Introduction
The number of studies investigating the effects of human activities on cetacean populations has grown substantially as human impacts on oceans and marine biodiversity become more evident (Lotze et al., Reference Lotze, Lenihan, Bourque, Bradbury, Cooke, Kay, Kidwell, Kirby, Peterson and Jackson2006; Parsons et al., Reference Parsons, Baulch, Bechshoft, Bellazzi, Bouchet, Cosentino, Godard-Codding, Gulland, Hoffmann-Kuhnt, Hoyt, Livermore, MacLeod, Matrai, Munger, Ochiai, Peyman, Recalde-Salas, Regnery, Rojas-Bracho, Salgado-Kent, Slooten, Wang, Wilson, Wright, Young, Zwamborn and Sutherland2015). Anthropogenic impacts, such as by-catch, fish stock depletion, habitat destruction, chemical and noise pollution, ship strikes and marine traffic can all have potentially negative consequences on cetaceans (Wright et al., Reference Wright, Soto, Baldwin, Bateson, Beale, Clark, Deak, Edwards, Fernandez, Godinho, Hatch, Kakuschke, Lusseau, Martineau, Romero, Weilgart, Wintle, Notarbartolo-di-Sciara and Martin2007; Bailey et al., Reference Bailey, Senior, Simmons, Rusin, Picken and Thompson2010; Thompson et al., Reference Thompson, Lusseau, Barton, Simmons, Rusin and Bailey2010; de Boer et al., Reference de Boer, Saulino, Leopold, Reijnders and Simmonds2012; Marçalo et al., Reference Marçalo, Katara, Feijó, Araujo, Oliveira, Santos, Ferreira, Monteiro, Pierce, Silva and Vingada2015; Baş et al., Reference Baş, Christiansen, Öztürk, Öztürk, Erdogan and Watson2017b; Oakley et al., Reference Oakley, Williams and Thomas2017).
Among these, marine traffic can have both short- and long-term negative effects on cetaceans (Lusseau, Reference Lusseau2003a; Neumann & Orams, Reference Neumann and Orams2006; Lusseau & Bejder, Reference Lusseau and Bejder2007; La Manna et al., Reference La Manna, Manghi, Pavan, Lo Mascolo and Sara2013; Dyndo et al., Reference Dyndo, Wisniewska, Rojano-Doñate and Madsen2015; Pennino et al., Reference Pennino, Roda, Pierce and Rotta2016; Baş et al., Reference Baş, Christiansen, Öztürk, Öztürk and McIntosh2017a, Reference Baş, Christiansen, Öztürk, Öztürk, Erdogan and Watson2017b; Cecchetti et al., Reference Cecchetti, Stockin, Gordon and Azevedo2017; Oakley et al., Reference Oakley, Williams and Thomas2017). Short-term effects include behavioural changes, such as an increase in travelling and a reduction in resting, foraging, socializing and nursing times (e.g. Neumann & Orams, Reference Neumann and Orams2006; Stensland & Berggren, Reference Stensland and Berggren2007; Baş et al., Reference Baş, Christiansen, Öztürk, Öztürk and McIntosh2017a, Reference Baş, Christiansen, Öztürk, Öztürk, Erdogan and Watson2017b; Cecchetti et al., Reference Cecchetti, Stockin, Gordon and Azevedo2017). Critically, due to their effect on the energy budget of individuals (Christiansen & Lusseau, Reference Christiansen and Lusseau2015; Baş et al., Reference Baş, Christiansen, Öztürk, Öztürk and McIntosh2017a, Reference Baş, Christiansen, Öztürk, Öztürk, Erdogan and Watson2017b), these changes in behaviour are likely to have long-term population-level consequences if human pressures remain constant (Lusseau & Bejder, Reference Lusseau and Bejder2007). Long-term effects could include area abandonment and decreased reproductive and nursing success (Bejder et al., Reference Bejder, Samuels, Whitehead, Gales, Mann, Connor, Heithaus, Watson-Capps, Flaherty and Krützen2006; Lusseau & Bejder, Reference Lusseau and Bejder2007; Stensland & Berggren, Reference Stensland and Berggren2007; Wright et al., Reference Wright, Soto, Baldwin, Bateson, Beale, Clark, Deak, Edwards, Fernandez, Godinho, Hatch, Kakuschke, Lusseau, Martineau, Romero, Weilgart, Wintle, Notarbartolo-di-Sciara and Martin2007).
