Introduction
Cabbage (Brassica oleracea L. Capitata Group) is a popular leafy vegetable worldwide. Many pathogens can infect cabbage (Keinath et al., Reference Keinath, Cubeta, Langston and Pimentel2006). Among them, Rhizoctonia solani Kühn and Pythium aphanidermatum Edson cause cabbage seedling damping-off, resulting in severe economic loss. The two pathogens have a wide host range and can be transmitted via infected soils/media, seeds or plant debris (Stephens et al., Reference Stephens, Herr, Schmitthenner and Powell1982). Cabbage seeds affected by either pathogen could decay, particularly in cold, wet soils. Affected seedlings quickly wilt, bend and eventually die.
Fungicides are commonly applied to control cabbage damping-off caused by R. solani and P. aphanidermatum. Cabbage seeds are treated or coated with fungicides in order to reduce seed or seedling loss due to seed-borne pathogens. Soil treatment with a broad-spectrum fumigant is also commonly conducted to reduce cabbage seedling damping-off. However, extensive use of toxic chemicals has increased concerns about food safety and the environment. Frequent application of toxic fumigants may also result in the emergence of resistant strains and impact non-target and beneficial soil microorganisms (Vaughn and Spencer, Reference Vaughn and Spencer1994). Thus, the use of soil fumigants, including methyl bromide, is restricted or being phased out in many countries (Whipps, Reference Whipps2001). To continue to battle plant diseases in agricultural production, it is imperative to develop alternative means with less harmful effects to humans and beneficial microorganisms and with better environmental fitness.
Biological control using volatile organic compound (VOC)-producing microorganisms has been reported with increasing success on various plant diseases (Wan et al., Reference Wan, Li, Zhang, Jiang and Huang2008; Arrebola et al., Reference Arrebola, Sivakumar and Korsten2010; Li et al., Reference Li, Ning, Zheng, Huang, Li and Hsiang2012; Di Francesco et al., Reference Di Francesco, Ugolini, Lazzeri and Mari2015; Kanchiswamy et al., Reference Kanchiswamy, Malnoy and Maffei2015). For example: Enterobacter species can produce ammonia, benzoic acid, methyl benzyl sulphide and dimethyl disulphide (DMDS), which have been implicated in suppressing Pythium damping-off (Howell et al., Reference Howell, Beier and Stipanovic1988). Pseudomonas species generate VOCs that are inhibitory to Sclerotinia sclerotiorum (Fernando et al., Reference Fernando, Ramarathnam, Krishnamoorthy and Savchuk2005). Bacillus subtilis is a prolific producer of VOCs that induce hyphal deformation and inhibit radial growth of various fungal pathogens, including Alternaria, Cladosporium, Fusarium, Paecilomyces, Pythium and Rhizoctonia (Fiddaman and Rossall, Reference Fiddaman and Rossall1993; Chaurasia et al., Reference Chaurasia, Pandey, Palni, Trivedi, Kumar and Colvin2005). Furthermore, some Bacillus strains can degrade the sulphur-containing amino acids, methionine and cysteine, and release methylmercaptan, dimethyl sulphide and DMDS, which are toxic to many plant pathogens (Endoh et al., Reference Endoh, Kasuga, Horinouchi, Yoshida, Habe, Nojiri and Omori2003; Anakwenze et al., Reference Anakwenze, Ezemba and Ekwealor2014). Dimethyl disulphide has also been reported to trigger induced systemic resistance in plants (Huang et al., Reference Huang, Tsay, Chang, Yang, Wu and Chen2012). Many bacteria can produce volatile metabolites, including alcohols, aldehydes, esters, carbohydrates, organic acids and sulphurs and their derivatives that are inhibitory to a wide range of plant pathogens (Edwards et al., Reference Edwards, Dainty and Hibbard1987; Howell et al., Reference Howell, Beier and Stipanovic1988). Gas-producing biological control agents could potentially be used to manage soil-borne diseases (Shafi et al., Reference Shafi, Tian and Ji2017).
Bacillus spp. are often used as biocontrol agents because they can produce thick-walled endospores that are resistant to adverse environments, including ultra-violet (UV), radiation, drought, high osmosis and temperature, and toxic chemicals (Driks, Reference Driks2004; Gardener and Driks, Reference Gardener and Driks2004). Bacillus mycoides Flügge, commonly found in soils and the plant rhizospheres, is a Gram-positive, saprophytic bacterium (Buyer, Reference Buyer1995). Bacillus mycoides is the only Bacillus sp. that produces spreading, root-like (rhizoidal) colonies on nutrient agar medium. These colonies resemble fungal filamentous growth and display a genetically controlled spiral pattern with either clockwise or counter clockwise curving (Di Franco et al., Reference Di Franco, Beccari, Santini, Pisaneschi and Tecce2002). Bacillus mycoides promotes plant growth and induces systemic acquired resistance against various plant diseases (Petersen et al., Reference Petersen, Shishido, Holl and Chanway1995; Bargabus et al., Reference Bargabus, Zidack, Sherwood and Jacobsen2004). Coating wheat seeds with B. mycoides has been shown to increase yield and reduce the damage caused by Gaeumannomyces graminis var. tritici and Fusarium culmorum (Czaban et al., Reference Czaban, Ksiezniak, Wroblewska and Paszkowski2004a, Reference Czaban, Ksiezniak and Paszkowski2004b). Because B. mycoides is often considered as a plant growth-promoting rhizobacterium (PGPR), little is known about the VOCs they produce and their roles in biocontrol efficacy. The objectives of the current study were to test whether B. mycoides can produce VOCs and to evaluate the efficacy of the bacterium in terms of reducing cabbage diseases. Two B. mycoides strains were cultured from tomato rhizospheres and demonstrated to produce volatile DMDS and ammonia that had growth inhibitory activities against R. solani and P. aphanidermatum. Greenhouse trials demonstrated that the newly identified B. mycoides strains were able to promote plant growth and reduce cabbage damping-off caused by P. aphanidermatum.
Materials and methods
Bacterial strains and growth conditions
Bacillus mycoides strains (CHT2401 and CHT2402) were isolated from tomato rhizospheres in central Taiwan (Ding and Huang, Reference Ding and Huang2017). Plant roots and soil (~5 g) were boiled in 100 ml water for 5 min. The resulting suspension was serially diluted tenfold and streaked three times, consecutively, on tryptic soy agar (TSA) (Difco, Sparks, MD, USA); the plates were then incubated at 30 °C for single colony formation. Bacterial strains were identified as B. mycoides based on distinct characteristics of filamentous and rhizoid colonies formed on agar medium (Di Franco et al., Reference Di Franco, Beccari, Santini, Pisaneschi and Tecce2002) and confirmed based on biochemical and physiological tests described in Bergey's Manual and the MicroLog bacterial identification system (Claus and Berkeley, Reference Claus, Berkeley, Sneath, Mair, Sharpe and Holt1986). The identity of CHT2401 and CHT2402 was examined by sequence analysis of an rRNA gene located in the16S–23S ribosomal DNA intergenic transcribed spacer (ITS) and a gyrB gene encoding a sub-unit B protein of DNA gyrase (Ding and Huang, Reference Ding and Huang2017). ITS rDNA was amplified by PCR with the primers ITSAr (5′-aaaatagctttttggtggag-3′) and ITSBf (5′-aaatttgtatgggcctatag-3′) as described by Cherif et al. (Reference Cherif, Borin, Rizzi, Ouzari, Boudabous and Daffonchio2002) and Rivas et al. (Reference Rivas, Velázquez, Zurdo-Piñeiro, Mateos and Martínez Molina2004). The gyrB gene fragment was amplified with the primers BCFW1 (5′-gtttctggtggtttacatgg-3′) and BCRW1 (5′-caacgtatgatttaattccacc-3′) as described by Yamada et al. (Reference Yamada, Ohashi, Agata and Venkateswaran1999). Bacterial cells were harvested with sterile water from TSA plates and their concentrations were adjusted to 2 × 108 colony-forming units (cfu)/ml by dilution.
