Postural orthostatic tachycardia syndrome, a dysautonomia responsible for profound dizziness and disability associated with activities of daily living, is estimated to occur in 500 000 to 3 000 000 people in America.Reference Robertson 1 , Reference Mar and Raj 2 However, for the amount of disease burden, this syndrome is treated by relatively few providers in America. 3 At the Children’s Hospital of Philadelphia, increasing numbers of patients presented to our diagnostic centre. With the addition of a paediatric cardiologist with experience in managing patients with postural orthostatic tachycardia syndrome in 2007 (J.R.B), the number of these patients referred to our Division of Cardiology increased significantly. A formal programme dedicated to the care of patients with postural orthostatic tachycardia syndrome was subsequently launched in January 2014. After 18 months, a retrospective review to determine specific demographic trends was undertaken to better understand the characteristics of this patient population. However, documentation of care for these patients previously managed through the Cardiology clinic since 2007 was also included to the extent that data regarding their care were able to be extracted from the medical record. The total volume of patients quickly escalated, and it was decided to further evaluate and characterise these data to be able to share our findings.
Materials and methods
Patients aged 18 years and under at the time of their initial evaluation at the Postural Orthostatic Tachycardia Syndrome Program at the Children’s Hospital of Philadelphia were evaluated. Patients were included if they were initially diagnosed between November 2007 and June 2016. They were evaluated by one of two paediatric cardiologists knowledgeable about postural orthostatic tachycardia syndrome. All data abstracted were derived from the electronic health record into a database, either manually or automatically from the electronic chart. These data included initial and subsequent symptoms, potential initiating factors, age at diagnosis, gender, time to diagnosis, associated diagnoses, and heart rate while supine, as well as during a 10-minute standing test. All of these data were obtained by the evaluating provider as part of the medical evaluation. Initial symptoms were defined as the first symptom or abnormal finding noticed by the patient and/or family, whereas subsequent symptoms were defined as those symptoms that presented later at any time after onset of the initial symptom but before the diagnosis. For this data evaluation, the term “dizziness” was synonymous with light-headedness, and used interchangeably by providers and patients, but was distinguished from vertigo by history. Symptoms were assessed using a checklist in the electronic health record in patients from 2014 forward; symptoms in patients before this date were assessed by the provider asking a series of specific questions that were later to become the electronic checklist. The standing test was supervised by the physician. The patient was supine for at least 3 minutes, at which time a resting pulse rate was obtained. Afterwards, the patient actively stood and was told to then stand motionless for 10 minutes. The patient was not allowed to hold onto any objects for support. The heart rate was obtained once per minute for 10 minutes, or less, if the patient became too symptomatic to maintain upright posture. Blood pressure was monitored in the 1st and 3rd minute, with patients excluded if orthostatic hypotension was noted in the first 3 minutes of upright position. The diagnosis of postural orthostatic tachycardia syndrome was made based on a combination of multiple current and historical disabling symptoms associated with orthostatic intolerance plus an increase in heart rate with upright position of at least 30 beats/minute. Patients who were already being treated for orthostatic intolerance, such as with fludrocortisone, midodrine, or beta blockade, were included in the study. Conversely, patients who were on vasoactive medications that could have falsely elevated heart rate at the time of diagnosis, such as tricyclic antidepressants, were excluded from the study. All data were obtained during the initial evaluation in the clinic of our programme. Data on race were self-reported. Patient data were further subdivided by gender and by the presence or absence of joint hypermobility, as there have been prior reports of female predominance in postural orthostatic tachycardia syndrome,Reference Schondorf and Low 4 joint hypermobility in postural orthostatic tachycardia syndrome,Reference Rowe, Barron, Calkins, Maumenee, Tong and Geraghty 5 and a higher incidence of hypermobility in females in general.Reference Dida, Dapper and Boboye 6 The presence or absence of joint hypermobility was assessed using Beighton testing. The diagnosis of hypermobile Ehlers–Danlos syndrome versus hypermobility spectrum disorder was made by providers in the Connective Tissue Disorders clinic at the Children’s Hospital of Philadelphia. In brief, hypermobility spectrum disorder was diagnosed in those patients who did not meet the more stringent criteria for hypermobile Ehlers–Danlos syndrome, and who thus had fewer criteria, either by Beighton score, by lack of other generalised systemic manifestations (skin findings, dental findings, etc.), by lack of family history, or by lack of musculoskeletal complications. Because these data were derived from the electronic health record, as obtained during the course of routine clinical care, and were de-identified, a waiver of consent was granted by the Institutional Review Board at the Children’s Hospital of Philadelphia. Statistical analysis was performed using Microsoft Excel plus the website, Social Science Statistics (http://www.socscistatistics.com/Default.aspx), with the Wilcoxon rank-sum test used for medians and the chi-square test for categorical variables; the significance level set was at p<0.05.
