Acute pulmonary hypertension and pulmonary hypertensive crisis may result in death if unsuccessfully treated, which is mostly caused by right heart failure and diminished left ventricular preload that compromise cardiac output and systemic perfusion.Reference Hill, Roberts and Preston1 Specific pharmacotherapy that mostly act as vasodilators to the pulmonary artery are widely used in addition to basic intensive care setting measures for cases of acute pulmonary hypertension. The standard use of inhaled nitric oxide for the treatment of post-operative pulmonary hypertension may be questioned by conflicting evidences, side effects, and the lack of feasibility in settings with limited resources.Reference Bizzarro, Gross and Barbosa2–Reference Chester, Yacoub and Moncada5 Therefore, the use of alternative agents such as inhaled nitroglycerin, an inactivated form of nitric oxide, is worth considering.Reference Bando, Ishii and Kitamura6 This article is aimed to review available data on the use of nitroglycerin inhalation in the acute treatment of pulmonary hypertension in children with CHD, as well as its potential benefit in the post-operative setting.
Treatment of acute pulmonary arterial hypertension
Treatment of acute pulmonary hypertension involves several basic measures in intensive care settings. These include general treatments such as oxygen supplementation, alkalisation, vasopressor, fluid management, muscle relaxant, sedation, intubation, and mechanical ventilation.Reference Kaestner, Schranz and Warnecke7–Reference Brunner, Perez and Richter9 Treatment of acute pulmonary hypertension also includes the use of specific pharmacotherapy that works mainly by causing vasodilation in the pulmonary vessels.Reference Suesaowalak, Cleary and Chang10–Reference Zamanian, Haddad and Doyle12
Specific pharmacotherapy that could be used in the treatment of acute pulmonary hypertension includes exogenous nitric oxide, prostanoids (prostacyclin analogues), and phosphodiesterase type 5 inhibitors. These agents work by inhibiting the contraction of smooth muscle cells in the pulmonary artery, hence reducing pulmonary arterial pressure and right ventricular afterload.Reference Kaestner, Schranz and Warnecke7,Reference Suesaowalak, Cleary and Chang10
Intravenous prostacyclin (prostaglandin I2) analogues such as alprostadil and epoprostenol could be used to treat acute pulmonary hypertension. Studies showed that both the use of intravenous alprostadil and prostaglandin E1 is associated with mean pulmonary arterial pressure reduction and lower mortality of post-operative pulmonary hypertension in CHD patients.Reference Dong, Ma and Ma13,Reference Kermode, Butt and Shann14 Chronic use of intravenous epoprostenol was shown to be beneficial for pulmonary hypertension in CHD patients. However, its benefits are mostly based on case reports.Reference Brunner, Perez and Richter9,Reference Yamauchi, Yamaki and Fujii15,Reference Frost, Quinones and Zoghbi16
Intravenous prostacyclin analogs lower systemic vascular resistance, and it gives rise to the possibility of requiring systemic vasopressors. In addition, intravenous prostacyclin can only be used in cases without severe ventilation perfusion mismatch as it may worsen the condition. Inhaled agents, in contrast, work more specifically to pulmonary vessels. Therefore, inhaled agents including aerosolised prostanoids such as alprostadil, epoprostenol, and iloprost, as well as aerosolised nitric oxide are widely used in the treatment of acute pulmonary hypertension.Reference Kaestner, Schranz and Warnecke7,Reference Brunner, Perez and Richter9
