Introduction
Adequate weed management may have positive consequences on agriculture sustainability, enhancing agro-ecosystem productivity and also by improving ecological services (Gerowitt et al., Reference Gerowitt, Bertke, Hespelt and Tute2003). The uncontrolled presence of natural flora in tree orchards, like in any crop, increases competition especially for water and nutrients, with a subsequent reduction of yields and fruit quality. However, weed flora, where appropriately managed, may have many beneficial effects on the agro-ecosystem. Previous studies on the most common tree crops in the Mediterranean region, including olives (Montemurro et al., Reference Montemurro, Fracchiolla and Guarini2002; Corleto and Cazzato, Reference Corleto and Cazzato2008b; Simoes et al., Reference Simoes, Belo, Pinto-Cruz and Pinheiro2014), wine grapes (Corleto and Cazzato, Reference Corleto and Cazzato2008a; Ferrara et al., Reference Ferrara, Fracchiolla, Al Chami, Camposeo, Lasorella, Pacifico, Aly and Montemurro2012), table grapes (Novello et al., Reference Novello, De Palma and Montemurro1997), citrus (Colloff et al., Reference Colloff, Lindsay and Cook2013) and almond orchards (Ramos et al., Reference Ramos, Benitez, Garcia and Robles2010), support reducing tillage intensity and the permanent or temporary presence of sward, either sown or natural, especially to preserve soil fertility and to increase the agro-ecosystem's diversity (Marshall et al., Reference Marshall, Brown, Boatman, Lutman, Squire and Ward2003; Norris, Reference Norris2005; Colloff et al., Reference Colloff, Lindsay and Cook2013). The ground flora reduces the soil erosion hazard (Hernandez et al., Reference Hernandez, Lacasta and Pastor2005; Ramos et al., Reference Ramos, Benitez, Garcia and Robles2010; Chauhan et al., Reference Chauhan, Singh and Mahajan2012) and improves the chemical, physical and microbiologic fertility of the soil (Ramos et al., Reference Ramos, Robles, Sánchez-Navarro and González-Rebollar2011; Soriano et al., Reference Soriano, Álvarez, Landa and Gómez2014).
Remarkable results in almond orchards are reported in Spain by Ramos et al. (Reference Ramos, Benitez, Garcia and Robles2010), suggesting that the temporary presence of weeds and the abandoning of frequently tilled management enhance soil properties. In a research on insect pollinators, almond orchards with living ground cover showed positive relationship between native bee abundance and the richness of ground cover plants (Saunders et al.).
The weed communities should ideally combine adequate and positive effects on the agro-environment with only marginal negative competitive effects on orchard (Fracchiolla et al., Reference Fracchiolla, Caramia, Lasorella and Montemurro2013). Such effects clearly depend upon the eco-physiological and morphological traits of species and their abundance in weed communities (Altieri and Letourneau, Reference Altieri and Letourneau1982; Marshall et al., Reference Marshall, Brown, Boatman, Lutman, Squire and Ward2003; Barberi et al., Reference Barberi, Burgio, Dinelli, Moonen, Otto, Vazzana and Zanin2010). Consequently, proper weed management should not only take into account the efficacy of weed control but also how the practices affect the weed population (Naylor and Drummond, Reference Naylor, Drummond and Naylor2002). This is the reason why integrated weed management (Swanton and Murphy, Reference Swanton and Murphy1996) considers weeds not as a mere ‘target’ but as a community that ‘needs to be managed’ that is composed by individuals interacting with each other, with the crop and with all agro-ecosystem components (Clements et al., Reference Clements, Weise and Swanton1994; Soriano et al., Reference Soriano, Álvarez, Landa and Gómez2014).