Fishing presents an additional type of anthropogenic pressure on cetaceans in critical ways. While the interaction between fisheries and dolphins may provide a unique foraging opportunity (Chilvers & Corkeron, Reference Chilvers and Corkeron2001; Chilvers et al., Reference Chilvers, Corkeron and Puotinen2003; Brotons et al., Reference Brotons, Grau and Rendell2008), it also presents associated risks of encirclement and by-catch, which may result in injury or mortality (Read, Reference Read2008; Escalle et al., Reference Escalle, Capietto, Chavance, Dubroca, De Molina A, Murua, Gaertner, Romanov, Spitz, Kiszka, Floch, Damiano and Merigot2015, Christiansen et al., Reference Christiansen, McHugh, Bejder, Eilidh, Siegal, Lusseau, McCabe, Lovewell and Wells2016). In areas of the Atlantic Ocean, the Mediterranean Sea, the Indian Ocean and the Black Sea, bottlenose dolphins (Tursiops truncatus) appear in close proximity, and interact with, both trawlers and purse seiners, typically engaging in foraging activities (Mattson et al., Reference Mattson, Thomas and St Aubin2005; Wise et al., Reference Wise, Silva, Ferreira, Silva and Sequeira2007; Birkun et al., Reference Birkun, Northridge, Willsteed, James, Kilgour, Lander and Fitzgerald2014; Marçalo et al., Reference Marçalo, Katara, Feijó, Araujo, Oliveira, Santos, Ferreira, Monteiro, Pierce, Silva and Vingada2015; Siegel et al., Reference Siegel, Detweiler, Breeden, West, Clark and Kirby2015; Pennino et al., Reference Pennino, Roda, Pierce and Rotta2016; Allen et al., Reference Allen, Pollock, Bouchet, Kobryn, McElligott, Nicholson, Smith and Loneragan2017). Some communities of bottlenose dolphins even specialize in this type of foraging (Chilvers & Corkeron, Reference Chilvers and Corkeron2001; Chilvers et al., Reference Chilvers, Corkeron and Puotinen2003; Jaiteh et al., Reference Jaiteh, Allen, Meeuwig and Loneragan2013). Similarly, in some areas, common dolphins (Delphinus delphis) have shown close association with fishing vessels, with bigger groups found in the presence of purse seiners and other fishing vessels (Lennert-Cody et al., Reference Lennert-Cody, Minami and Hall2004; Hamer et al., Reference Hamer, Ward and McGarvey2008; de Boer et al., Reference de Boer, Saulino, Leopold, Reijnders and Simmonds2012). The attraction response behaviours of both bottlenose and common dolphins towards fishing vessels contrast with avoidance responses often displayed towards other vessel types documented in the study area (Baş et al., Reference Baş, Christiansen, Öztürk, Öztürk, Erdogan and Watson2017b). There are considerably fewer studies regarding behavioural responses of other species, such as harbour porpoises (Phocoena phocoena), to specific boat types. Baş et al. (Reference Baş, Christiansen, Öztürk, Öztürk and McIntosh2017a) demonstrated the effect of general marine traffic presence on the behavioural budget of harbour porpoises in the Istanbul Strait, revealing a significant increase in porpoises' avoidance behaviours in the presence of marine traffic, whilst Oakley et al. (Reference Oakley, Williams and Thomas2017) reported that harbour porpoise behaviour is hardly affected by boat type.
The three odontocete species, namely bottlenose dolphins, short-beaked common dolphins and harbour porpoises, and the subspecies recorded in the Istanbul Strait, are considered at risk according to the IUCN (Bearzi, Reference Bearzi2003; Birkun, Reference Birkun2008, Reference Birkun2012; Birkun & Frantzis, Reference Birkun and Frantzis2008; Bearzi et al., Reference Bearzi, Fortuna and Reeves2012). Moreover, despite its ecological importance (Öztürk & Öztürk, Reference Öztürk and Öztürk1996), the Strait is under heavy pressure from human activity (Kara, Reference Kara2016; Baş et al., Reference Baş, Christiansen, Öztürk, Öztürk, Erdogan and Watson2017b), with an average of 130 commercial cargo vessels and 2500 domestic vessels passing through the Strait daily (Baş et al., Reference Baş, Christiansen, Öztürk, Öztürk, Erdogan and Watson2017b). In addition, there are 2742 registered fishing vessels within the Marmara region (Figure 1), including the Strait, and 2018 vessels within the Western Black Sea, including the vessels registered in the Strait (Turkish Fishery Statistics, 2017). Together, these regions comprise 33% of the total registered fishing fleet within Turkish waters. Trawlers account for 182 and 229 vessels and purse seiners account for 122 and 76 vessels in the Marmara and Western Black Sea, respectively. However, aside from artisanal fishing, only purse seiners are legally allowed to operate within the Strait, from Beykoz to the northern exit to the Black Sea and the southern bordering waters of the Marmara Sea (Öztürk et al., Reference Öztürk, Karakulak, Öztürk, Öztürk and Ozkan2002). Moreover, the Istanbul Strait is an important fishing area for Turkey, both for industrial and artisanal fishing, with 17 fishing ports located along its 31 km long coastline (Öztürk et al., Reference Öztürk, Karakulak, Öztürk, Öztürk and Ozkan2002). Purse seiners in the area are rarely found transiting between fishing grounds as they rely on these waters for the majority of their catch. Therefore, the Istanbul Strait is a unique study area to understand the effect of fishing boats in a high-density marine traffic area on several cetacean species. Previous studies in the area revealed the effect of general marine traffic on cetacean behaviour (Baş et al., Reference Baş, Christiansen, Öztürk, Öztürk and McIntosh2017a, Reference Baş, Christiansen, Öztürk, Öztürk, Erdogan and Watson2017b) and the consequences of interactions between dolphins and turbot fisheries within the neighbouring waters of Istanbul and the Black Sea (Tonay, Reference Tonay2016). Although the effect of industrial fishing practices has been studied worldwide, no research effort has yet focused on the relationship between cetacean behaviour and purse seiners' presence in this area.