Results
A total of 722 patients diagnosed with postural orthostatic tachycardia syndrome during the inclusion period were evaluated, with 708 patients meeting the criteria of being of age 18 years or less. All patients who were already taking medications that could potentially modify heart rate or blood pressure still met heart rate criteria for postural orthostatic tachycardia syndrome. Females comprised 77.5% of the total group, with a female to male ratio of 3.45:1. The median age of presentation to our clinic was 15.7 years, with a range of 6 to 18. The median age of onset of symptoms was 13.1 years. The median duration of symptoms before diagnosis was 2.0 years (Table 1). Race was overwhelmingly represented by Caucasians (94.1%), with minimal representation by Black/African-Americans, Asians, and Native Americans (Table 2). Only 3.4% of patients identified their ethnicity as Hispanic.
Table 1 Demographic data.
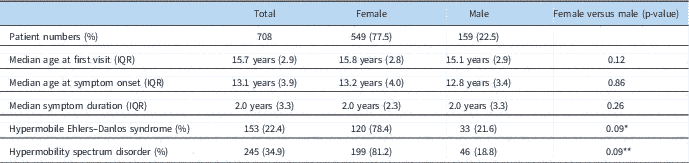
IQR=interquartile range
* p-Value for gender in patients with hypermobile Ehlers–Danlos syndrome versus those without
** p-Value for gender in patients with hypermobility spectrum disorder versus those without
Table 2 Race/ethnicity.

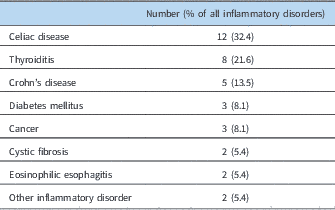
At least one-third of the patients were noted to have a potential temporally related trigger or inciting factor (Table 3). An infection seemed to be associated with the onset of symptoms in nearly one-quarter of the patients (Table 4). Another 11% had a concussion as their inciting event, and nearly 3% had a surgical procedure or traumatic event (non-concussion), such as a bony fracture, after which they noted progressive onset of symptoms. Joint hypermobility was demonstrated in more than half of the patients, with approximately 22% being diagnosed with hypermobile Ehlers–Danlos syndrome, and nearly another 35% having any joint hypermobility without meeting Beighton criteria for Ehlers–Danlos syndrome (Table 1). Another 5% of patients had an inflammatory disorder, such as celiac disease, thyroiditis, or Crohn’s disease (Table 5).
Table 3 Triggers.
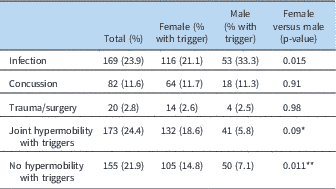
* p-Value for gender in patients with hypermobility with triggers versus those without triggers
** p-Value for gender in patients without hypermobility with triggers versus those without triggers
Table 4 Infectious triggers.
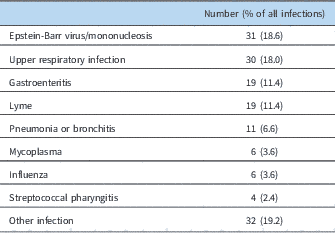
Table 5 Associated inflammatory disorders.