For large-scale preparation, B. mycoides strains were cultured in roasted soybean powder milk (SPM) (Yong Chengxing, Taichung, Taiwan) broth. Soy powder (10 g) was mixed with 100 ml water, boiled for 20 min, filtrated through three layers of cheesecloth, and sterilized by autoclaving. Bacteria were incubated at 30 °C for 7 days on a rotary shaker set at 200 rpm. Other media used for culturing bacteria included: nutrient agar (NA) (Difco), Luria–Bertani agar (LA) (ZymesetBiotek, Taipei, Taiwan), King's B agar (KBA, Sigma-Aldrich, St. Louis, MO, USA) (King et al., Reference King, Ward and Raney1954) and soy powder milk agar (SPMA) (Atlas, Reference Atlas1993).
Fungal strains, growth conditions and inoculum preparation
The RST04 isolate of R. solani AG-4 was cultured from a diseased cabbage in central Taiwan. RST04 was grown on potato dextrose agar (PDA, Difco) at 30 °C and inoculum was prepared as described previously (Hsieh et al., Reference Hsieh, Lin, Lin, Chung and Huang2016). Briefly, RST04 was cultured in a 250 ml flask containing sterilized shredded potato (100 g) for 7 days, mixed with sterilized peat moss (1:10, w/v) (Bas Van Buuren No. 4, Maasland, Netherlands) and 400 ml water, and incubated at room temperature (~25 °C). Flasks were swirled manually with a sterile glass rod for 1 min every 2–3 days. After 4 weeks, the culture was further mixed with equal volume of unsterilized peat moss to make infected medium.
The Pa01 isolate of P. aphanidermatum (Edson) Fitzp. was recovered as a single colony from a diseased lettuce seedling showing damping-off symptoms in central Taiwan. Pa01 isolate was cultured on V8 juice agar (Atlas, Reference Atlas1993). Infested medium containing P. aphanidermatum was prepared as follows: agar medium covered with mycelium was cut to small pieces and mixed with sterilized peat moss (100 ml). For zoospore formation, Pa01 was cultured on V8 agar plates under a 12 h light cycle at 30 °C for 3–4 days.
Volatile antimicrobial assays
Assays for the production of VOCs and their effects against plant pathogens were performed using a plate-to-plate method (Stinson et al., Reference Stinson, Ezra, Hess, Sears and Strobel2003; Di Francesco et al., Reference Di Francesco, Ugolini, Lazzeri and Mari2015). Bacterial suspensions (1 ml, 108 cfu/ml) were streaked on agar medium (TSA, SPMA, KBA, LA, NA, or PDA), cultured at 30 °C for 24 h and used for antimicrobial assays. Rhizoctonia solani RST04 grown on PDA and P. aphanidermatum Pa01 isolate grown on V8 for 2 days were used for sensitivity assays. Petri dish lids were removed and the coverless plates attached to each other. The gap between plates was sealed with four layers of parafilm (American National Can, Chicago, IL, USA) and the plates were incubated at 30 °C for 2–3 days. Agar plates (PDA and V8) with no bacteria were attached to agar plates with R. solani RST04 or P. aphanidermatum Pa01 as controls. Each treatment contained at least five replicates. The toxicity of commercially available DMDS (98 g/ml, Sigma-Aldrich) or ammonia was assessed similarly by placing DMDS on a cover glass or by placing ammonia (25 g/ml) on a filter paper disc that was then attached to cuture plates containing R. solani or P. aphanidermatum. Equal volumes of water applied onto cover glasses or paper filter discs were used as negative controls. Percentage of growth inhibition of R. solani or P. aphanidermatum was determined by dividing the relative difference of the growth between control and treatment by the growth of the control and multiplied by 100.
The viability of R. solani and P. aphanidermatum was assessed every 12 h after treatment by transferring agar plugs (3 mm in diameter) covered with mycelium onto freshly prepared PDA and V8, respectively. The effect of VOCs on the production of zoospores by P. aphanidermatum was assessed as follows: after 2-day incubation with or without B. mycoides, ten agar discs (1 cm in diameter) covered with P. aphanidermatum mycelium were transferred to a glass petri dish containing 20 ml sterilized water and incubated at 24 °C for 12 h. The zoospores were completely encysted after centrifugation at 1500 rpm (Sigma 3K15 rotor, Osterode am Harz, Germany) for 20 min, and resuspended and the number of zoospores was determined microscopically.
Identification of volatile organic compounds by solid-phase microextraction/gas chromatography–mass spectrophotometry
Bacillus mycoides strains (1 ml, 108 cfu/ml) were cultured on SPMA or TSA at 30 °C for 2 days. Volatile organic compounds were collected by a headspace solid-phase microextraction (HS-SPME) device (SUPELCO, Bellefonte, PA, USA) packed with 50/30 µm divinylbenzene/carboxen/polydimethylsiloxane (SUPELCO) fibres for 15 min and analysed by a gas chromatography–mass spectrophotometry (GC/MS). The HS-SPME device was detached from plates and inserted directly into the injector (200 °C) of a Model CP-3800 GC and a Saturn 2000 MS (VARIAN, CA, USA) connected to an electron capture detector (Model 902B, ECD, splitless mode). Nitrogen was used as a gas carrier and flowed at 2 ml/min through a VF-5MS capillary column (30.0 m × 0.25 mm ID, 50/30 µm film thickness, Agilent, Santa Clara, CA, USA). Analytical temperatures modified from Kai et al. (Reference Kai, Effmert, Berg and Piechulla2007) were set as follows: initial column temperature at 50 °C for 2 min, followed by an increment of 5 °C/min up to 220 °C with a final temperature at 220 °C for 1 min. Controls consisted of SPMA and TSA plates without bacteria. The identities of VOCs were verified by comparing them with chemical databases deposited in the GC-MS library (Saturn 2000, USA) and by referencing the National Institute of Standards and Technology (NIST) mass spectral database. Gas collected from agar plates (with no bacteria) attached to agar plates with R. solani or P. aphanidermatum were used as negative controls (Fig. 1). Each treatment contained at least three replicates. Dimethyl disulphide was purchased from Tokyo Kasei Kogyo (Tokyo, Japan).
Production and quantification of ammonia
The production of ammonia by B. mycoides strains cultured on SPMA or TSA at 30 °C was assessed after a 2-day incubation on agar plates. Volatile organic compounds were collected through a Colour Detecter tube No. 3La (GASTEC Co., Kanagawa, Japan) attached to a Gastec model GV-100 gas sampling pump (GASTEC). Ammonia, after reacting with Nessler's reagent, was quantitatively determined by colorimetry. Gas collected from agar plates (with no bacteria) attached to agar plates with R. solani or P. aphanidermatum was used as the negative control. Each treatment contained at least three replicates. Ammonia solution was purchased from Hayashi Pure Chemical Industries (Osaka, Japan).