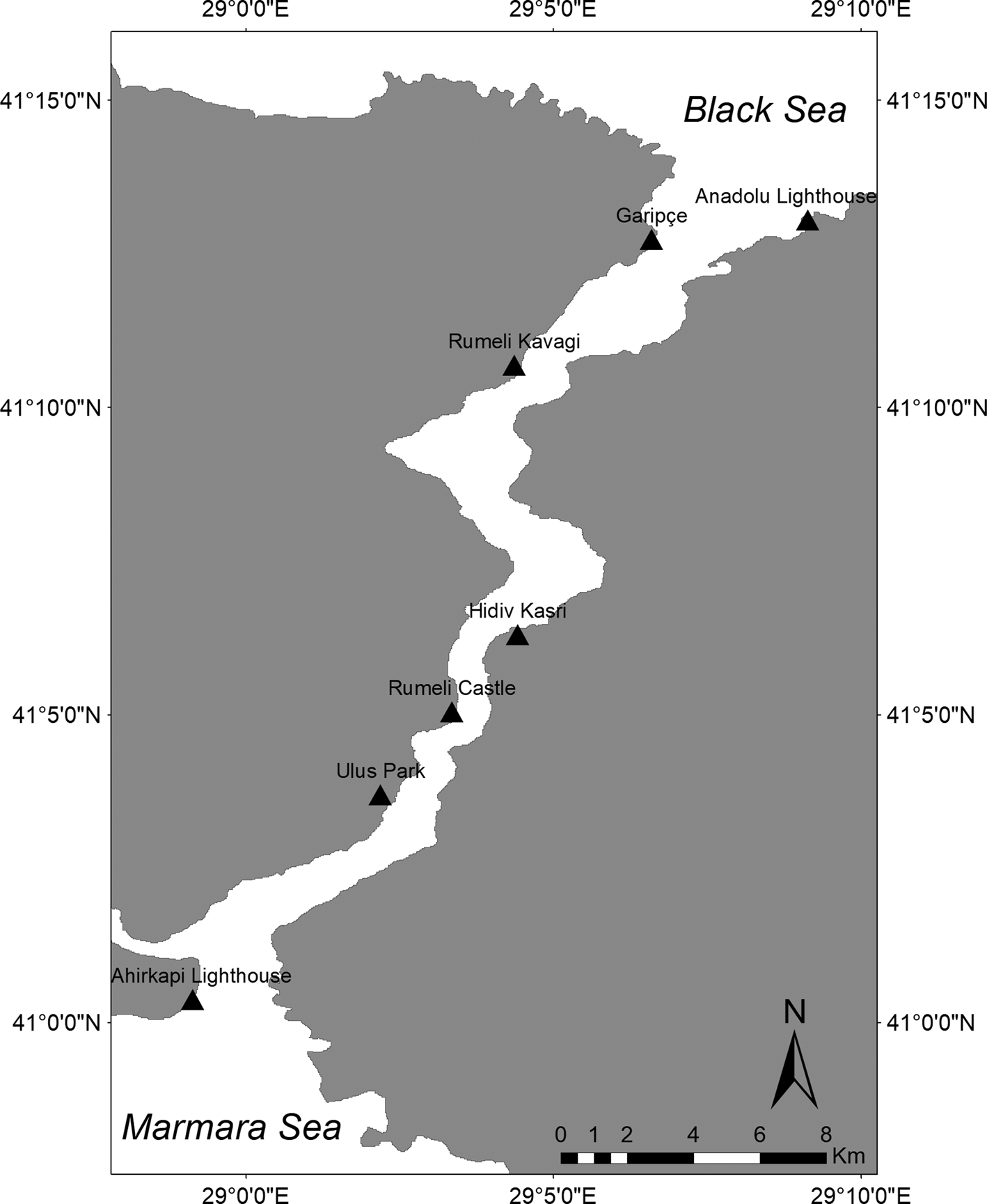
Fig. 1. Location of the study area. Triangles represent the survey stations. Ahirkapi Lighthouse (41°0′22″N 28°59′8″E; 38 m), Ulus Park (41°3′42″N 29°2′1″E; 30 m), Rumeli Castle (41°5′3″E 29°3′21″N; 44 m), Hidiv Kasri (41°6′18″ N 29°4′25″E; 90 m), Rumeli Kavagi (41°10′41″N 29°4′22″E; 45 m), Garipçe (41°12′44″N; 29°6′36″E; 45 m) and Anadolu Lighthouse (41°13′3″N 29°9′8″E; 55 m).
The behavioural responses of cetaceans to purse seiners is a critically important research topic due to the potential for injury and mortality associated with this type of marine vessel. Thus, the current study aims to (1) provide a description of the behavioural changes occurring in the Strait when odontocetes are in the presence of purse seiners and (2) consider the possible consequences of close encounters with purse seiners in one of the busiest waterways of the world, the Istanbul Strait.
Methods and materials
Data collection
Survey platforms
Systematic land surveys were conducted weekly, throughout the day, in the Istanbul Strait and adjacent waters between September 2011 and September 2013 (Figure 1). Seven different locations on the coastline were chosen as land stations to get a representative coverage of the whole Strait (Figure 1). Data were collected using a Sokkia DT5A Electronic Theodolite. Theodolite stations were selected at least 30 m above sea level. Reference points and exact theodolite locations were kept constant throughout the study. Surveys lasted between 3 to 4 h during daylight hours, starting and ending at sunrise and sunset and environmental variables (Beaufort, cloud cover and glare) were collected hourly.
The theodolite was linked to the tracking software Pythagoras v.1.2 to transform the theodolite data to geographic coordinates and record the geographic location of animals and marine vessels (Würsig et al., Reference Würsig, Cipriano, Würsig, Pryor and Norris1991; Lerczak & Hobbs, Reference Lerczak and Hobbs1998).
Behavioural sampling
Instantaneous focal group scan sampling (Altmann, Reference Altmann1974) was used during the behavioural data collection, with 3 min sampling intervals. A set of individuals was defined as a group when the animals were engaged in similar behaviour and were closer than 50 m from one another (Baş et al., Reference Baş, Christiansen, Öztürk, Öztürk and McIntosh2017a, Reference Baş, Christiansen, Öztürk, Öztürk, Erdogan and Watson2017b). The predominant behaviour (the behaviour of >50% of the group) was recorded every 3 min. If the group was not seen between 3 and 20 min, the 3 min interval was restarted when the next sighting was recorded. If the group was not visible for longer than 20 min, the next sighting was considered a different group for analytical purposes. Sampling ended when the group of animals left the site or when the environmental conditions forced the survey to stop. When two species were seen at the same time, both species' behaviours were recorded. However, if the distance between the focal groups was less than 1000 m, the behavioural data were excluded from the analyses to minimize the possible effect of inter-species interactions.
