Introduction
The phenomenon of intense nutrient enrichment of habitat caused by bird colonies is well known and documented (Sobey & Kenworthy Reference Sobey and Kenworthy1979; Ishida Reference Ishida1996; Ligeza & Smal Reference Ligeza and Smal2003; Ellis Reference Ellis2005; Laiviņš & Čekstere Reference Laiviņš and Čekstere2008). It has been thoroughly studied and described in the case of bird colonies affecting soil and flora of treeless oceanic and sea islands and seashores in various regions of the world (Breslina & Karpovich Reference Breslina and Karpovich1969; Sobey & Kenworthy Reference Sobey and Kenworthy1979; Hogg & Morton Reference Hogg and Morton1983; Hogg et al. Reference Hogg, Morton and Venn1988; Paradis & Larenzoni Reference Paradis and Larenzoni1996; Norton et al. Reference Norton, Delange, Garnock-Jones and Given1997; Vidal et al. Reference Vidal, Médail, Tatoni, Roche and Vidal1998; Abbot et al. Reference Abbot, Marchant and Cranfield2000; Ellis Reference Ellis2005). However, despite the commonness of bird colonies in trees, only very few papers discuss the impact of nesting on forest ecosystems (Olech Reference Olech1990; Maesako Reference Maesako1991; Ishida Reference Ishida1996; Mun Reference Mun1997; Laiviņš & Čekstere Reference Laiviņš and Čekstere2008; Żółkoś & Meissner Reference Żółkoś and Meissner2008, Reference Żółkoś and Meissner2010; Adamonytė et al. Reference Adamonytė, Iršėnaitė, Motiejūnaitė, Taraškevičius and Matulevičiūtė2013) and no study has been undertaken on the influence of such colonies on epiphytic lichen biota.
Lichens are very sensitive to environmental changes, caused by natural factors as well as human impact, especially the increase or decrease in air pollution (van Dobben Reference van Dobben1996; Rusu et al. Reference Rusu, Jones, Chimonides and Purvis2006; Wolseley et al. Reference Wolseley, James, Theobald and Sutton2006, Reference Wolseley, Sutton, Leith and van Dijk2010; Motiejūnaitė Reference Motiejūnaitė2007; Sparrius Reference Sparrius2007; Otnyukova & Sekretenko Reference Otnyukova and Sekretenko2008; van Herk Reference van Herk2009; Olsen et al. Reference Olsen, Berthelsen, Andersen and Søchting2010). However, little has been published on the influence of bird colonies on the occurrence and species composition of lichen biota, and what has been published focused mainly on the detection of changes in thallial growth caused by the deposition of bird droppings or the development of ornithocoprophilous lichen communities on rocks (e.g. Grönlie Reference Grönlie1948; Barkman Reference Barkman1958; Armstrong Reference Armstrong1984, Reference Armstrong1994, Reference Armstrong2000; Seppelt et al. Reference Seppelt, Broady, Pickard and Adamson1988; Olech Reference Olech1990; Valladares & Sancho Reference Valladares and Sancho1993; Nash Reference Nash2008). The impact of bird colonies on the epiphytic lichen biota does not appear to have been studied previously, although some research has focused on myxomycetes in the great cormorant colony in Lithuania (Adamonytė et al. Reference Adamonytė, Iršėnaitė, Motiejūnaitė, Taraškevičius and Matulevičiūtė2013).
When studying the distribution of the grey heron (Ardea cinerea) in Poland and its influence on the plant vegetation within its colonies (Żółkoś & Markowski Reference Żółkoś and Markowski2006; Żółkoś & Meissner Reference Żółkoś and Meissner2008), considerable changes in the lichen composition of epiphytic communities in most of the investigated stands were observed. In two cases it was clear that the lichen biota changed not only on the trees inhabited by birds, but also on those which had been abandoned a few years before. The bird colonies studied at both localities were established in the same type of pine forest and thus a more detailed investigation on the impact of nutrient enrichment derived from bird excrement on epiphytic biota was undertaken.