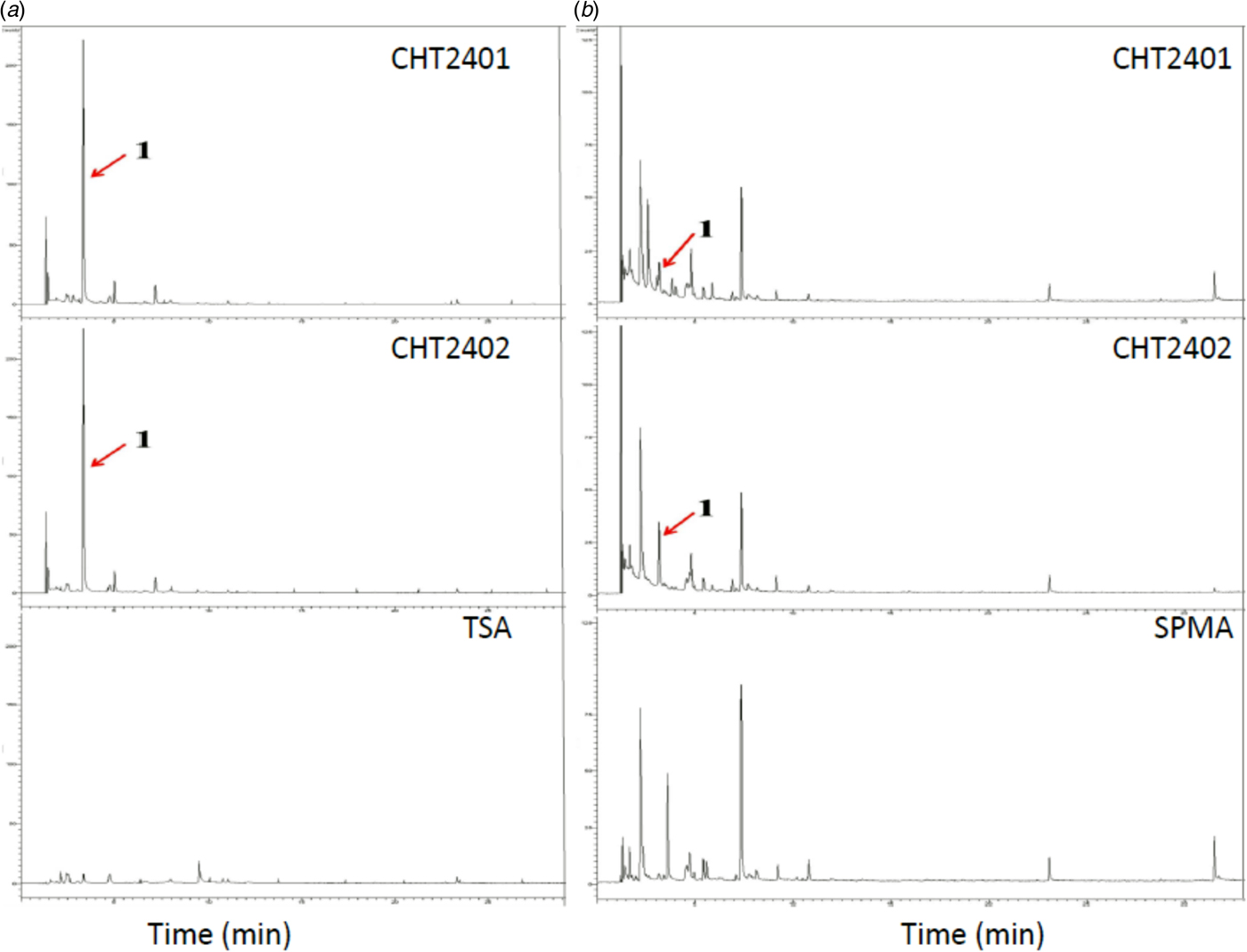
Fig. 1. Gas chromatography profiles of VOCs obtained from Bacillus mycoides strains CHT2401 and CHT2402. (a) B. mycoides was cultured on tryptic soy agar (TSA) for 2 days at 30 °C. (b) B. mycoides was cultured on soy powder milk agar (SPMA). Peak 1 indicated by an arrow was later identified as dimethyl disulphide. Colour online.
Electron microscopy
Rhizoctonia solani and P. aphanidermatum mycelium treated with or without B. mycoides (108 cfu/ml), DMDS or ammonia were examined by scanning electron microscopy (SEM). Mycelium was sampled directly from cover glass, filter paper or from agar plates by a 3 mm punching device, fixed on a stage with glue and frozen in liquid nitrogen. Samples were frozen in an E7400 Cry-transfer system chamber (Cryotrans, Bio-Rad, Hercules, CA, USA) set at −180 °C. To prevent the formation of ice crystals, samples were maintained at −100 °C for 5–10 min. After coating with gold ions for 50 s, samples were examined using a Topcon ABT-150S SEM (Topcon, Tokyo, Japan). For transmission electron microscopy (TEM), samples were fixed with 2.5 g/ml glutaraldehyde in 0.1 M phosphate buffer (pH 7.2) at 4 °C overnight. After washing three times with phosphate buffer, samples were fixed further with 1 g/ml osmium tetraoxide for 2 h and embedded in 50 g/ml LR white resins (Sigma-Aldrich) at 4 °C for 12 h after being dehydrated with an ethanol series. Samples were sectioned to 60–90 nm, consecutively stained with 2 g/ml uranium dioxide for 50 min and lead citrate for 15 min, and examined using a JEM-1400 TEM (JEOL, Tokyo, Japan).
Assays for plant growth
Bacillus mycoides cells (108 cfu/ml) grown in soy powder milk (SPM) broth for 7 days were mixed with peat moss (Bas Van Buuren No. 4) to make a 0.5, 1, 5 or 10 g/ml mix. The mix containing ~15–20 g/ml water was placed in a plastic bag, incubated at room temperature for 24 h and used to fill a 16-compartment (4 × 4) plastic tray. The effect of B. mycoides on plant growth was assessed on cabbage (B. oleracea L. Capitata group cv. Tops), asparagus bean [Vigna unguiculata (L.) Walp. subsp. sesquipedalis (L.) Verdc. cv. Known-You dwarf], edible rape (Brassica campestris L. Japonica group cv. Ya Tsai No. 4), lettuce (Lactuca sativa L.), and tomato (Lycopersicon esculeutum Mill cv. Known-You 301). Plants grown in untreated peat moss were used as controls. All seeds were purchased from Known-You Seed Co. (Kaohsiung, Taiwan). Plants were maintained in a greenhouse and fresh weight and height were determined 21–30 days post planting. Each treatment contained four replicates.
Disease control assays
Rhizoctonia solani or P. aphanidermatum was cultured and mixed with B. mycoides (108 cfu/ml) or water (H2O). The mixtures were incubated in a plastic bag for varying days (0, 2, 4 or 6 days) and used to fill a 16-compartment (4 × 4) tray. Cabbage seeds were planted individually in each hole. Inoculated plants were maintained in a greenhouse located in the Chung Hsing University, Taichung, Taiwan (24.1′N, 120.6′E, 2000 m a.s.l.). Disease incidence (the number of diseased plants) was assessed 21–30 days post inoculation (dpi). Disease incidence was calculated by dividing the number of diseased plants by the total number of test plants. Each treatment contained four replicates.