The behaviour states used are presented in Table 1. Surface feeding and diving were grouped under foraging, following Lusseau (Reference Lusseau2003a), Constantine et al. (Reference Constantine, Brunton and Dennis2004), Stockin et al. (Reference Stockin, Lusseau, Binedell, Wiseman and Orams2008) and Meissner et al. (Reference Meissner, Christiansen, Martinez, Pawley, Orams and Stockin2015). Other diving related behaviours, such as travel diving, were grouped with travelling behaviour, as the animals followed a clear directional movement. In addition, socializing, milling and resting had to be grouped under resting-socializing following Baş et al. (Reference Baş, Christiansen, Öztürk, Öztürk, Erdogan and Watson2017b) due to low sighting numbers. Behaviours such as travel diving were determined by checking the first and second behavioural recording of the group, based on time and distance, from theodolite data.
Table 1. Definition of behaviours recorded in this study, adapted from Lusseau (Reference Lusseau2003a) and Christiansen et al. (Reference Christiansen, Lusseau, Stensland and Berggren2010)

Marine vessel sampling
Marine traffic data were collected in two stages. First, vessel presence and type were recorded every 10 min throughout surveys and independent of dolphin presence. Second, vessel presence and density were recorded for each behavioural sampling unit during a species sighting. The nearest marine vessel type to the focal group, as well as the number of vessels at different distances from the animals (100, 400 and 1000 m), were recorded.
Data analysis
Behavioural transitions probabilities
Markov chain analyses were used to quantify the one-way dependence of a behavioural state on the previous behavioural state (Baş et al., Reference Baş, Christiansen, Öztürk, Öztürk and McIntosh2017a, Reference Baş, Christiansen, Öztürk, Öztürk, Erdogan and Watson2017b). In the case of bottlenose dolphins, three behavioural states were analysed (travelling, resting-socializing and foraging), whilst only two behaviours were analysed (travelling and foraging) for common dolphins and harbour porpoises, due to limited recordings of resting-socializing behaviour in these two species. The behavioural states were recorded as: preceding (P) (time: t min) and succeeding behaviour (S) (time: t + 3 min) and pooled under control or impact groups. The impact group was defined as any observation made in the presence of purse seine vessels as the closest vessel. Behavioural transitions recorded in the presence of any other type of vessels or boats were discarded. The control group was defined as any observation with no marine vessel presence of any type within 1000 m radius of the focal group (Baş et al., Reference Baş, Christiansen, Öztürk, Öztürk and McIntosh2017a, Reference Baş, Christiansen, Öztürk, Öztürk, Erdogan and Watson2017b). The control group was comprised of behavioural transitions (P to S) that occurred after at least 9 min (3 sampling units) of vessel absence on at least 1000 m radius to the focal group, i.e. the fourth sampling unit. This 9 min interval was longer than those in previous studies and therefore more conservative (Meissner et al., Reference Meissner, Christiansen, Martinez, Pawley, Orams and Stockin2015) to ensure that the effect of other vessels in such a busy area was minimal. The impact group comprised only the behavioural transitions that occurred within 400 m of purse seiners, independently of the seiner behaviour, with no other vessels within 400 m. The radius of 400 metres was chosen in order to optimize the amount of data usable, and still maintain the possibility of studying the highest effect of the vessels. Recordings where the closest vessel of any type was within 400–1000 m of the group of the animals were discarded for Markov chain analyses.
Subsequently, data were pooled to create two contingency tables for control and impact chains to analyse the temporal dependence between behavioural states (Lusseau, Reference Lusseau2003a; Christiansen et al., Reference Christiansen, Lusseau, Stensland and Berggren2010; Meissner et al., Reference Meissner, Christiansen, Martinez, Pawley, Orams and Stockin2015; Baş et al., Reference Baş, Christiansen, Öztürk, Öztürk, Erdogan and Watson2017b).
Transition probabilities were calculated for both impact and control contingency tables according to the following equation:
where i is the preceding behaviour, j is the succeeding behaviour (i and j range from 1 to n being the total of behavioural states in the analysis), p is the probability of transition from preceding to succeeding behaviour, and aij is the number of transitions observed from behavioural state i to j (Lusseau, Reference Lusseau2003a; Baş et al., Reference Baş, Christiansen, Öztürk, Öztürk, Erdogan and Watson2017b). The differences between the transition probabilities of control and impact chains were analysed with a chi-square test (Lusseau, Reference Lusseau2003a; Christiansen et al., Reference Christiansen, Lusseau, Stensland and Berggren2010; Baş et al., Reference Baş, Christiansen, Öztürk, Öztürk, Erdogan and Watson2017b). Control transitions were then compared to the corresponding impact transitions with a two-sample test for equality of proportions with continuity correction (Fleiss, Reference Fleiss and Fleis1981; Lusseau, Reference Lusseau2003a; Christiansen et al., Reference Christiansen, Lusseau, Stensland and Berggren2010; Baş et al., Reference Baş, Christiansen, Öztürk, Öztürk, Erdogan and Watson2017b).