In this paper, the substantial modifications in the species composition and abundance of lichens on Scots pine caused by the deposit of bird droppings, as well as the changes after the cessation of that process, are described.
Material and Methods
Study area
In northern Poland, 33 of 76 breeding colonies of grey heron were settled within the Scots pine tree stands (Żółkoś et al. Reference Żółkoś, Meissner, Kalisiński, Górska, Melin, Ibron and Wysocki2010). All of them were visited, but only two colonies could be clearly divided into two parts: 1) an area with a currently functioning colony and 2) a fragment abandoned by birds 2–3 years earlier. Therefore, only those two colonies of grey heron near Przesieki (53°00′59″N, 15°58′59″E) and Skrzeszewo (54°24′39″N, 17°49′29″E) villages (both in the Pomerania region) were taken into consideration. Both colonies are located at the periphery of the pine tree stands planted in post-agricultural grounds, on slightly inclined slopes with southern (Skrzeszewo) and south-western (Przesieki) exposure.
In Skrzeszewo, the major part of the tree stand consisted of 40-year-old pines, but a group of c. 90-year-old pines mixed with oaks was also present at the margin of the existing colony. The trees studied were separated from the adjacent meadow by an undergrowth of oaks and rowans. Herons firstly inhabited only the group of older pines, but when the younger ones reached a height similar to existing pines, birds also used them for nesting. In 2008 the colony comprised of 24 nests.
In Przesieki, the whole tree stand was c. 40 years old. Herons initially inhabited trees growing on the upper part of the slope, and then subsequently inhabited other trees further down. There were 57 nests observed in 2007.
Methods
The research was carried out in the late summers of 2007 and 2008. The age of the Scots pines in both colonies was established by counting annual growth rings of wood collected with the aid of a Pressler drill.
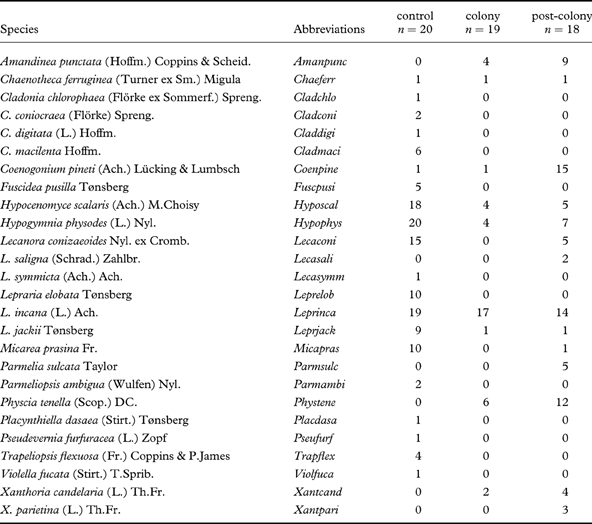
In both forest complexes, three plots were marked out: 1) a control area, 2) a colony (with currently functioning nests) and 3) a post-colony. In each of these, 10 pines were randomly chosen. In Przesieki, one of the trees investigated in the colony plot was excluded from analyses as it did not support any epiphytic lichens; in Skrzeszewo, within the post-colony area, only 8 trees had been inhabited by grey heron in the past. All lichen taxa on the selected trees were listed, from the trunk base to a height of 2 m, and the cover of each species was estimated according to the following 5-point scale: 1=<1%, 2=1–10%, 3=11–30%, 4=31–50% and 5=>50%.
No lichen species found on the trees studied reached the fifth cover degree, and on one tree in the colonized area in Przesieki no lichen was observed at all. Most of the lichen species were noted directly in the field, but for some taxa (e.g., Fuscidea pusilla, Lepraria spp.) it was necessary to collect samples for determination by thin-layer chromatography (TLC) according to Orange et al. (Reference Orange, James and White2001). Micarea prasina was regarded in a wide sense.