Statistical analysis
Data presented in the current study were analysed by analysis of variance using SAS/STAT software (version 9.0). Significance of treatments was determined based on Fisher's protected LSD test (P < 0.05).
Results
Toxic volatile organic compounds produced by B. mycoides
Two B. mycoides strains, CHT2401 and CHT2402, were isolated from tomato rhizospheres. Plate-to-plate assays revealed that two plant pathogens, R. solani and P. aphanidermatum, upon exposure to either CHT2401 or CHT2402 culture, displayed severe growth retardation (P < 0.05) (Fig. 2). Bacillus mycoides grown on TSA or SPMA produced VOCs that inhibited radial growth of both pathogens by >90%. When grown on KBA or LA, B. mycoides displayed moderate growth inhibition of both pathogens. Bacillus mycoides strains grown on NA or PDA had little or no inhibitory effects on R. solani or P. aphanidermatum. Rhizoctonia solani and P. aphanidermatum co-cultured with B. mycoides in plate-to-plate assays reduced aerial hyphae and formed abnormal colonies (data not shown). Rhizoctonia solani and P. aphanidermatum resumed normal growth after they were transferred onto freshly prepared PDA and V8 agar, respectively (data not shown). Volatile organic compounds produced by both B. mycoides strains grown on TSA or SPMA also suppressed the formation of zoospores by P. aphanidermatum at rates >95% (Table 1).
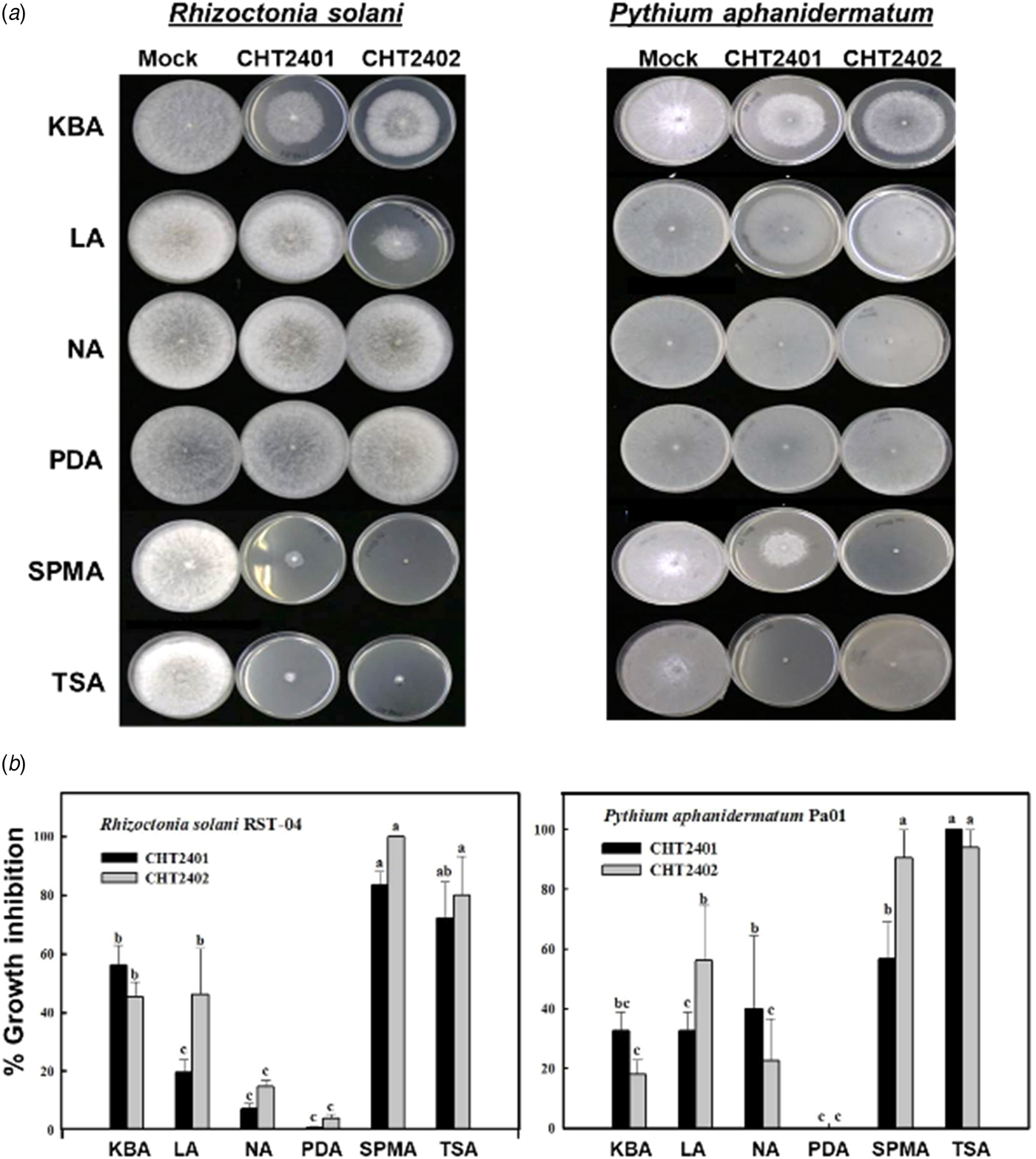
Fig. 2. The toxicity of VOCs produced by the CHT2401 and CHT2402 strains of Bacillus mycoides to plant pathogens: Rhizoctonia solani and Pythium aphanidermatum. (a) Plate-to-plate assays reveal growth inhibition of the pathogens after being exposed to B. mycoides cultured on tryptic soy agar (TSA), soy powder milk agar (SPMA), King's medium B agar (KBA), Luria–Bertani agar (LA), nutrient agar (NA) or potato dextrose agar (PDA). Agar plates with no bacteria were attached to agar plates with plant pathogens as controls. Only representatives are shown. (b) Quantitative analysis of growth inhibition of plant pathogens exposed to VOCs produced by B. mycoides. The data presented are the mean ± standard error of the mean (S.E.M.).
Table 1. Suppression of zoospore formation in Pythium aphanidermatum after exposure to VOCs produced by two Bacillus mycoides strains (CHT2401 and CHT2402) cultured on tryptic soy agar (TSA) or soy powder milk agar (SPMA)

a Pythium aphanidermatum treated with water was used as controls.
b Significantly different at P < 0.05 according to Fisher's protected LSD test. Experiments were conducted twice showing similar results.
Identification of volatile organic compounds produced by B. mycoides
The VOCs produced by B. mycoides grown on TSA were identified after gas trapping and GC/MS separation, revealing similar profiles for samples collected from two different B. mycoides strains (Fig. 1). One of the unique volatile substances found in the bacterium-grown medium was identified as DMDS by referencing the GC-mass spectra database library and further verified by comparison with the authentic standard and mass spectra of DMDS in the NIST database (Fig. 3). The GC/MS analysis revealed that B. mycoides strains grown on SPMA produced more complex VOC profiles than those grown on TSA. Gas phase collected from SPMA alone (without bacterium) also resulted in many unknown compounds, which were not found in the samples collected from TSA. However, the quantity of DMDS produced by the bacterial strains cultured on SPMA was much lower compared with volatiles produced when cultured on TSA.