At the time of diagnosis, 80% of patients reported having had one of six initial symptoms, with dizziness and headaches the most frequent of the initial complaints in half of the patients (Fig 1). Subsequent symptoms were wide-ranging, with 66% of patients demonstrating at least 10 symptoms, 50% of patients exhibiting 14 or more symptoms, and 30% of patients having as many as 26 symptoms (Fig 2).
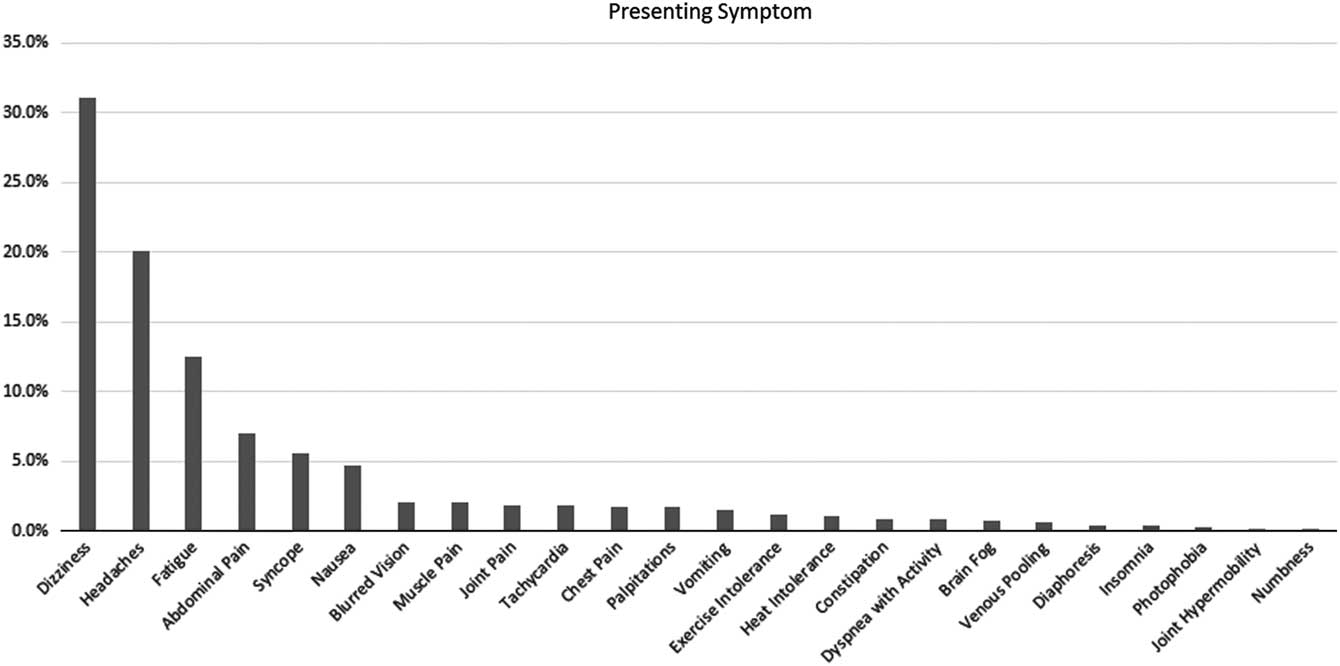
Figure 1 Frequency of the first symptom noted upon the onset of postural orthostatic tachycardia syndrome.