Habitat conditions were characterized using the indicator values estimated for lichen species by Wirth (Reference Wirth2010), and according to the method of ecological indicator values developed for vascular plants by Ellenberg (Reference Ellenberg1992). The indicator values are a suitable tool for quantitative estimation of environmental changes, as shown primarily for vascular plants (e.g., Schaffers & Sýkora Reference Schaffers and Sýkora2000; Żółkoś & Meissner Reference Żółkoś and Meissner2008), but also for lichens (Berthelsen et al. Reference Berthelsen, Olsen and Søchting2008).
Multivariate analysis was performed to explore relationships among species, samples representing three distinguished investigated areas and the environmental variables. Firstly, Detrended Correspondence Analysis (DCA) was used to identify the length of the ordination axis. The gradient length of the first axis was 3·86 SD and this justified the use of Canonical Correspondence Analysis (CCA) (Jongman et. al. Reference Jongman, ter Braak and van Tongeren1995) to characterize variation among the 26 lichen species in 57 samples constrained by 4 traits. Values of the 5-point cover scale were treated as the species data. For each sample, the weighted average values of Wirth's (Reference Wirth2010) indicators for moisture (F), light (L), eutrophication (N) and reaction (acidity) (R) were calculated. In the CCA, apart from Wirth's indicator values used as environmental variables, information about three investigated areas (colony, post-colony and control) was also included as supplementary variables. The significance of the environmental variables for explaining variation in species distribution was tested using a Monte-Carlo permutation test with 499 unrestricted permutations. Multivariate analysis was conducted to identify traits used in CCA, of which absolute t-values > 2·1 were used to indicate important canonical coefficients (ter Braak & Šmilauer Reference ter Braak and Šmilauer2002). Significant differences among samples within the three areas were detected by a one-way ANOVA and the Tukey post-hoc test, with the use of sample scores for the first canonical axis. Data were square-root transformed to normalize variables and a constant value of 3 was added to bring minimum values after transformation to above zero. Thus, the transformation followed the equation: x′=√(x+3), where x is the original value and x′ the value after transformation. All tests for significance were set at P<0·05.
In order to demonstrate the diversity of lichen species composition in the defined areas, the DCA analysis, with a 5-degree scale of species abundance used as species data and the three investigated areas as supplementary variables, was performed.
The analyses were carried out using CANOCO ver. 4.5 (ter Braak & Šmilauer Reference ter Braak and Šmilauer2002), while other calculations were performed in Statistica software ver. 10.
Results
The epiphytic lichen biota of the localities studied was diverse. In total, 26 taxa were noted. The number of taxa found on each plot are presented in Figure 1.
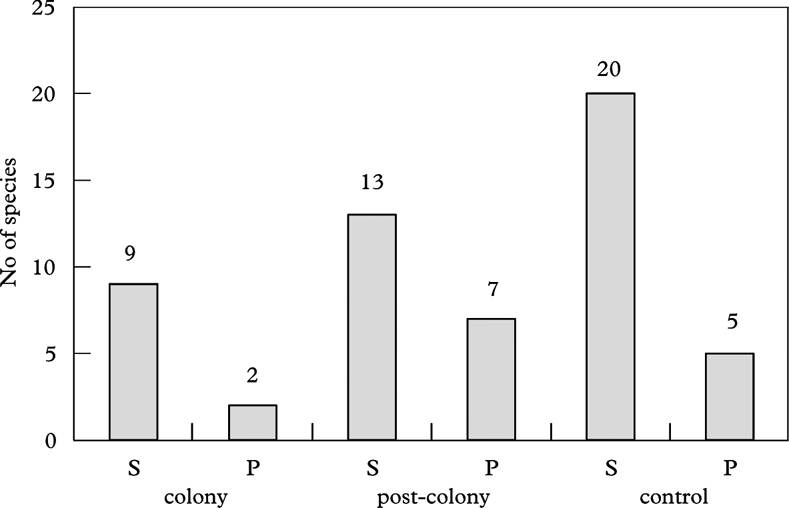
Fig. 1. Number of species within particular plots in Skrzeszewo (S) and Przesieki (P).