Inhaled nitric oxide has long been accepted as the standard therapy for post-operative pulmonary arterial hypertension as recommended by the 2004 European expert consensus.Reference Abman, Hansmann and Archer17–Reference Germann, Braschi and Della20 Haemodynamic improvement and better clinical outcome with inhaled nitric oxide use on post-operative pulmonary hypertension cases undergoing surgical repair for CHD have been shown by a number of randomised controlled trials.Reference Russell, Zwass and Fineman21–Reference Journois, Baufreton and Mauriat23 A 2014 meta-analysis involving four randomised trials, however, concludes no significant difference in mortality, incidence of pulmonary hypertensive crisis, mean pulmonary arterial pressure, and mean arterial pressure. The meta-analysis also recognised the potential for increased methaemoglobin levels in nitric oxide-inhaled patients, despite questionable clinical importance.Reference Bizzarro, Gross and Barbosa2 Another study, a retrospective cohort published in 2019, shows that inhaled nitric oxide is not associated with significantly lower length of stay in children undergoing cardiac surgery.Reference Wong, Loomba and Evey3
The high cost of inhaled nitric oxide administration via currently available technology is another limitation of the therapy. Rebound pulmonary hypertension following the rapid withdrawal of inhaled nitric oxide is another potential issue with the use of this therapy. Conflicting evidence on the use of inhaled nitric oxide and its high cost challenge the use of inhaled nitric oxide as the standard therapy for post-operative pulmonary hypertension. Therefore, an alternative to inhaled nitric oxide treatment is needed, particularly in settings with limited resources.Reference Hill, Preston and Roberts4,Reference Chester, Yacoub and Moncada5
Nitroglycerin inhalation
Inhalation of nitroglycerin is a possible alternative to the use of inhaled nitric oxide. It is a vasodilator that works in a similar fashion to inhaled nitric oxide, as nitroglycerin is basically in its inactivated form. Activation of nitroglycerin involves the role of aldehyde dehydrogenase-2 that catalyses the nitric oxide-donating mechanism. Nitric oxide donated from nitroglycerin, in turn, works by activating cyclic guanosine monophosphate-dependent signalling pathways in the smooth muscles of pulmonary vessels, allowing selective vasodilation of pulmonary circulation.Reference Bando, Ishii and Kitamura6
The former intravenous route of nitroglycerin administration has long been known to cause vasodilation that is beneficial for the treatment of pulmonary hypertension. A study by Ilbawi et al in 1985 showed that intravenous administration of nitroglycerin reduces pulmonary vascular resistance, an effect observed to a significantly (p < 0.001) higher degree in those with elevated pulmonary arterial pressure compared to those with normal pulmonary arterial pressure. However, the study also reported a significant decrease of systemic vascular resistance with the use of a higher dose, which according to the study, is the dose that produces significant clinical effects such as cardiac index and pulmonary arterial pressure improvement.Reference Ilbawi, Idriss and DeLeon24 A previous study by Pearl et al in 1983 also showed significant systemic haemodynamic effect with the use of intravenous nitroglycerin. In addition to significant (p < 0.01) increase of stroke volume by 40%, cardiac index by 40%, decrease of pulmonary vascular resistance by 40%, and mean pulmonary arterial pressure by 15%, intravenous nitroglycerin also cause a comparable systemic effect, namely the reduction of systemic vascular resistance by 37% and systemic mean arterial pressure by 15%.Reference Pearl, Rosenthal and Schroeder25 The considerable systemic effect that was shown by intravenous nitroglycerin use made it necessary for inhalation to be considered as an alternative route of administration.Reference Omar, Gong and Sun26–Reference Singh, Choudhury and Saxena28