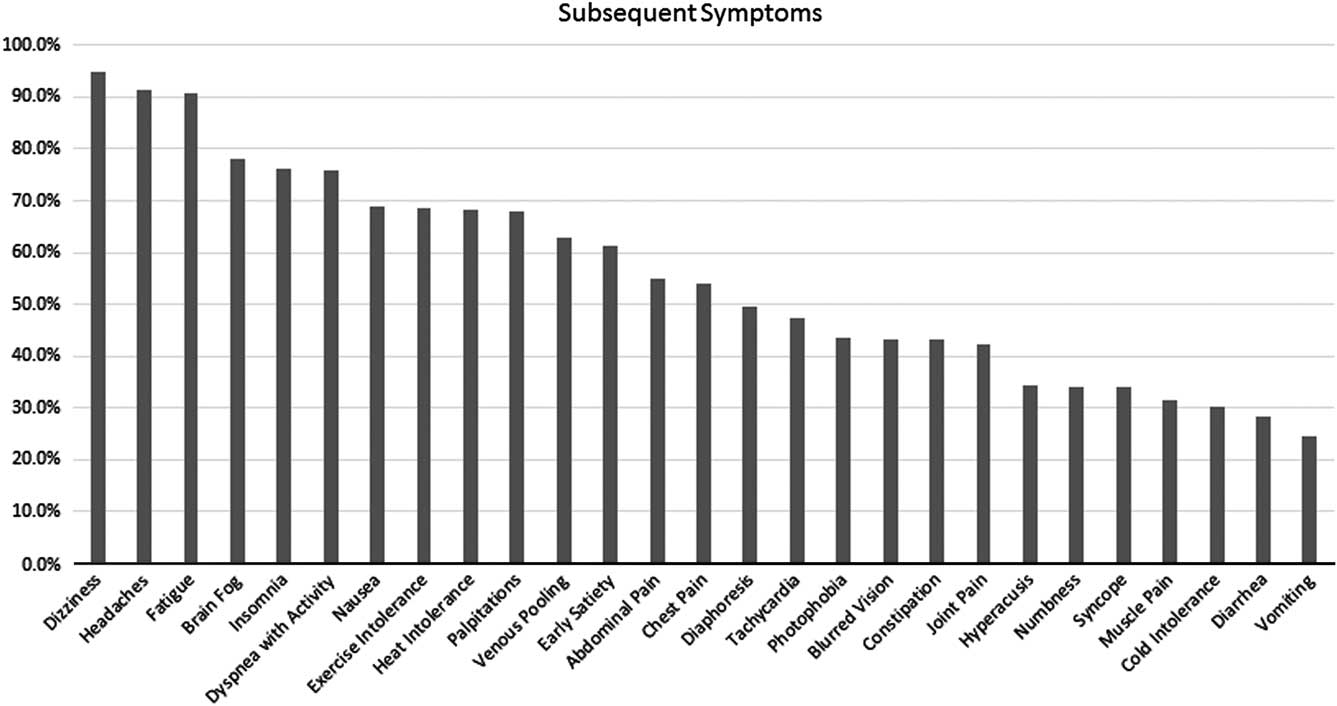
Figure 2 Frequency of occurrence of other symptoms associated with postural orthostatic tachycardia syndrome at the time of initial evaluation.
When further evaluated by gender, boys were more likely to have an infectious triggering event (33%) as compared with girls (21%, p=0.015). Otherwise, there were no differences noted in age at first visit, age of onset of symptoms, or duration of symptoms (Table 3).
When evaluated as to the presence of joint hypermobility, there was a higher frequency of females (4.1:1) as compared with those without hypermobility (2.9:1, p=0.044). Patients with hypermobility also had a longer median duration of symptoms, and thus an earlier age of onset of symptoms (Table 6). Hypermobile males also seemed to present earlier than females. However, there was no difference in incidence of triggers or associated inflammatory disorders. Patients with hypermobility were more likely to exhibit joint and/or muscle pain (49 and 37%, respectively) as compared with those patients without joint hypermobility (34 and 25%, respectively).
Table 6 Demographic information based on the presence or absence of joint hypermobility.