Ordination of samples in the CCA diagram (Fig. 2) shows the differences in the lichen biota inhabiting particular trees in the three areas studied. The first four ordination axes accounted for 90% of the variation in the species versus environment relationship. The overall inertia was 2·811. Eigenvalues of the axes show that the first and second axes had the greatest input in the variation (66%) of the data set analyzed (eigenvalues of the first and second axes are 0·55 and 0·22, respectively).
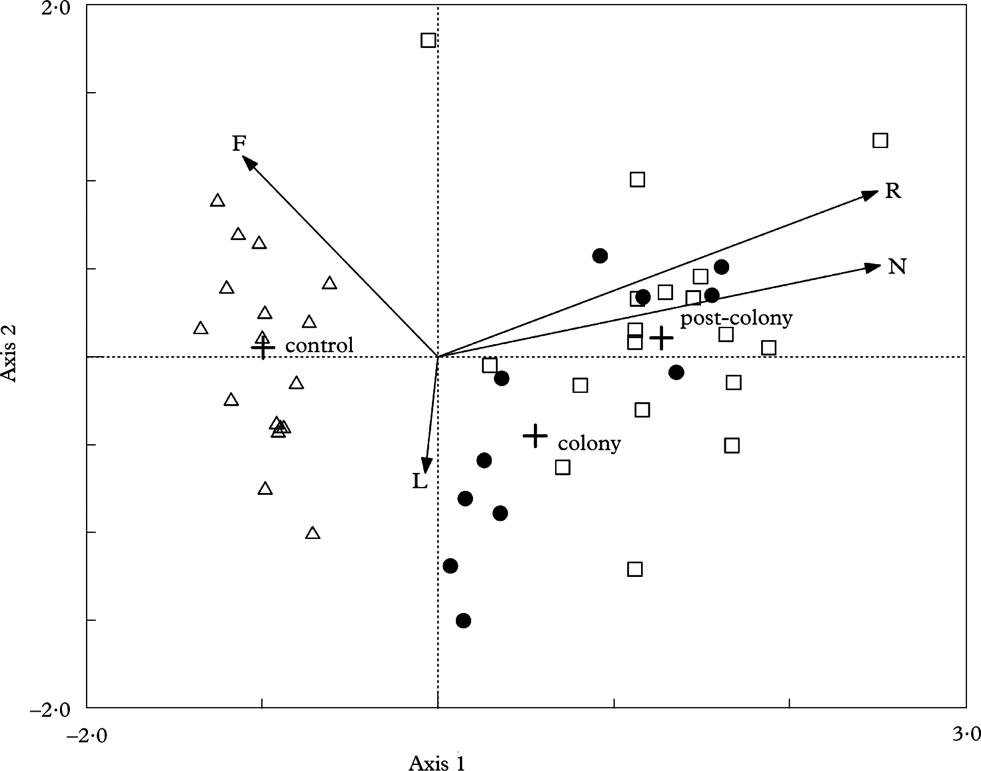
Fig. 2. Samples and environmental variables on the biplot of canonical correspondence analysis (CCA) of the first and second axes. The symbols represent plots: •, colony; □, post-colony; △, control. Arrows represent supplementary variables (Wirth's indicators): F=moisture, L=light, N=eutrophication (nitrogen), R=reaction (acidity).
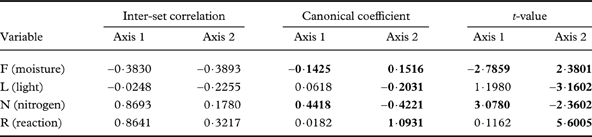
The first ordination axis shows a strong positive correlation with the nutrient-enrichment and a negative correlation with the moisture indicator. The second axis has the strongest positive correlation with the moisture indicator and negative correlation with light (Table 1). A Monte-Carlo permutation test confirmed that the first canonical axis (F=0·55, P=0·002) and the overall model (F=5·93, P=0·002) were statistically significant. Moreover, the averages of sample scores in the defined groups (control, colony and post-colony) differed significantly (ANOVA; F 2,54=86,98; P<0·0001), and these differences were statistically significant among all groups (Tukey post-hoc test; P<0·04 in all cases).