Adult studies on the use of nitroglycerin inhalation for acute treatment of pulmonary hypertension were all mostly in perioperative settings, namely corrective surgery of valvular defects. Significant decrease in mean pulmonary arterial pressure and pulmonary vascular resistance with nitroglycerin inhalation was shown in all of these studies. However, they were excluded from further discussion in this critical appraisal that is aimed to focus on pulmonary hypertension in paediatric CHD cases.Reference Yurtseven, Karaca and Kaplan29–Reference Fikry, Ramadan and Elhadedy32
Paediatric studies on the use of nitroglycerin inhalation for acute treatment of pulmonary arterial hypertension in CHD are limited. Between the year 1999 to recently, only three studies were done on paediatric population, namely the study of Omar et al in 1999, Goyal et al in 2006, and Singh et al in 2010 (Table 1). None of the studies on children was conducted in a perioperative setting, but rather in catheterisation laboratory. The study by Omar and Goyal et al were both done on uncorrected cases of the ventricular septal defect, while the Singh et al study was done on uncorrected cases of the partial atrioventricular septal defect and patent ductus arteriosus (Table 1).Reference Omar, Gong and Sun26–Reference Singh, Choudhury and Saxena28
Table 1. Characteristics of studies on the use of inhaled nitroglycerin for the acute treatment of pulmonary hypertension in paediatric CHD
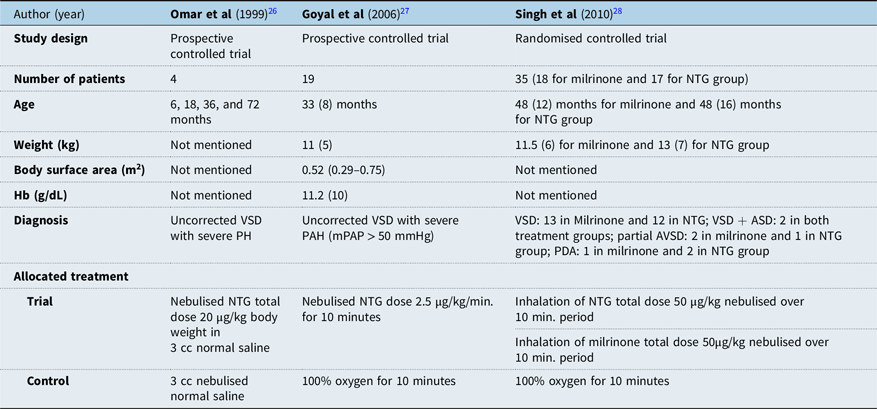
ASD = Atrial septal defect; NTG = Nitroglycerin; PAH = Pulmonary arterial hypertension; PH = Pulmonary hypertension; VSD = Ventricular septal defect.
Only one of the three paediatric studies was a randomised control trial (Table 1). The other studies involved paired samples without allocation of patients into trial and control group. None of the three articles mentions an effort of blinding and objective measurements. However, it can be generally observed that the later studies made improvements in their methods compared to prior studies. Earlier study by Omar et al in 1999 did not mention the equality of treatments outside the control and trial treatment, rendering it open to question on validity. In contrast, later studies by Goyal et al in 2006 and Singh et al in 2010 provided information regarding equal treatments aside from those allocated for trial such as the use of anaesthesia and sedation. The more recent study by Singh et al in 2010 improved its study design to a randomised controlled trial, randomising patients into treatment groups as compared to using paired data as shown in the study of Omar et al in 1999 and Goyal et al in 2006.Reference Omar, Gong and Sun26,Reference Goyal, Kiran and Chauhan27 The study of Singh et al also explained randomisation method in sufficient detail, mentioning the use of computer-generated random numbers that were kept in sealed envelopes (Table 2).Reference Singh, Choudhury and Saxena28
Table 2. Critical appraisal of studies on the use of inhaled nitroglycerin for the acute treatment of pulmonary hypertension in paediatric CHD

DAP = Diastolic arterial pressure; HR = Heart rate; IM = Intramuscular; MAP = Mean arterial pressure, mPAP = Mean pulmonary arterial pressure; NTG = Nitroglycerin; PAP = Pulmonary arterial pressure; PCWP = Pulmonary capillary wedge pressure; PVR = Pulmonary vascular resistance; SAP = Systolic arterial pressure; SVR = Systemic vascular resistance.
Significant decrease of mean pulmonary arterial pressure compared to pre-NTG inhalation value was observed in all of the studies (Table 3). The largest decrease is shown by the study of Omar et al in 2003Reference Omar, Gong and Sun26 from 47 ± 4 mmHg to 38 ± 4 mmHg (p = 0.005). This is followed by Goyal et alReference Goyal, Kiran and Chauhan27 that showed a decrease from 63.2 (8.1) mmHg to 55.1 (8.9) mmHg (p < 0.001). The slightest decrease is shown by Singh et alReference Singh, Choudhury and Saxena28 from 61.2 ± 16.1 mmHg to 57.1 ± 13.2 mmHg (p < 0.05). The study of Omar et al, which showed the highest fall of mPAP, compared inhaled NTG with 3 cc nebulised normal saline, while the study of Goyal et al and Singh R et al used 100% oxygen therapy for 10 minutes.