Fig. 3. Identification of dimethyl disulphide (DMDS) by gas chromatography–mass spectrophotometry (GC-MS). (a) Mass spectra of DMDS available in the National Institute of Standards and Technology (NIST). (b) Mass spectra of commercially available DMDS. (c) Mass spectra of samples (peak 1 in Fig. 9) obtained from the CHT2401 or CHT2402 strain of Bacillus mycoides, showing similar profiles as those of commercially available DMDS.
In addition to DMDS, B. mycoides strains (CHT2401 and CHT2402) grown on TSA or SPMA also emitted ammonia (Fig. 4). Compared with the CHT2401 strain, the CHT2402 isolate produced a higher level of ammonia (Table 2). The medium used to culture B. mycoides strains also impacted ammonia accumulation; bacteria cultured on TSA medium produced a higher level of ammonia compared with those grown on SPMA.

Fig. 4. Detection of ammonia released from the CHT2401 and CHT2402 strains of Bacillus mycoides grown on tryptic soy agar (TSA) or soy powder milk agar (SPMA) by a GASTEC device. Formation of yellow colour indicates the production of ammonia. Colour online.
Table 2. Ammonia produced by two Bacillus mycoides strains (CHT2401 and CHT2402) cultured on tryptic soy agar (TSA) or soy powder milk agar (SPMA)

a The medium was used as controls.
b Significantly different at P < 0.05 according to Fisher's protected LSD test. Experiments were conducted twice showing similar results.
Dimethyl disulphide and ammonia are toxic to plant pathogens
Co-incubation of R. solani or P. aphanidermatum with commercially available DMDS resulted in a marked inhibition of growth (Fig. 5). Radial growth of R. solani and P. aphanidermatum on a glass slide was suppressed completely by 8 and 1.5 µl of DMDS, respectively, for 3 days. Co-incubation of the pathogens with commercially available ammonia solution (25 g/ml) for 2 days also suppressed pathogen growth. Although R. solani was less sensitive to ammonia compared with P. aphanidermatum, 10 µl of ammonia completely inhibited the growth of both pathogens.

Fig. 5. The toxicity of (a) commercially available dimethyl disulphide and (b) ammonia to plant pathogens Rhizoctonia solani or Pythium aphanidermatum.
Scanning electron microscopy analysis revealed that R. solani hyphae, after co-incubation with B. mycoides cultured on TSA or SPMA medium, displayed distinct morphological abnormalities, including empty cytoplasm with poor rigidity, shrinkage and curling (Fig. 6). Similar abnormalities were observed in R. solani hyphae treated with commercially available DMDS or ammonia. Pythium aphanidermatum hyphae, after co-incubation with B. mycoides or exposure to DMSD or ammonia, were smaller and thinner than those of controls (Fig. 7). Some cells within the hyphae became swollen. Hyphae also appeared to shrink or rupture. Further analysis by TEM revealed that individual cells within the R. solani hyphae, after co-incubation with B. mycoides or DMDS, had normal cell walls and contained fewer organelles compared with those of controls (Fig. 8). However, P. aphanidermatum hyphae, after co-incubation with B. mycoides or treatment with DMSD, had thicker cell walls, displayed cell wall deformation and contained fewer organelles than those of controls (Fig. 9).

Fig. 6. Volatile compounds produced by the CHT2402 strain of Bacillus mycoides induce deformation of Rhizoctonia solani hyphae. Scanning electron microscopy (SEM) images of (a) untreated hyphae; (b) hyphae treated with CHT2402; (c) hyphae treated with commercially available dimethyl disulphide; and (d) hyphae treated with commercially available ammonia.
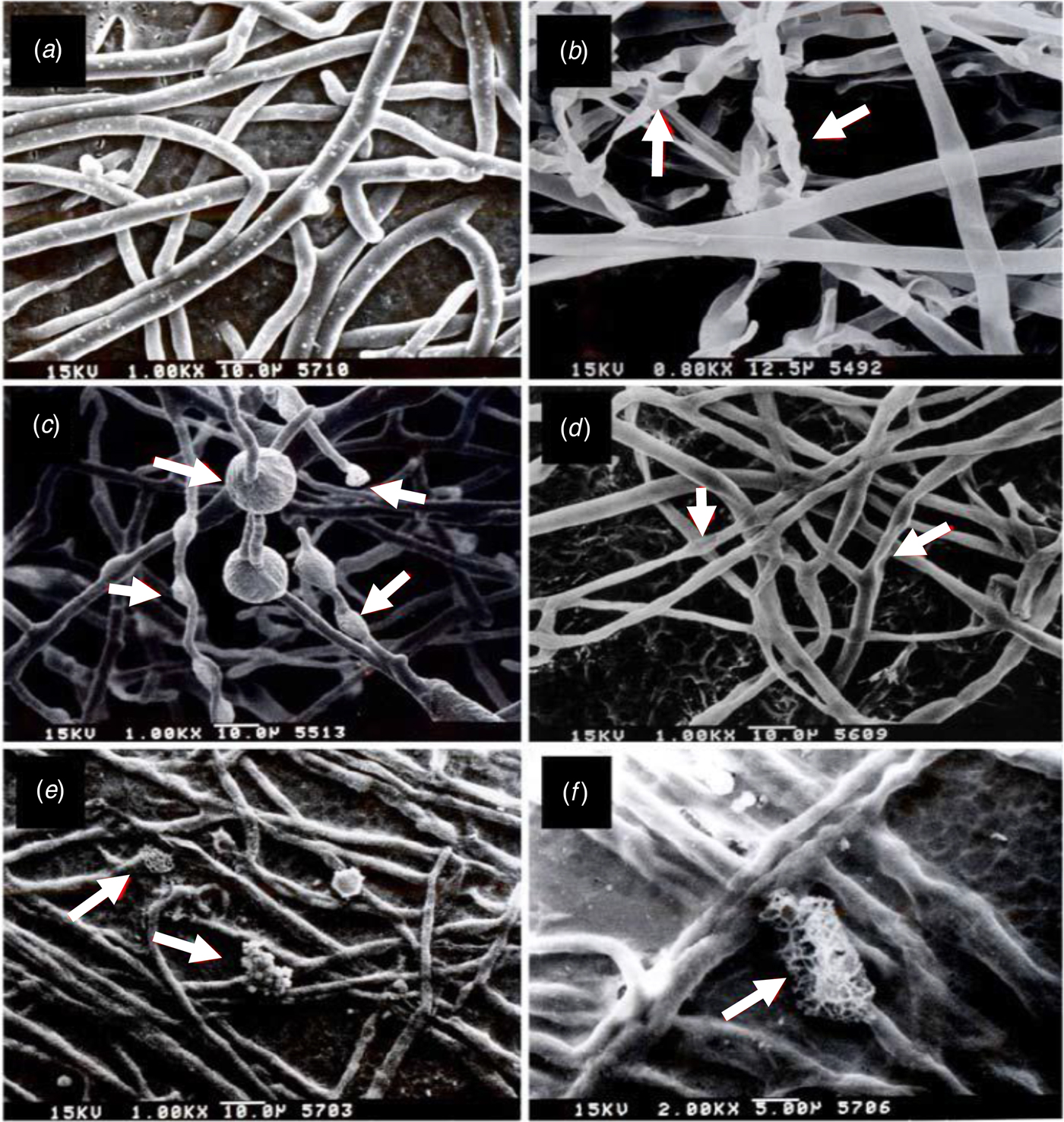
Fig. 7. Volatile compounds produced by the CHT2402 strain of Bacillus mycoides induce deformation of Pythium aphanidermatum hyphae. Scanning electron microscopy (SEM) images of (a) untreated hyphae; (b) and (c) hyphae treated with CHT2402; (d) hyphae treated with commercially available dimethyl disulphide; and (e) and (f) hyphae treated with commercially available ammonia.