Table 1. Statistics for Wirth's values used in the detrended correspondence analysis (DCA) of the first and second axes. Bold values indicate variables with significant correlation and canonical coefficients (t-value >2·1).
Samples from the colony and post-colony areas are grouped to the right of the CCA diagram, in the range of highest values of reaction and fertility, while trees from both control areas are recorded on the left-hand side of the diagram.
Ordination of samples in the DCA diagram (Fig. 3) points at changes in the lichen biota inhabiting particular trees in the three plots. Four ordination axes explain 28% of the total variation in the data set. Eigenvalues of the axes show that the first and second axes had the greatest input in the explanation of variation in the data set analyzed (eigenvalue of the first axis is 0·62 and of the second is 0·20). The first one, placed on the left-hand side of the diagram, contains taxa connected with the post-colony and colony plots. These are nitrophytes, such as Physcia tenella and Xanthoria spp. (van Herk Reference van Herk1999), as well as species typical for deciduous trees, such as Parmelia sulcata and Amandinea punctata (however, excluding Coenogonium pineti, which can occur on pines but grows in strongly shaded places) (Fig. 3, Appendix 1). The opposite group consists of species associated with pines. These are acidophytes such as Lepraria jackii, L. elobata, Parmeliopsis ambigua and Pseudevernia furfuracea. Between those two groups there are taxa found within all three areas studied (control, post-colony and colony), although with varied abundance. Among them, the most frequent are Hypogymnia physodes and Hypocenomyce scalaris. The species most commonly occurring in the whole study area is Lepraria incana (Appendix 1).
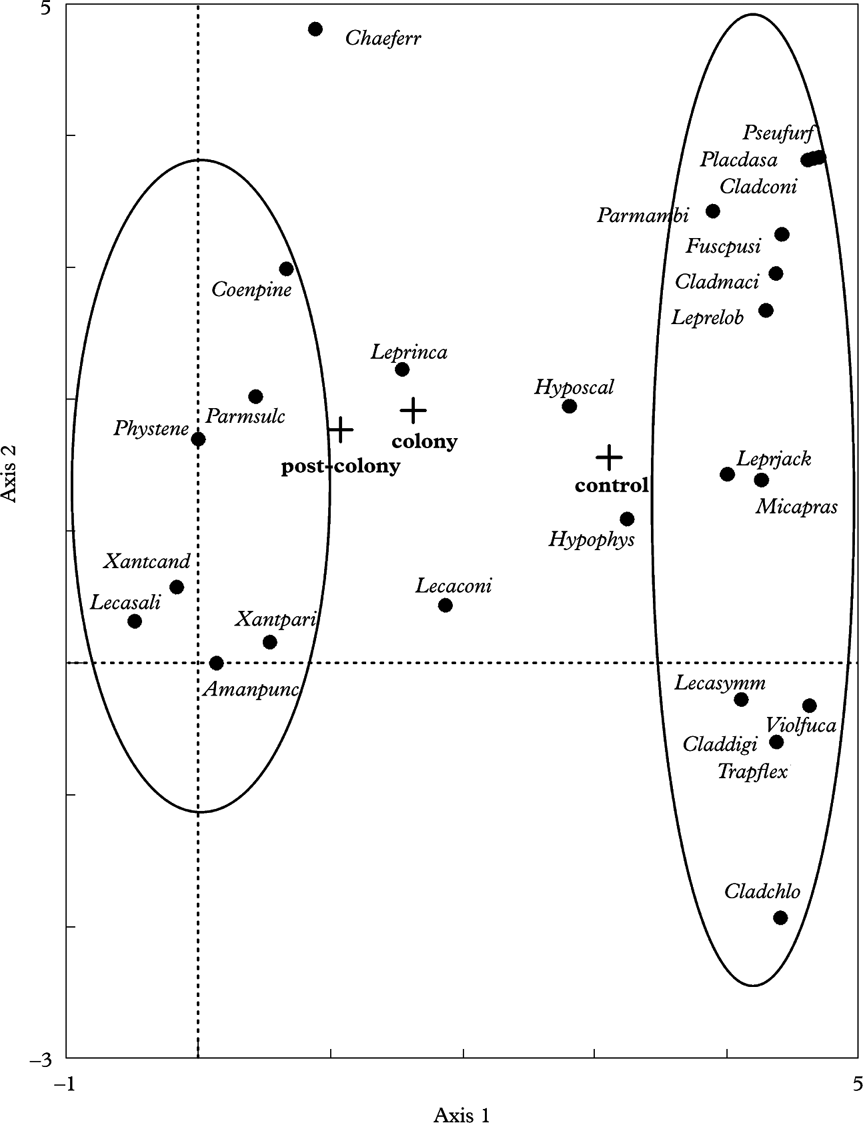
Fig. 3. Lichen species on the diagram of detrended correspondence analysis (DCA) of the first and second axes. The circle on the left-hand side encloses nitrophytes and that on the right-hand side encloses acidophytes. Abbreviations of species names are given in Appendix 1.
Discussion