Behavioural budgets
Eigen-analysis of both the control and impact matrices was performed to analyse changes in behavioural budgets (Lusseau, Reference Lusseau2003a, Reference Lusseau2004). The differences between control and impact budgets were then tested with a chi-square test (Fleiss, Reference Fleiss and Fleis1981; Lusseau, Reference Lusseau2003a; Baş et al., Reference Baş, Christiansen, Öztürk, Öztürk, Erdogan and Watson2017b). Each specific behavioural state from the control budget was compared with the corresponding impact behavioural state from the impact budget using a two-sample test for equality of proportions with continuity correction. 95% confidence intervals were calculated for the estimated proportion of time spent within each behavioural state (Lusseau, Reference Lusseau2003a).
Bout lengths
The average time spent in each behavioural state (bout length) tii was estimated for the two chains, as described by Lusseau (Reference Lusseau2003a):
with
 $${\rm SE} = \sqrt {\displaystyle{{\,p_{ii}x\lpar 1-p_{ii\rpar }} \over {n_i}}} $$
$${\rm SE} = \sqrt {\displaystyle{{\,p_{ii}x\lpar 1-p_{ii\rpar }} \over {n_i}}} $$where i is the preceding behaviour and ni the number of samples. Bout lengths were then compared between impact and control group using a Student's t-test.
Cumulative behavioural budgets
Cumulative behavioural budgets account for the time animals spend on both the control and impact budgets during a defined time period, assuming that vessel presence will not significantly vary during the night time (Lusseau, Reference Lusseau2003a; Meissner et al., Reference Meissner, Christiansen, Martinez, Pawley, Orams and Stockin2015; Baş et al., Reference Baş, Christiansen, Öztürk, Öztürk and McIntosh2017a, Reference Baş, Christiansen, Öztürk, Öztürk, Erdogan and Watson2017b). Following Lusseau (Reference Lusseau2004) and Christiansen et al. (Reference Christiansen, Lusseau, Stensland and Berggren2010), the cumulative behavioural budget was calculated according to:
where a is the proportion of time that animals spend exposed to fishing vessels (in the 400 m radius) and b is the proportion of time (1–a) animals spend away from fishing vessels. The level of vessel exposure at which the behavioural budget becomes significantly affected can be determined by artificially varying this proportion from 0 to 100% (Lusseau, Reference Lusseau2004). χ2 analyses and two-sample tests for equality of proportions with continuity correction for each behavioural state were run to assess the level of fishing vessel exposure at which significant changes in the cumulative behavioural budgets occur (Fleiss, Reference Fleiss and Fleis1981; Christiansen et al., Reference Christiansen, Lusseau, Stensland and Berggren2010).
Possible confounding effects
Seasonal and diurnal changes in purse seine vessel abundance were investigated using χ2 to look for independence of data. Multinomial regression models of behaviour with respect to vessel abundance over 400 m were run for each species to look for changes in behaviour. Finally, log-linear models of behaviour were run for all three species to identify which explanatory variables had an effect on succeeding behavioural states. The variables used in these regressions (season, section, station, preceding behaviour, vessel presence and vessel abundance) were dropped in a stepwise fashion until the models yielded the smallest AIC value. All regressions were run on R as generalized linear models.
Results
A total of 308 days (1631 h) were spent surveying for cetaceans in the Istanbul Strait, of which bottlenose dolphins, common dolphins and harbour porpoises were sighted on 164 days (204 h), 63 days (65.8 h) and 85 days (58.6 h) respectively. Bottlenose dolphins were recorded in the presence of purse seiners (<400 m) 207 times out of 895 sightings, common dolphins 26 out of 299, and harbour porpoises 29 out of 288. Therefore, the exposure levels of bottlenose dolphins, common dolphins and harbour porpoises to purse seine vessels were 23.1%, 8.7% and 10.1% respectively.
Behavioural transition probabilities
Bottlenose dolphins' behavioural transitions significantly changed in the presence of purse seiners (Goodness-of-fit test, χ2 = 216.67, df = 4, P = 0.0001). In the presence of purse seine vessels four of nine behavioural transitions were affected (Figure 2). Two of the transitions, travelling to travelling (Z-test = 8.77, P = 0.003) and foraging to travelling (Z-test = 6.51, P = 0.01), significantly decreased in the presence of purse seine vessels. In contrast, the probability of changing from travelling to foraging (Z-test = 16.11, P = 0.0001) and foraging to foraging (Z-test = 11.31, P = 0.0008) significantly increased.

Fig. 2. Transition matrices for control (C) and impact (I) chains of T. truncatus behaviours. Behaviours were travelling (TR), resting-socializing (SOC) and diving-surface feeding (FOR). Numbers represent probabilities. Underlined (green) numbers represent significant decreases and overlined (red) numbers represent significant increases.
Results for common dolphins and harbour porpoises, showed that behavioural transitions significantly changed when exposed to purse seine vessels (Goodness-of-fit test, χ2D. delphis = 75.10, df = 1, P = 0.0001; Goodness-of-fit test, χ2P. phocoena = 19.33, df = 1, P < 0.0001). However, no significant differences were found for any specific pairwise transition (Figure 3).

Fig. 3. Transition matrices for control and impact chains of D. delphis and P. phocoena behaviours. Numbers represent the probability of transition. Behaviours were travelling (TR) and diving-surface feeding (FOR).
Behavioural budgets
The behavioural budgets of all three species were significantly affected by exposure to purse seine vessels: (χ2T. truncatus = 178.30, df = 2, P < 0.0001; χ2D. delphis = 47.32, df = 1, P < 0.000; χ2P. phocoena = 10.98, df = 1, P < 0.001).