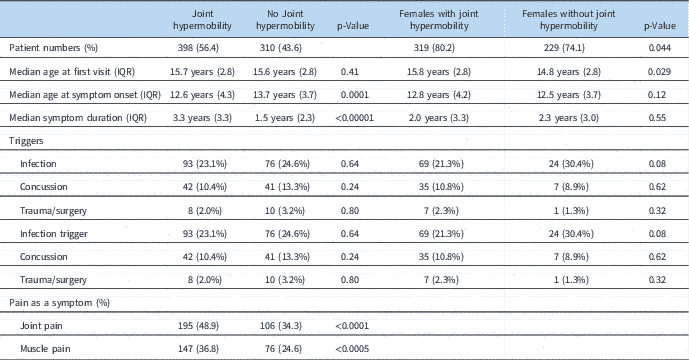
IQR=interquartile range
Discussion
Postural orthostatic tachycardia syndrome was first described in the modern literature by Schondorf and Low in 1993,Reference Schondorf and Low 4 and may have been described in American Civil War soldiers as long ago as 1871.Reference Da Costa 7 Since that time, there have been several studies that have looked at various characteristics of these patients, although a number of them either include adults or adults plus adolescents; those that review paediatric patients only are relatively limited in number of patients and scope of evaluation. With the increasing volume of patients being evaluated and diagnosed at our programme, we have been able to collect a wide range of data on a large paediatric-specific population. To our knowledge, this is the largest clinical paediatric database of patients with postural orthostatic tachycardia syndrome in the United States that has evaluated and shared its data.
The data demonstrated confirmation of widely disparate race and ethnic distribution in patients with postural orthostatic tachycardia syndrome, which has been noted in prior studies.Reference Kanjwal, Saeed, Karabin, Kanjwal and Grubb 8 The overwhelming predominance of Caucasian patients was interesting, and establishes these findings in a study of only paediatric patients. The reason for this is unclear, although other disease processes, such as fibromyalgia,Reference Friedman, Tewi and Ahn 9 cyclic vomiting,Reference Venkatesan, Sengupta and Lodhi 10 chronic idiopathic nausea,Reference Kovacic, Miranda, Chelimsky, Williams, Simpson and Li 11 and cystic fibrosis, Reference O’Sullivan and Freedman 12 also demonstrate a significant predominance of Caucasian patients. A curious hypothesis involves a difference in energetics noted in the mitochondria of Caucasian patients versus those of other races or ethnicities.Reference Wallace 13 It has been suggested that a difference in energy creation, which may have been an asset in Ice Age survival, may be a liability in the case of chronic pain, inflammatory and autoimmune processes, and other diseases that may be related to postural tachycardia.
Our patient population displayed a number of findings not previously reported. The ratio of female to male patients, at 3.45:1, was lower than typically reported; prior studies have noted this ratio as high as 4:1 or 5:1,Reference Schondorf and Low 4 , Reference Raj 14 although one study has reported it as low as 2.7:1.Reference Burkhardt, Fischer and Brands 15 Interestingly, when further subdivided into those patients with joint hypermobility versus those without, patients with hypermobility were more frequently female. The median age of symptom onset is similar to those previously described.Reference Li, Zhang, Hao, Jin and Du 16 However, the duration of symptoms before diagnosis, which has not otherwise been previously discussed in the medical literature, lasted over a 3-year period in those patients with hypermobility, but much less in those without joint hypermobility. The reason for this difference is unclear. It is noteworthy that we also found that patients with hypermobility were more likely to have either joint or muscle pain. Thus, it is possible that other symptoms associated with joint hypermobility, such as pain, may be more likely tolerated in hypermobile patients, and thus do not alert the patient or family to other chronic concerns. The presence of joint hypermobility itself has been described in other studies Reference Schondorf and Low 4 , Reference Gazit, Nahir, Grahame and Jacob 17 , Reference Kanjwal, Karabin, Kanjwal and Grubb 18 ; over half of our patients demonstrated its presence, with a majority of patients having hypermobility spectrum disorder.Reference Castori, Tinkle, Levy, Grahame, Malfait and Hakim 19 In those patients who were diagnosed with co-morbid Ehlers–Danlos syndrome, it was uniformly the hypermobile type; there were no patients demonstrating either classic or vascular subtypes. As has also been found in a mixed study of paediatric and adult patients, the age of onset of symptoms in those patients with hypermobility is lower than that of those patients without.Reference Kanjwal, Karabin, Kanjwal and Grubb 18 The aetiology of this earlier onset and longer duration of symptoms is unknown. However, many patients with hypermobility do seem to experience multiple non-autonomic symptoms earlier on.Reference Henderson, Austin and Benzel 20