The results of multivariate analyses confirm that changes in the lichen biota inhabiting pine trunks observed in the functioning colony and post-colony plots, compared to the control plots, were connected with the direct and indirect influences of the colonies. Heron droppings falling from the tree crowns and flowing down the trunks cause changes in the acidity and fertility of the tree bark (Adamonytė et al. Reference Adamonytė, Iršėnaitė, Motiejūnaitė, Taraškevičius and Matulevičiūtė2013), resulting in the appearance of nitrophyte species and lichen epiphytes which occur typically on deciduous trees.
The biodiversity of lichens within the functioning colony area was always the lowest and their cover was minimal, compared to the other areas. This was connected with the change in habitat conditions, and the elimination of lichen thalli could be the direct effect of the toxic influence of bird excrement. The main factor was most probably the urea, which is a highly toxic nitrogen product that generally inactivates proteins by affecting hydrogen bonds, but also the ammonia level and bark pH influence the lichen biota (Nash Reference Nash2008 and literature cited therein). Only some lichens, such as Xanthoria parietina (Gaio-Oliveira et al. Reference Gaio-Oliveira, Dahlman, Palmqvist and Máguas2004), are able to survive in such highly modified habitats due to their high urease activities (Rai Reference Rai and Galun1988) and their tolerance of high concentrations of nitrogen substances; this explains the occurrence of such lichens in the post-colony plots.
Differences in the lichen species composition between functioning colonies might be explained by the fact that the rate and quality of changes to the ecosystem caused by the colony are connected not only with the species of nesting bird, but also with the density of nests and the age of the colony (Sobey & Kenworthy Reference Sobey and Kenworthy1979; Maesako Reference Maesako1991; Ishida Reference Ishida1996; Mun Reference Mun1997; García et al. Reference García, Marañón, Ojeda, Clemente and Redondo2002; Żółkoś & Meissner Reference Żółkoś and Meissner2008, Reference Żółkoś and Meissner2010). This probably also applies to the lichen biota studied. The lower number of taxa growing in Przesieki than in Skrzeszewo could be connected with higher nest density and, in consequence, a stronger influence of the colony.
