrence even in sediments from rural headwaters. The distribution of individual PAH together with isomeric and LMW/HMW ratios confirmed pyrolytic PAH sources comprising mainly petroleum combustion PAH in the upper Clyde and inner estuarine domains. In contrast, PAH distributions and ratios suggested a mixture of petroleum and coal combustion sources in the urban tributaries. The application of BaP/BghiP >0.6 to infer traffic emissions from either diesel and or unleaded vehicle exhaust particulates suggested at face value that the entire river-estuarine continuum received vehicular PAH. However, this interpretation is not entirely proven because high BaP/BghiP values have been reported from burnt moorlands (Vane et al. Reference Vane, Rawlins, Kim, Moss-Hayes, Kendrick and Leng2013). It is, therefore, plausible that the two high BaP/BghiP values in the rural upper Clyde domain are from burning and that the values of between 0.6 and 2 actually represent a mix of both vehicular and burnt moorland vegetation sources (Fig. 8).
A comparison of the extent of PCB chlorination (% homologue groups) showed that the urban tributary sediments were more variable than the upper or estuarine Clyde. We hypothesise that this is either because (1) there are a greater variety of possible PCB sources in the urban setting, which yield a heterogeneous environmental mixture due to the many different formulations used in the original products, or that (2) the microbial and weathering processes acting on PCBs may differ in the freshwater-estuarine domains as compared to the more anoxic urban tributaries.
This study clearly demonstrates that urban tributaries are an important sink and potential source of organic contaminants. Our results imply that a comprehensive understanding of estuarine and, ultimately, coastal and marine sediment pollution chemistry in the Clyde and other UK estuaries that lie within close proximity of a major city can only be achieved by considering the manifold anthropogenic inputs from urban tributaries.
4. Acknowledgements
The Clyde estuary and urban tributary surveys were co-funded by Glasgow City Council (GCC), and boat time was provided by the Scottish Environment Protection Agency and Glasgow Humane Society. The authors would like to thank Paul Mellon, Simon Watson and Donald Linn of GCC for their support. BGS colleagues, Dr Dave Jones, Bob Lister, Sarah Nice and Andreas Scheib are thanked for sample collection, and all student volunteers are thanked for their assistance in sample collection. Diarmad Campbell, project manager of CUSP, is thanked for his support throughout. This paper is published with the permission of the Executive Director of the British Geological Survey (BGS), Natural Environment Research Council (NERC). BGS/NERC reference: PRP17/051.