A number of putative triggers was found in our patients. An infectious aetiology appeared to be a temporally related trigger for symptoms of the greater syndrome in nearly one-quarter of the patients. The patient history typically obtained suggested that they were medically well before their intercurrent infection, and then were never symptomatically “normal” afterwards, despite “recovery” from what appeared to be the infectious symptoms (e.g. rhinorrhoea, cough, etc.). Symptoms such as dizziness, tachycardia, fatigue, diarrhoea, myalgias, headache, abdominal pain, and other symptoms typically associated with this syndrome would persist without resolution. Subsequently, the syndrome would progress with the development of more symptoms. We speculate that this onset of symptoms of postural orthostatic tachycardia syndrome after infection could be suggestive of an autoimmune aetiology, at least in a subset of patients. Autoantibodies such as anti-cholinergic ganglionic and anti-beta adrenergic receptor antibodies Reference Vernino, Low, Fealey, Stewart, Farrugia and Lennon 21 , Reference Fedorowski, Li and Yu 22 have been increasingly reported, although this association has not confirmed this mechanistic pathway. This may also be additionally supported by the findings of concurrent inflammatory disorders in our population. Interestingly, when separated by gender, males were more likely to have an infection as a trigger for their postural orthostatic tachycardia syndrome. The aetiology for this novel finding is unclear.
Concussions were seen as a trigger in one-tenth of patients. Typically, the symptoms of concussion resolve within 6–8 weeks, although there are some patients who required either vestibular therapy for residual vertigo or visual therapy for convergence insufficiency. Yet, in those patients with postural orthostatic tachycardia syndrome, the dizziness and headaches typically persisted, with the onset of further symptoms developing and lasting after the typical (or expected) 2-month recovery time. These patients had successful vestibular and/or visual therapy with resolution of their attendant symptoms, yet still had findings of postural tachycardia. A similar trigger is seen in patients having surgery or those who have had trauma, such as bony fractures. Despite having gone through recovery from their surgical procedure or having appropriate bone healing, symptoms of the syndrome developed and progressed. Consistent with the aforementioned hypothesis of an autoimmune aetiology, concussions have also been associated with an inflammatory and autoimmune response,Reference Goryunova, Bazamaya and Sorokina 23 and thus may be another manifestation of this autoimmune mechanism.
The six most common presenting symptoms of postural orthostatic tachycardia syndrome (dizziness, headache, fatigue, abdominal pain, syncope, and nausea) have also been noted as the most common initial symptoms elsewhere.Reference Burkhardt, Fischer and Brands 15 , Reference Pianosi, Goodloe, Soma, Parker, Brands and Fischer 24 They essentially comprise the three main systems that are typically affected with this syndrome: cardiovascular, neurologic, and gastrointestinal. These systems were further involved as symptoms subsequently developed. In nearly one-third of patients, as many as 26 symptoms were reported. The large symptom burden exemplifies the significant morbidity, as well as potential disability, associated with this syndrome. In surveying these symptoms, the overwhelming majority involved those that were not typically demonstrated by findings on physical examination, except for tachycardia, venous pooling, and joint hypermobility. This concept of “invisible” symptomatology makes postural orthostatic tachycardia syndrome difficult both for providers to diagnose, and even to validate. It also makes it difficult for family members, friends, and educators to understand, and limits the ability to provide effective family, social, and academic support.
A smaller study of 150 paediatric patients from China performed in 2014 demonstrated similar historical and clinical findings,Reference Li, Zhang, Hao, Jin and Du 16 including a history of traumatic brain injury in 12.6% and infection in 33.09% of patients as precipitating events. The mean age at diagnosis was 11.2 years, and the median duration of symptoms was 9 months. Dizziness was noted as the predominant symptom. It is curious to note that the female to male ratio was distributed much more evenly, with girls making up 52.67% of the patients; this finding is not discussed in the article, especially in light of the findings of multiple prior studies. However, despite this one difference, our study appears to corroborate a number of these findings on a larger scale.