Bottlenose dolphins and common dolphins showed an increase in foraging in the impact budget (Z-testT. truncatus = 59.8, P < 0.0001, control = 43%, impact = 74%; Z-testD. delphis = 5.98, P = 0.01, control = 37%, impact = 67%), and a decrease in travelling (Z-testT. truncatus = 36.2, P < 0.0001, control = 46%, impact = 22%; Z-testD. delphis = 5.98, P = 0.01, control = 63%, impact = 32%). In addition, bottlenose dolphins showed a decrease in the proportion of resting-socializing behaviour (Z-test = 8.12, P = 0.004, control = 11%, impact, 4%) in the presence of purse seine vessels (Figure 4).

Fig. 4. Behavioural budgets of control and impact chains for bottlenose dolphins (T. truncatus), common dolphins (D. delphis), and harbour porpoises (P. phocoena). Behaviours were travelling (TR), resting-socializing (SOC) and diving-surface feeding (FR). Error bars represent 95% confidence intervals. Stars indicate significant differences in proportion of behaviour between control and impact chains (P < 0.05).
Despite significant differences in their total behavioural budget, harbour porpoises showed no significant results when looking at the differences between the proportions of foraging (Z-test = 0.38, P = 0.54, control = 60%, impact = 45%) and travelling behaviours (Z-test = 0.38, P = 0.54, control = 40%, impact = 55%) in both situations (Figure 4).
Bout lengths
The average bout lengths of bottlenose dolphin engaged in foraging and travelling showed significant differences between impact and control chains, while resting-socializing did not (Figure 5). Foraging bout length increased from 8.67 ± 0.07 SD min in control situations, to 17.36 ± 0.1 SD min in impact situations (Student's t-test = −59.93, df = 501, P < 0.0001), while travelling bout length decreased from 8.82 ± 0.07 SD min in control situations, to 5.58 ± 0.18 SD min in impact situations (Student's t-test = 17.14, df = 400, P < 0.0001). Common dolphins revealed a similar result, with significant changes in the presence of purse seine vessels (Figure 5). Their foraging bout length increased from 8.46 ± 0.16 SD min to 13.5 ± 0.42 SD min in impact situations (Student's t-test = −10.10, df = 86, P < 0.0001), while travelling decreased from 14.52 ± 0.09 SD min to 6.6 ± 0.45 SD min in impact situations (Student's t-test = 20.73, df = 193, P < 0.0001).

Fig. 5. Bout lengths of each behaviour during control and impact chains for bottlenose dolphins (T. truncatus), common dolphins (D. delphis), and harbour porpoises (P. phocoena). Behaviours were travelling (TR), resting-socialising (SOC) and diving-surface feeding (FR). Error bars represent 95% confidence intervals. Stars indicate significant differences in bout length between control and impact chains (P < 0.05).
Finally, harbour porpoises showed a significant change in foraging bout length in the presence of purse seine vessels reduced from 11.8 ± 0.1 SD min to 7.5 ± 0.66 SD min (Student's t-test = 7.32, df = 165, P < 0.0001) (Figure 5). Travelling bout length increased, although not significantly, from 8.06 ± 0.13 SD min to 9 ± 0.58 SD min during impact situations (Student t-test = −1.55, df = 133, P = 0.1).
Cumulative behavioural budgets
The cumulative budget of bottlenose dolphins showed significant changes at the recorded purse seine vessel exposure level of 23.1% (χ2 = 9.2, df = 2, P = 0.018), with a significant difference in foraging and travelling behaviours (Figure 6). With the effect built linearly, resting-socializing behaviour was estimated to show a significant difference at an exposure level of 34%. Further, common dolphins, at an exposure level of 8.7%, and harbour porpoises, at an exposure level of 10.1%, did not show significant differences in their cumulative behaviour budgets (χ2D. deplhis = 0.28, df = 1, P = 0.59; χ2P. phocoena = 0.07, df = 1, P = 0.8). However, with the effect built linearly for common dolphins, both travelling and foraging behaviours were found to be affected significantly at a 29% level of exposure. For harbour porpoises, the significant effect on foraging and travelling was found at a level of exposure of 60%.

Fig. 6. Effect of marine vessels on the cumulative behavioural budget of T. truncatus during different levels of exposure. The y-axis displays the significance level of the difference between the cumulative behavioural budget and the control behavioural budget for the three behavioural states (see legend) at different purse seine vessels' exposure levels. The red line (upper horizontal) represents the statistical threshold for significance (P < 0.05). The blue line (right vertical) indicates the current exposure level of dolphins to purse seine vessels in the Istanbul Strait.
Possible confounding effects
Purse seine vessel traffic did not depend on time of day (Goodness-of-fit test, χ2 = 0.379, df = 4, P = 0.768). A significant dependence of seiner traffic on season was found (Goodness-of-fit test, χ2 = 85.06, df = 3, P < 0.001) with most traffic in the months of autumn (1 September to 1 December), followed by winter (1 December to 1 March), then spring (1 March to 1 June) and summer (1 June to 1 September). No significant pairwise differences were found.
Results showed significant effects of general vessels abundance further than 400 m on the impact group. In the case of bottlenose dolphins, analysis showed a significant decrease in resting-socializing behaviours with the increase in vessel number further than 400 m (Z-test = −1.993, P = 0.04625). On the other hand, common dolphins showed a significant increase of travelling behaviour with the increase of vessel abundance further than 400 m (Z-test = 2.075, P = 0.0380).
The same variables (preceding behaviour, vessel presence and vessel abundance) best explained the succeeding behaviour of the three species, with the log-linear models accounting for these variables showing the lowest AIC values (AIC = 2819, AIC = 668 and AIC = 761 for bottlenose dolphins, common dolphins and harbour porpoises respectively). The other variables considered (season, section and station) were dropped from the models for all three species. The AIC values of the different models tested are shown in Table 2.