Table 3. Results of studies on the use of inhaled nitroglycerin for the acute treatment of pulmonary hypertension in paediatric CHD
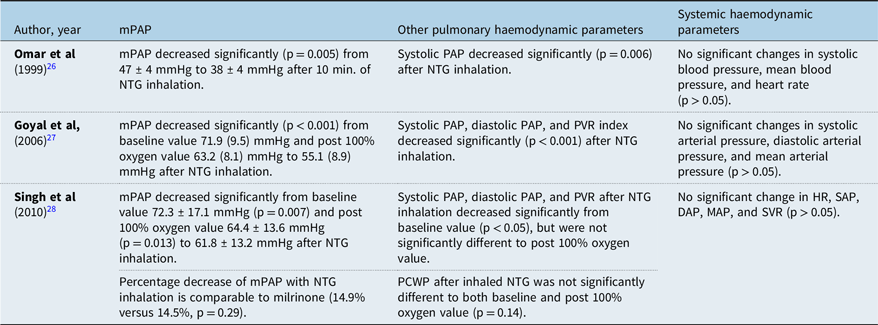
DAP = Diastolic arterial pressure; HR = Heart rate; IM = Intramuscular; MAP = Mean arterial pressure; mPAP = Mean pulmonary arterial pressure; NTG = Nitroglycerin; PAP = Pulmonary arterial pressure; PCWP = Pulmonary capillary wedge pressure; PVR = Pulmonary vascular resistance; SAP = Systolic arterial pressure; SVR = Systemic vascular resistance.
The dose of inhaled NTG does not seem to show a clear correlation with the degree of mPAP change in the studies. It can be observed that Omar et al used the lower dose of 20 μg/kg, but showed higher mPAP change compared to Singh et al that used 50 μg/kg (Table 3). The relationship between NTG dose and the degree of mPAP change, as well as the appropriate dosing, thus require further study.Reference Omar, Gong and Sun26–Reference Singh, Choudhury and Saxena28
Inhalation of nitroglycerin also affects other haemodynamic measurements besides mean pulmonary arterial pressure. Both the studies of Goyal et al and Singh et al are consistent regarding the reduction of pulmonary vascular resistance after nitroglycerin inhalation, while the earlier study of Omar et al did not provide observation on pulmonary vascular resistance (Table 3). Systemic haemodynamic values, such as heart rate and mean arterial pressure, consistently showed no significant change after nitroglycerin inhalation in all studies. However, data from these paediatric studies were obtained over a very brief period of time in the catheterisation laboratory. Information regarding the frequency of dosing over time, potential for tachyphylaxis, rebound phenomenon, side effects with repeated dosing, and other important aspects if inhaled nitroglycerin was to be used in post-operative period remains unavailable.Reference Omar, Gong and Sun26–Reference Singh, Choudhury and Saxena28
Conclusion
Following our review of the literature, we concluded that nitroglycerin inhalation can reduce pulmonary arterial pressure in uncorrected paediatric cases of CHD during catheterisation procedures. Despite the absence of data supporting improved clinical outcomes in comparison to standard medication such as nitric oxide inhalation and other treatments such as iloprost or milrinone inhalation, the use of inhaled nitroglycerin is worth considering when the availability of the aforementioned medications are limited. Results from studies on uncorrected paediatric CHD cases, supported by similar observations in adult post-operative studies, suggest the potential benefits of nitroglycerin inhalation for post-operative CHD cases in children. Further study on the clinical outcomes of nitroglycerin inhalation such as mortality and length of stay, the appropriate dosing, and its potential benefit for post-operative pulmonary arterial hypertension in paediatric CHD is therefore needed.
Acknowledgements
The authors would like to thank Steven M. Schwartz, MD, Pediatric Cardiac ICU, Hospital for Sick Children, University of Toronto, Ontario, Canada for his support during the writing of this manuscript,
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of interest
None.
Ethical statement
The writing of this review did not involve any action that may risk ethical violation.