Even with the large number of patients and patient data in our study, limitations do exist. The retrospective nature of this study, as with any retrospective evaluation, can limit the ability to ensure capture of all data. Early in our programme, there was variability in symptom-related questions asked in obtaining the patient history. As time and experience progressed, an increasing and consistent number of symptoms was routinely evaluated, which was further enhanced by the advent of utilising a checklist in the electronic health record. In those patients who were evaluated before the use of the electronic health record checklist, further historical symptoms were then subsequently obtained retrospectively. Furthermore, obtaining historical features depends upon the recall of the patients and parents. With the duration of symptoms sometimes lasting several years, accurate recall of these events can be difficult. Therefore, our data may not be fully complete, and likely represent a lower limit to a number of these triggers and symptoms. In addition, owing to the progressive nature of the syndrome, further symptoms may still have developed subsequent to the first visit, and were not able to be included in the frequency of subsequently reported symptoms.
The utilisation of an increase of at least 30 beats/minute as the threshold for the diagnosis of postural orthostatic tachycardia syndrome provides another point of discussion. Although the Heart Rhythm Society published threshold heart rate criteria of at least 40 beats/minute for adolescents in 2015,Reference Sheldon, Grubb and Olshansky 25 the diagnostic threshold of 30 beats/minute had been previously utilised in our clinic, as patients were seen and diagnosed before the publication of this statement; thus, these criteria were maintained for consistency. It is important to note that these 2015 criteria were based on tilt table test data, which were not utilised in our clinic; instead, a 10-minute stand was used to confirm the diagnosis. The utilisation of a 10-minute standing test may alter the perception of these data, as the use of a standing test has not been validated. However, standing testing has been used in other studies for the diagnosis of this syndrome.Reference Raj, Black, Biaggioni, Harris and Robertson 26 – Reference George, Bivens and Howden 28 In fact, a disparity between tilt table testing and standing testing has been shown,Reference Plash, Diedrich and Biaggioni 29 with tilt table testing showing higher mean heart rates as compared with standing. In addition, as standing is more of a physiologic condition than tilt table testing, it is not unreasonable to use this as the diagnostic assessment for these patients.
One last limitation in our study may be that our patients overwhelmingly come from our regional catchment in the Northeast United States of America. There may be variability of patients seen in other parts of the country, which we are not able to ascertain.
Overall, further prospective evaluation and data collection would be ideal to capture all aspects of information required to best delineate the various characteristics of patients with postural orthostatic tachycardia syndrome. That said, our study is able to provide a snapshot of a large paediatric practice managing these patients. These patients are typically Caucasian, have a high incidence of joint hypermobility, and are often female. Those patients with hypermobility are also more likely female and tend to have a younger onset of symptoms, as well as a longer duration of symptoms before diagnosis. The symptoms of these patients span several body systems, and can be numerous; the majority of symptoms involve the cardiovascular, neurologic, and gastrointestinal systems. Over one-third of patients can identify a specific temporally related trigger. There is also a small percentage of patients with co-morbid inflammatory or autoimmune disease. Besides comprising a very large paediatric sample and confirming a number of previously known data seen in mixed paediatric and adult, as well as adult-only, studies, our study specifically points out many more subtleties among this heterogeneous population of patients that may point towards finding a true pathophysiology. Understanding the clinical characteristics of patients with postural orthostatic tachycardia syndrome can better help in the diagnosis and therapeutic approach, as well as in the search for both an aetiology and a directed cure for this debilitating disorder.
Acknowledgements
The authors would like to thank Hannah Boris, Robert Rennie, and Elizabeth Schoenberg for data compilation, and Andrew Glatz, MD, MSCE, for his thoughtful review and recommendations in the creation of this manuscript.
Financial Support
Students received financial support for their database work from the Division of Cardiology at the Children’s Hospital of Philadelphia and from Dysautonomia International.
Conflicts of Interest
None.
Ethical Standards
No human experimentation was performed in the course of the acquisition of data for this review. The Institutional Review Board of the Children’s Hospital of Philadelphia granted a waiver of consent, as data were de-identified and collected as part of routine clinical care.