Table 2. AIC values of log-linear models of succeeding behaviour. Explanatory variables were dropped in a stepwise manner until no significant change (ΔAIC≤3) in AIC value was found

Discussion
Based on the results just described, and on the knowledge on the species studied, it seems that bottlenose and common dolphins showed a significant increase in foraging behaviour whilst travelling behaviour declined significantly in the vicinity of purse seine vessels. Bottlenose and common dolphins also showed a significant increase in bout length, with foraging almost doubling whilst travelling decreased. Harbour porpoises showed a significant reduction in their foraging bout length, in line with previous results on general marine traffic (Baş et al., Reference Baş, Christiansen, Öztürk, Öztürk and McIntosh2017a). Bottlenose dolphins showed an alteration in their cumulative budget at the current purse seine vessel exposure levels (23.1%), for both foraging and travelling behaviours with an expected effect on socializing-resting behaviours at an exposure level of 41%. Significant behavioural budget alterations were expected to arise at exposure levels of 29% for common dolphins and 60% for harbour porpoises. These differences reveal that bottlenose dolphins might show greater sensitivity than the other species to the presence of purse seiners.
Bottlenose dolphins and common dolphins appear to forage more in the vicinity of purse seine vessels, which may be related to high density of prey inside and around the nets (Chilvers & Corkeron, Reference Chilvers and Corkeron2001; Chilvers et al., Reference Chilvers, Corkeron and Puotinen2003; Brotons et al., Reference Brotons, Grau and Rendell2008; Christiansen et al., Reference Christiansen, McHugh, Bejder, Eilidh, Siegal, Lusseau, McCabe, Lovewell and Wells2016). However, it is important to highlight that the current study did not consider the activity of the purse seiners. It is not possible, therefore, to determine a causative relationship. Despite this, when several variables related to the behavioural transitions were considered, purse seiner presence was still among the best explanatory variables. Therefore, even though we fail to pinpoint the exact cause, purse seines are somewhat related to the observed behavioural budget changes. Independently of the cause, the increase in foraging apparent in our results is likely to affect the animals' total behavioural budgets. Changes in behavioural budget due to human pressure are likely to cause long-term consequences both at the individual and population levels (Lusseau & Bejder, Reference Lusseau and Bejder2007; Pirotta et al., Reference Pirotta, Booth, Costa, Fleishman, Kraus, Lusseau and Simmons2018). In this case, the increase of foraging behaviour, along with the decrease of travelling behaviour, could result in changes in the home range if pressures remain constant (Lusseau & Bejder, Reference Lusseau and Bejder2007). Furthermore, bottlenose dolphins showed a significant decline in their socializing and resting behaviours, the most sensitive behaviours to human impact (Lusseau, Reference Lusseau2004). This alteration to important life functions can potentially affect the social structure of this species. A decrease in socializing behaviours may result in weaker individual bonds, which may lead to more frequent intraspecific aggression behaviours, gaps in knowledge transmission (Samuels et al., Reference Samuels, Bejder and Heinrich2000), and reduced reproductive output and pregnancy rates (Lusseau, Reference Lusseau2004). Moreover, the possible feeding opportunities provided by purse seine vessels, together with potential prey depletion, could push bottlenose dolphins to display highly risky foraging behaviours, such as feeding inside fishing nets, or in close proximity to fishing vessels (Jaiteh et al., Reference Jaiteh, Allen, Meeuwig and Loneragan2013). These types of behaviours can be linked with an increase of by-catch risk, one of the main human threats for the three species in the neighbouring waters of the Black Sea (Özturk & Özturk, Reference Öztürk and Öztürk2002; di Sciara & Birkun, Reference di Sciara and Birkun2010; Özturk, Reference Öztürk2013). Moreover, interactions between small cetaceans and purse seiners, including feeding within the nets, have been described in the Mediterranean Sea; however, in the Black Sea, this type of interaction has been mostly described for bottlenose and common dolphins, while porpoises do not seem to be attracted to the fishing vessels (di Sciara, Reference di Sciara2002).
In contrast with the behavioural alterations of bottlenose and common dolphins, harbour porpoises, when exposed to purse seiners, showed a significant decrease in time spent foraging and a non-significant increase in the time spent travelling, signalling an overall decrease in time dedicated to energy intake (Neumann & Orams, Reference Neumann and Orams2006; Stensland & Berggren, Reference Stensland and Berggren2007; Baş et al., Reference Baş, Christiansen, Öztürk, Öztürk and McIntosh2017a, Reference Baş, Christiansen, Öztürk, Öztürk, Erdogan and Watson2017b; Cecchetti et al., Reference Cecchetti, Stockin, Gordon and Azevedo2017). Moreover, these results align with previous results that suggest harbour porpoises in the Strait avoid marine traffic (Baş et al., Reference Baş, Christiansen, Öztürk, Öztürk and McIntosh2017a) and are consistent with the suggestion that harbour porpoises do not alter their behaviour in response to particular boat types (Oakley et al., Reference Oakley, Williams and Thomas2017), generally just avoiding all vessels. Such behavioural changes, even without significant effects on cumulative behavioural budgets, may limit energy intake in the short term (Lusseau & Bejder, Reference Lusseau and Bejder2007). Further, the apparent increases in travelling behaviour may point to early signs of temporal area avoidance due to purse seine vessel presence and could lead the animals to adopt a long-term avoidance strategy, such as the abandonment of the area (Lusseau & Bejder, Reference Lusseau and Bejder2007).