Fig. 8. VOCs produced by Bacillus mycoides CHT2402 strain induce deformation of Rhizoctonia solani hyphae. (a) Transmission electron microscopy (TEM) of R. solani hyphae cultured on PDA, which was attached to a tryptic soy agar (TSA) for 2 days at 30 °C. (b) TEM image of R. solani hyphae cultured on PDA, which was attached to a soy powder milk agar (SPMA). (c) and (d) Hyphae after being exposed to B. mycoides CHT2402 strain cultured on TSA, showing fewer organelles. (e) Hyphae after being exposed to B. mycoides CHT2402 strain cultured on SPMA. (f) Hyphae after being exposed to commercially available dimethyl disulphide.
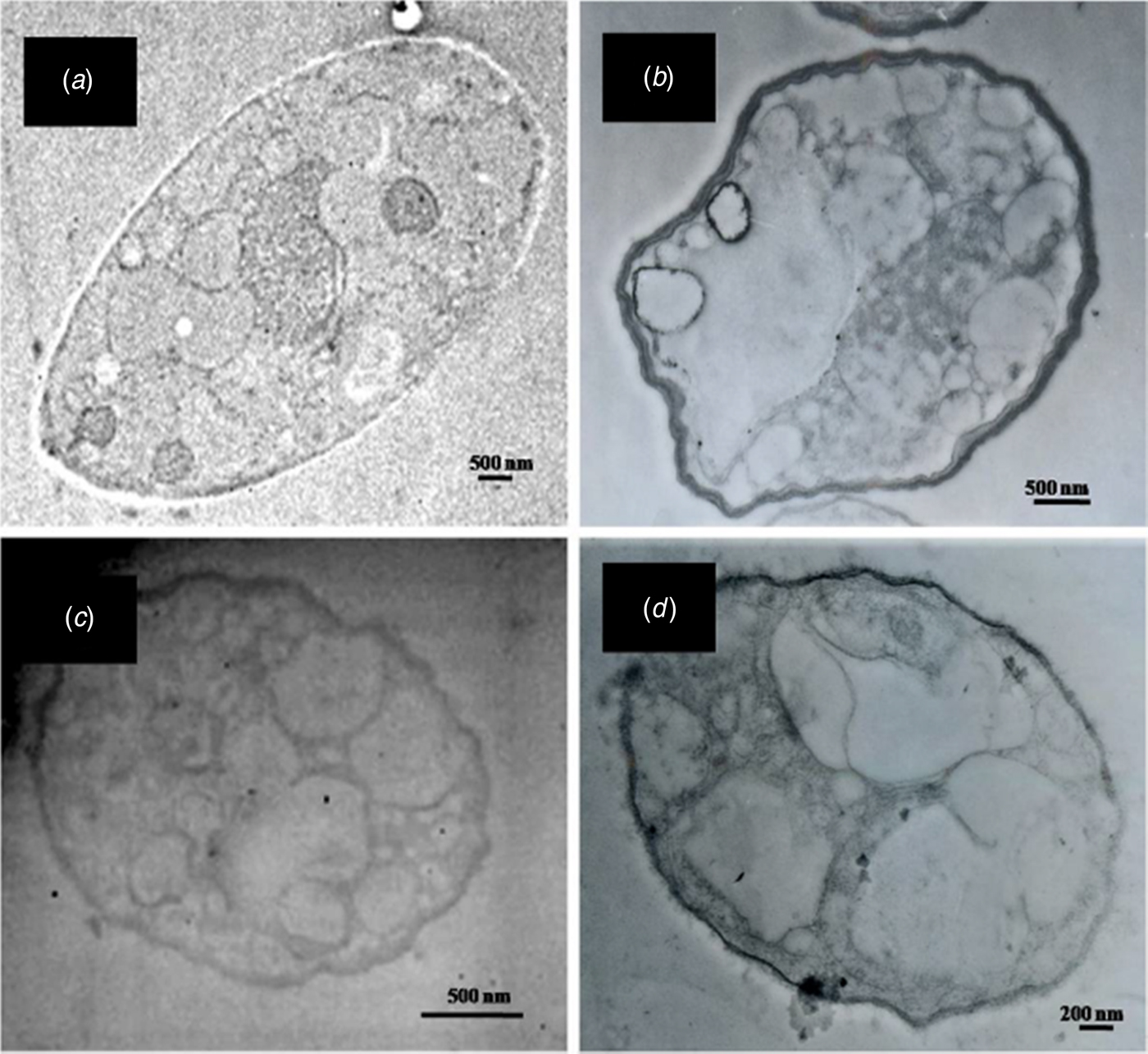
Fig. 9. VOCs produced by B. mycoides CHT2402 strain induce deformation of Pythium aphanidermatum hyphae. (a) Transmission electron microscopy (TEM) of P. aphanidermatum cultured on V8, which was attached to a tryptic soy agar (TSA) plate for 2 days at 30 °C. (b) Hyphae after exposure to B. mycoides CHT2402 cultured on TSA show deformed cell shapes with thicker cell wall and enlarged vacuoles. (c) Hyphae after exposure to B. mycoides CHT2402 cultured on SPMA. (d) Hyphae after exposure to commercially available dimethyl disulphide.
Bacillus mycoides promotes plant growth
Bacillus mycoides strains were grown in SPM broth for 7 days, mixed with peat moss and used to grow cabbage, asparagus bean, edible rape, lettuce and tomato. Plants grown in B. mycoides-treated materials were taller and had greater fresh weight compared with the controls at 21 days after planting (Table 3). Soy powder milk alone also enhanced growth of all plants except tomato (Fig. 10). Application of culture suspensions of B. mycoides CHT2402 (1:10, v/v) increased the height and weight of cabbage and edible rape twofold. The higher concentration of bacterial suspensions displayed a greater ability to promote cabbage growth (Fig. 11).

Fig. 10. Soil application of Bacillus mycoides CHT2401 and CHT2402 strains grown in soy powder milk (SPM) compared with those treated with water (CK). Bacterial suspensions (108 cfu/ml) mixed with peat moss (1:20, v/v) were incubated in plastic bags at room temperature (25–28 °C) for 24 h. The soil mixtures were used to fill a 16-compartment (4 × 4) plastic tray and 2–3 seeds were planted in each well. After germination, extra seedlings were removed to maintain one seedling per well. Plants were maintained in a greenhouse and watered daily. No fertilizers were added during the experiments. Photo was taken 21–30 days post germination.