In the post-colony plots of both localities, the most frequent species were from nutrient-rich habitats (nitrophytes), which appear as the result of still high nutrient enrichment and less acidic bark. On the other hand, the occurrence of species typically occurring on pines (acidophytes) in both post-colony plots might be connected with the termination of the bird droppings on trunks, the dilution and flush of accumulated substances by the superficial flow of rain, as well as by the disappearance of the modified fragments of bark, which have peeled from the trees. In such places, conditions on the trunks were similar to those on trees never inhabited by herons, and probably for that reason lichens typical for pine appeared.
The DCA clustered some species in between the groups of acidophytes (the right-hand side of the diagram) and nitrophytes (the left-hand side of the diagram). All those taxa are considered as acidophytes (van Herk Reference van Herk1999), and in the localities studied they occur in two or three plot types (i.e. colony, post-colony and control), although usually with a different frequency. The only species common to all the plots studied at both localities is Lepraria incana, which has a relatively wide habitat amplitude and often grows on pines (Kukwa Reference Kukwa2006). In the control area it is a typical taxon, while its occurrence in the colony and post-colony areas is probably not a result of its resistance to excrement deposition. Lepraria incana, as many other representatives of that genus, occurs in habitats not directly exposed to rain or water-flow down the trunk (e.g. Laundon Reference Laundon1992). This lichen species grows in bark fissures, and thus its occurrence in the colony and post-colony areas is probably the result of its survival in places unchanged by the heron droppings. Hypogymnia physodes, Hypocenomyce scalaris and Lecanora conizaeoides were also commonly recorded, but the last was absent in the colony plots. Chaenotheca ferruginea was found in all three plot types, but in each case only on single trees. Their occurrence in the control plot is not surprising, and the presence in other plots can be explained only by reasons stated in the previous paragraph.
There are many examples in the literature describing the bark or soil enrichment in phytocoenoses influenced by bird colonies, as well as showing qualitative and quantitative changes in the flora of vascular plants; that is, significantly higher numbers of nitrophilous species, nitrophytes and species of wide ecological amplitude (Sobey & Kenworthy Reference Sobey and Kenworthy1979; Bukaciński et al. Reference Bukaciński, Rutkowska and Bukacińska1994; Ishida Reference Ishida1996; Mun Reference Mun1997; Laiviņš & Čekstere Reference Laiviņš and Čekstere2008; Żółkoś & Meissner Reference Żółkoś and Meissner2008). Bird colonies also affect community structure, shown by the occurrence of a highly dense shrub layer (Żółkoś & Meissner Reference Żółkoś and Meissner2008; Żółkoś et al. Reference Żółkoś, Meissner, Olszewski and Remisiewicz2013). This indirect impact of strong shading of pine trunks explains the common appearance of the hygrophilous and shade-preferring Coenognium pineti (see Wirth Reference Wirth2010) in the post-colony areas.
To conclude, grey heron colonies have a significant impact on the epiphytic lichen biota associated with Scots pine. Firstly, the number of taxa and their coverage decreases, as species typical of Scots pine disappear, and those with wide ecological amplitude remain; next, the nitrophilous species untypical for pines appear. After a bird colony is abandoned, taxa indicating strong local deacidification and nutrient enrichment of the bark start to occur. The colonization of trunks by species typical for pines in the post-colony plots is also observed, which is probably connected with the disappearance of the modified bark. Furthermore, the indirect influence of the colonies caused by changes in the forest structure due to the appearance of a shrub layer, favours the appearance of shade-preferring species.
Changes in lichen biota of the colony and post-colony areas in Skrzeszewo and Przesieki were very similar to each other. One may assume that such a model of the influence of a grey heron colony on epiphytic lichen biota of Scots pines is a recurrent phenomenon elsewhere.
The authors would like to thank Joanna Bloch-Orłowska for the translation of the manuscript, Professor Mark Seaward for making valuable linguistic corrections, as well as Włodzimierz Meissner and two anonymous reviewers for the very helpful comments on the manuscript.
Appendix 1
List of epiphytic lichen species and their abundance in three plot types (colony, post-colony and control area) in the bark of trees in studied areas in Poland. Abbreviations are those used in the detrended correspondence analyses (DCA).