Alteration in behavioural transitions of bottlenose dolphins and harbour porpoises and the related consequences on their behavioural budget in the presence of marine vessels were already reported in the Istanbul Strait with a considerable increase in avoidance behaviours (Baş et al., Reference Baş, Christiansen, Öztürk, Öztürk and McIntosh2017a, Reference Baş, Christiansen, Öztürk, Öztürk, Erdogan and Watson2017b). The current study brings further insight into the plasticity of dolphin behaviour by narrowing down the analyses to the effect of purse seine vessels only and documenting behavioural reactions towards this specific type of fishing vessel. It is important to highlight that the current study only focused on the presence of purse seiners, whilst other vessel types were not considered and discarded from the analysis. Further studies should focus on the behavioural changes caused by each vessel type that is present, considering their activity states to accurately define the consequence of specific vessel presence and suggest effective conservation strategies in the Strait.
It is important to note that diving behaviour was previously categorized as both a vertical avoidance strategy (Lusseau, Reference Lusseau2003b; Baş et al., Reference Baş, Christiansen, Öztürk, Öztürk and McIntosh2017a, Reference Baş, Christiansen, Öztürk, Öztürk, Erdogan and Watson2017b) and a foraging strategy (Chilvers & Corkeron, Reference Chilvers and Corkeron2001; Lusseau, Reference Lusseau2003a; Constantine et al., Reference Constantine, Brunton and Dennis2004; Stockin et al., Reference Stockin, Lusseau, Binedell, Wiseman and Orams2008; Meissner et al., Reference Meissner, Christiansen, Martinez, Pawley, Orams and Stockin2015), including foraging in association with fishing vessels (Chilvers & Corkeron, Reference Chilvers and Corkeron2001; Chilvers et al., Reference Chilvers, Corkeron and Puotinen2003). Therefore, the increase in foraging in the Strait in association with purse seine vessels does not oppose the idea of diving as an avoidance strategy in vicinity of other vessel types. However, this points to the need for better understanding of diving behaviours along with the necessity of improved methodology for recording underwater activity, such as the use of acoustics analysis.
The current study represents the first attempt at an in-depth analysis of the effects of exposure to purse seiners on cetaceans using Markov chains. However, some limitations must be acknowledged. First, fewer recordings of resting and socializing behaviours for common dolphins and harbour porpoises may have resulted in the lack of significance in specific behavioural transitions in both of those due to the use of only two behavioural states in the analysis (foraging and travelling). However, the small number of recordings of these key behavioural states can be related to them occurring at night or to low probabilities of these behaviours occurring in the Istanbul Strait. Therefore, further studies with a wider study range are fundamental to understanding if resting and socializing behaviours are altered because of the heavy human pressure in the Strait and/or if the behaviours are simply happening somewhere else. Moreover, as the activity of the purse seine vessels was not recorded, we cannot identify the specific cause of the observed behavioural changes. However, our results point towards an important question about the behaviour of odontocetes in the area. The animals are reacting to seiners, and in some cases, differently than to other vessels. Therefore, future research on the activity of traffic is essential to increase our understanding of odontocetes' behaviour in the area, and to understand specifically if alterations in behaviour are related to the activity of vessels or the vessel itself.
There are other factors that might affect this study and that have to be acknowledged. First, we observed seasonal fluctuations in purse seine vessels. Purse seiner abundance increases in autumn and winter, the main fishing seasons in the Strait, as migratory species such as bluefish and bonito increase in abundance (Öztürk et al., Reference Öztürk, Karakulak, Öztürk, Öztürk and Ozkan2002; Dede et al., Reference Dede, Öztürk, Akamatsu, Tonay and Öztürk2014). Considering behavioural fluctuations of cetaceans in these seasons may provide a deeper understanding of the effects of fishing activity. Second, the effect of increased vessel abundance over 400 m from each species is statistically unaccounted for in the Markov Chains analysis. However, we suggest that as the Istanbul Strait is known for its extremely dense traffic, a background level of noise coming from different types of vessels has to be expected in any study undertaken in the area and that this result does not invalidate our study, as animals are constantly exposed to this disturbance. Finally, as the best fit log-linear models for all three species only included preceding behaviour, vessel presence and vessel abundance as explanatory variables but dropped season, station and section, we suggest that succeeding behavioural states are indeed affected by anthropogenic activities more strongly than by environmental factors.
These limitations are an opportunity to improve research in the area in the future. It would be interesting to study the effect of other types of fishing, specifically in the Strait, artisanal boats, as the differences in fishing technique might affect cetacean behaviour in different ways. In addition, further studies with individual identification techniques and social structure analysis are required to investigate if this reaction towards purse seine vessels is specific to certain individuals or is more generalizable.
This study broadens our understanding of the indirect interaction between cetaceans and fisheries, showing the need for a wider study of the area, especially considering the economic and ecological importance of the Strait for both humans and animals.
Acknowledgements
We would like to thank all the research assistants, fishermen and villagers who helped throughout the process of collecting the data and Istanbul University for their financial support. We also thank Directorate General of Coastal Safety for permission to use the Ahırkapı Lighthouse. We also thank Nadia Frontier, Jessica Rayner and Elisa Gaggioli for the advice on the initial writing. Finally, we would like to give a special thanks to Fredrik Christiansen for his involvement on the creation of the codes needed and used for the analysis.
Author contribution statement
A. Akkaya, C. Olaya Meza, F. Affinito, B. Öztürk and A.A. Öztürk conceived the ideas and designed methodology; A. Akkaya collected the data; C. Olaya Meza and F. Affinito analysed the data; C. Olaya Meza led the writing of the manuscript; A. Akkaya, F. Affinito, B. Öztürk and A.A. Öztürk revised the manuscript. All authors contributed critically to the drafts and gave final approval for publication.