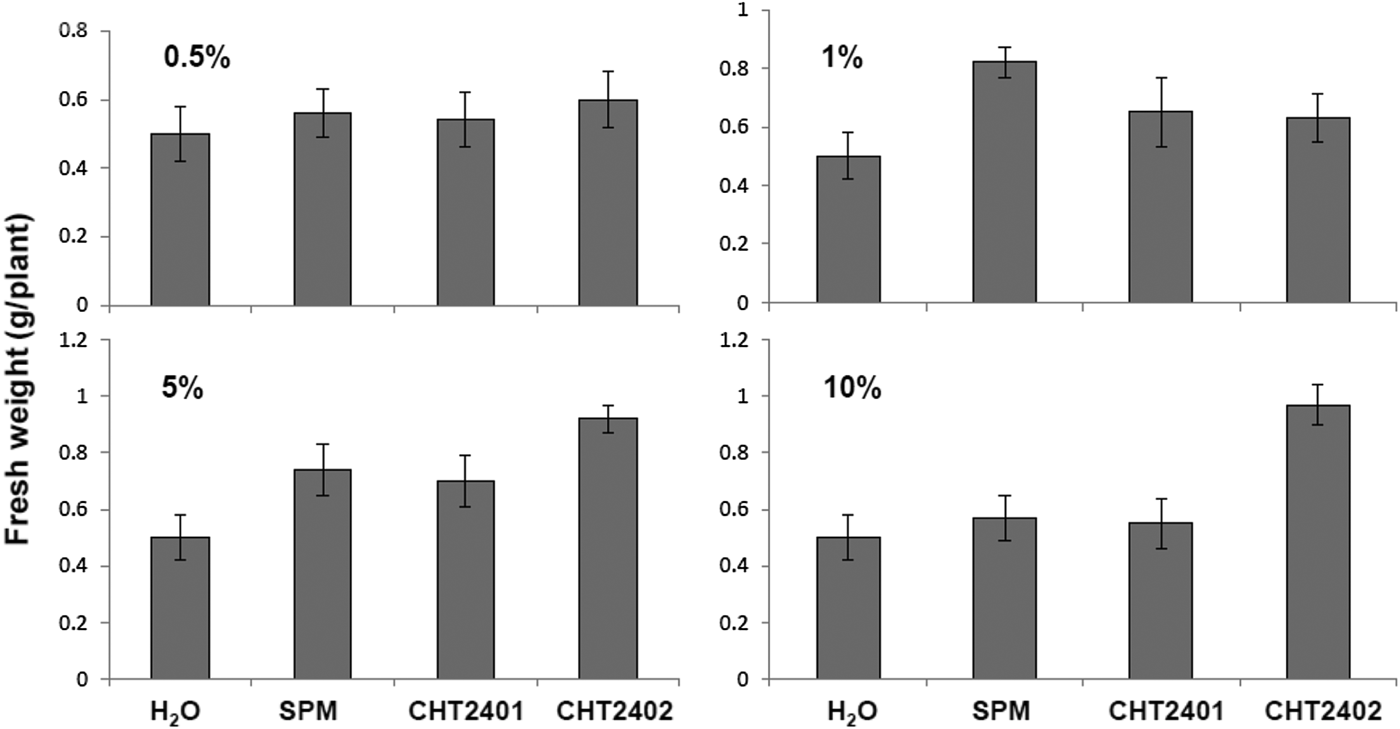
Fig. 11. Effect of Bacillus mycoides concentrations on the growth of cabbage seedlings. CHT2401 and CHT2402 strains were grown in soy powder milk (SPM) for 7 days. Bacterial suspensions (108 cfu/ml) were mixed with peat moss to make a 0.5, 1, 5 or 10% mixture. Cabbage seedlings were planted for 30 days, harvested and evaluated for fresh weight. Peat moss mixed with H2O or SPM was used as controls.
Table 3. Effect of Bacillus mycoides strains (CHT2401 and CHT2402) cultured in soy powder milk (SPM) broth on the growth of plants

a SPM medium and water were used as controls.
b Significantly different at P < 0.05 according to Fisher's protected LSD test. Experiments were conducted twice showing similar results.
Bacillus mycoides reduces cabbage seedling damping-off caused by P. aphanidermatum
Experiments were undertaken to evaluate if B. mycoides would be effective for controlling seedling damping-off caused by P. aphanidermatum or R. solani on cabbage. Cabbage seedlings grown in peat moss mixing with B. mycoides culture suspension (5 g/ml) had higher fresh weight compared with the controls regardless of the presence of the pathogens (Fig. 12). When challenged with P. aphanidermatum, B. mycoides significantly (P ⩽ 0.05) reduced damping-off incidence, by 28%. Increasing the duration of B. mycoides and P. aphanidermatum incubation in plastic bags increased the efficacy of disease reduction. When B. mycoides was incubated with P. aphanidermatum in a plastic bag for 6 days, damping-off incidence, indicated by wilting and loss of seedlings, was reduced by 45% (Fig. 13). Seedlings grown in soil amended with B. mycoides developed less severe sunken lesions on the stem than the controls. The results were similar using either CHT2401 or CHT2402. When challenged with R. solani, B. mycoides failed to reduce the incidence of seedling damping-off as assessed at 30 days post seed germination (data not shown).

Fig. 12. Effect of Bacillus mycoides CHT2401 and CHT2402 strains on the growth of cabbage seedlings. Bacterial suspensions (108 cfu/ml) grown in soy powder milk (SPM) for 7 days were mixed with peat moss to make a 5% mixture. Cabbage seedlings were planted for 30 days, harvested and evaluated for shoot and root fresh weights 21 days after planting. Peat moss mixed with H2O or SPM was used as controls.

Fig. 13. Effect of Bacillus mycoides on cabbage seedling damping-off caused by Pythium aphanidermatum. (a) Cabbage seedlings were grown in peat moss mixed with P. aphanidermatum and culture suspensions (1:20, v/v) of B. mycoides CHT2401 or CHT2402 strain cultured in soy powder milk (SPM). Seedlings grown in peat moss mixed with water (CK) or SPM alone were inoculated with P. aphanidermatum and used as controls. (b) Quantitative analysis of P. aphanidermatum incidence and damping-off of cabbage seedlings. The affected seedlings showed necrotic lesions on the basal stem, wilting and eventually dead. The percentage of damping-off incidence was calculated by dividing the number of diseased plants by the total number of plants tested. The data presented are the mean ± standard error of the mean (S.E.M.).
Discussion
It is widely accepted that the excessive application of fungicides in agricultural production may have a severe impact on the ecological environment and human health. Many fungicides have been banned or discontinued due to environmental concerns and food safety. Developing eco-friendly and effective products as alternatives to fungicides is crucial for sustainable agriculture (Pal and Gardener, Reference Pal and Gardener2006; Kanchiswamy et al., Reference Kanchiswamy, Malnoy and Maffei2015). In the current study, two B. mycoides strains were identified from plant rhizospheres and experiments were conducted to evaluate their abilities to produce antimicrobial VOCs and their potential as biocontrol agents for controlling cabbage seedling damping-off. Since B. mycoides strains and the pathogens were cultured on separate agar plates and did not physically contact each other, the inhibitory effects were probably due to the production of toxic VOCs.
In vitro assays demonstrated that DMDS and ammonia produced by B. mycoides strains have anti-microbial activity against two destructive plant pathogens. However, B. mycoides strains grown on different media displayed varying abilities to suppress the growth of P. aphanidermatum and R. solani. Bacillus mycoides strains had the best growth inhibitory effect against the test pathogens when cultured on TSA or SPMA. When grown on KBA or LA, B. mycoides displayed a moderate growth inhibitory effect against the test pathogens. When grown on NA or PDA, B. mycoides had little or no growth inhibitory effects. The results indicated that the medium used to culture B. mycoides impacts the production of VOCs consistent with the finding that growth medium can affect the quantity and the type of VOCs produced by a given microorganism (Claeson et al., Reference Claeson, Sandstrom and Sunesson2007; Blom et al., Reference Blom, Fabbri, Eberl and Weisskopf2011; Audrain et al., Reference Audrain, Farag, Ryu and Ghigo2015). Studies have also revealed that the types of VOCs (1-undecene, benzoic acid, 2-hydroxy- and methyl-ester, methane, thiobis- and benzyl methyl sulphide) produced by Enterobacter spp. and Pseudomonas putida vary considerably depending on the types of medium used to culture them (WZ Yang, personal communication).
The varying levels of DMDS and ammonia produced by B. mycoides grown on different media could be due to the changes in biosynthetic pathways. Methanethiol derived from the sulphur-containing amino acid methionine is the major precursor for the biosynthesis of dimethyl sulphide (Schulz and Dickschat, Reference Schulz and Dickschat2007). Other sulphur-containing amino acids such as cysteine might serve as a precursor for DMDS biosynthesis (Meldau et al., Reference Meldau, Meldau, Hoang, Underberg, Wünsche and Baldwin2013). Thus, the presence of sulphur may interfere with DMDS accumulation. Ammonia is mainly synthesized through the nitrogen fixation process in bacteria and its production is also impacted by nutritional components (Howell et al., Reference Howell, Beier and Stipanovic1988). Both B. mycoides strains cultured on TSA or SPMA produced VOCs that were identified as DMDS and ammonia by GC-MS and Gas-tech, respectively. Bioactivity assays using commercially available DMDS and ammonia confirmed their toxicity to P. aphanidermatum and R. solani. Thus, it was concluded that DMDS and ammonia are two predominant compounds produced by B. mycoides and are responsible for growth reduction of the test pathogens.
Bacillus mycoides strains produce DMDS and ammonia that suppress radial growth, cause hyphal deformation, and result in organelle degeneration in both R. solani and P. aphanidermatum. Scanning electron microscopy analysis revealed that pathogen hyphae, upon exposure to VOCs show poor rigidity, shrinkage, curling and swelling. The results suggest that DMDS and ammonia damage the cell membrane, which could result in electrolyte leakage. Transmission electron microscopy analysis indicated that DMDS and ammonia could also affect cell wall integrity, resulting in deformed cells with thicker cell walls and enlarged vacuoles in P. aphanidermatum. Such deformations were not observed in R. solani using TEM. The discrepancy could be attributed to fundamental differences in the cell wall composition between R. solani and P. aphanidermatum. Pythium aphanidermatum is an oomycete whose cell wall is mainly composed of cellulose, β-1,3-glucan and β-1,6-glucan (Blaschek et al., Reference Blaschek, Käsbauer, Kraus and Franz1992). The cell wall of R. solani is mainly made up of chitin, β-1,3-glucan, β-1,6-glucan, mannan and proteins (Adams, Reference Adams2004). Volatile organic compounds produced by B. subtilis have also been shown to cause hyphal deformation in other fungi, including Alternaria alternata, Cladosporium oxysporum, Fusarium oxysporum and R. solani (Fiddaman and Rossall, Reference Fiddaman and Rossall1993; Auger et al., Reference Auger, Arnault, Diwo-Allain, Ravier, Molia and Pettiti2004).
A successful biological control agent could produce secondary metabolites that are toxic to target pathogens. In addition, biological control agents could promote plant growth and induce host resistance to pathogens. In the current study, B. mycoides cultured in roasted SPM (10 g/ml) and mixed with peat moss enhanced the growth of cabbage, edible rape, asparagus bean, lettuce and tomato. The mechanism of growth stimulation in plants after treatment with CHT2401 or CHT2402 remains unknown. The mechanisms of plant growth promotion by a microorganism could be attributed to the production of plant growth regulators (i.e. indole acetic acid, cytokinin and ethylene) or VOCs, due to the enhancement of nutrient absorption from soils, or due to the elevation of disease resistance (Siddiqui, Reference Siddiqui2006). Many VOCs including 2,3-butanediol, 3-methyl-1-butanol and acetone produced by B. subtilis stimulate plant growth (Ryu et al., Reference Ryu, Farag, Hu, Reddy, Klopper and Paré2004; Farag et al., Reference Farag, Ryu, Sumner and Pare2006). However, those VOCs were not detected in the samples collected from B. mycoides cultures by SPME/GC-MS analysis. DMDS produced by a Bacillus sp. B55 strain promoted plant growth by enhancing sulphur uptake/assimilation/metabolism (Meldau et al., Reference Meldau, Meldau, Hoang, Underberg, Wünsche and Baldwin2013). In contrast, ammonia produced by bacteria inhibited plant growth (Weise et al., Reference Weise, Kai and Piechulla2013).
Although B. mycoides suppressed the growth of R. solani and P. aphanidermatum in vitro, greenhouse trials revealed that B. mycoides fails to reduce damping-off incidence caused by R. solani. In vitro assays using commercially available DMDS and ammonia also revealed that R. solani is less sensitive to those compounds than P. aphanidermatum. This may explain why B. mycoides can reduce cabbage damping-off caused by P. aphanidermatum but not that by R. solani. Another possible explanation is that DMDS and ammonia can reduce the formation of zoospores that are crucial for infectivity of P. aphanidermatum. Another reason that B. mycoides strains cultured in SPM did not reduce R. solani-induced damping-off could likely be due to differences in the composition of medium used to culture B. mycoides. Previously, it was found that CHT2401 and CHT2402 cultured in soybean or maize meal, but not in potato dextrose or nutrient broth, reduced tomato Fusarium wilt caused by F. oxysporum f. sp. lycopersici and powdery mildew (Ding and Huang, Reference Ding and Huang2017). A combination of spent blewit mushroom compost and B. aryabhattai has recently been shown to control Pythium damping-off in cucumber (Chen et al., Reference Chen, Lin and Huang2015). A B. mycoides strain isolated from the rice rhizosphere has recently been shown to produce biosurfactants, suppress the formation of zoospores by P. aphanidermatum and reduce damping-off by 35% in cucumber (Peng et al., Reference Peng, Chou, Liu, Jen, Chung and Huang2017). It was also found that increasing the duration of B. mycoides and P. aphanidermatum incubation in plastic bags could increase the efficacy of disease reduction. Taken together, it appears that the efficacy of B. mycoides for controlling different plant diseases might be improved through fermentation processes. Moreover, preliminary studies have found that treating plants with B. mycoides could lead to the induction of systemic acquired resistance (Jenn-Wen Huang, unpublished data). Nevertheless, the results derived from the current study provide information showing the potential of using B. mycoides as a biocontrol agent in controlling certain plant pathogens.
Financial support
This research was supported by a grant from the National Science and Technology Program for Agricultural Biotechnology, National Science Council (NSC) in Taiwan (No. NSC 96-2317-B-005-018) to JWH and in part by the Ministry of Education, Taiwan, R.O.C. under the Higher Education Sprout Project.
Conflict of interest
None.
Ethical standards
Not applicable.


