The effects of different management techniques on weed composition have been shown in many cropping systems (Zanin et al., Reference Zanin, Otto, Riello and Borin1997; Fried et al., Reference Fried, Kazakou and Gabac2012) and tree crops, such as citrus (Mas et al., Reference Mas, Poggio and Verdù2007), apple orchards (Minãrro, Reference Minãrro2012), grapevines (Montemurro et al., Reference Montemurro, Castrignanò, Cucci, Ferrara, Tei and Onofri1994; Gago et al., Reference Gago, Cabaleiro and García2007) and olive groves (Allen et al., Reference Allen, Randall, Amable and Devereux2006; Fracchiolla et al., Reference Fracchiolla, Caramia, Lasorella and Montemurro2013). The results are obviously correlated to the geographic location of the tested fields, although all trials show a marked influence of cultural practices on weed community composition.
The effects on weed flora are expected to be more stable if the practices have been carried out over a long period of time. Therefore, the aim of this paper is to compare the influence of different soil management practices, protracted over more than 30 yrs in an almond orchard, on the diversity and composition of weed communities and soil seed bank. The experimental field was located in the South of Italy, under a typical Mediterranean climate, and it was managed with practices with few or no disturbing activities to the soil.
Materials and Methods
The research was conducted in an experimental field including an almond varietal collection, located in ‘La Piantata’ experimental farm near Bari (South-East of Italy), of the Agricultural Research Council of Italy. Plant spacing was 7 × 7 m2 and the crop was rainfed. The soil had a clay texture and a topsoil nearly 0.30 m depth. Within a long-term study started in 1976, the following soil management practices were compared:
-
• Residual herbicide (ReH): no-tillage, keeping the soil totally weed-free throughout the year by using residual herbicides (pre-emergence) to prevent plant emergence or foliar (post-emergence) herbicides in the case of weeds already emerged (see Appendix).
-
• Foliar herbicide (FoH): no-tillage, with post-emergence chemical weed control in spring, using foliar herbicides (post-emergence of weeds) (see Appendix).
-
• Mowing (Mow): no-tillage, with mowing of natural weed flora in spring.
-
• Cover cropping (Cov): cover cropping, with faba bean (Vicia faba L. var. minor Beck) sown in November and green manured in springtime at bloom stage.
-
• Conventional tillage (Til): conventional soil tillage, using traditional techniques in the area where the experimental field is located.
Plots were of 147 m2 (strip 7 m wide that included one row of almond plants 21 m long) and were arranged in the field following the randomized block experimental design with four replicates. All the treatments were applied to the whole plots, whereas a central area of 56 m2 (a strip 4 m wide that included a central row of almond plants 14 m long) was identified for soil sampling and flora survey.
Herbicides were diluted in 400 liters ha−1 of water and applied with a hand pump sprayer backpack. Active ingredients and doses are listed in the Appendix.
For mowing, a tractor driven rotary cutting with blades mounted on a horizontal axle was used, to cut weeds to a height of about 5 cm above the ground.
In the conventional tillage treatment, several ploughings were performed each year: deep ploughings (about 20 cm depth), from late autumn to early winter to increase the water storage and shallow disk ploughings (10 cm depth) for weed removal in the other periods.
Seed bank analysis
In October 2010, for each replicate and within the sampling area, 10 soil samples were taken randomly and from a depth between 0 and 30 cm, using circular soil probe apparatus of 5 cm in diameter.
For the determination of the seed bank, the direct observation of the plantlets emerging from each soil sample was applied (Roberts and Neilson, Reference Roberts and Neilson1982).
Soil samples were placed into trays where the soil did not exceed 4 cm depth and was periodically wetted. Trays were kept in the greenhouse for 25 months, under such conditions as not to exceed an internal temperature of 35°C during the hottest periods. Irrigation was periodically stopped to simulate drought periods, so the soil was mixed up and newly wetted, so as to favor the interruption of dormancy of most seeds (Cantele et al., Reference Cantele, Zanin and Zuin1996). In the hottest months (July and August), trays were not irrigated so as to prevent the emergence of new plantlets and avoid their quick decline with the subsequent loss of data. The plantlets emerging from each tray were identified, counted and removed. The data collected for each species were expressed in terms of number of seeds per square meter of soil.
Flora field surveys
In October 2010–2011 and in May 2011–2012, namely at the end of the peak vegetative growth periods and prior to control practices, weed communities were assessed on the sampling area (56 m2) for each replicate. Percent covers of species were visually estimated; sporadic species were recorded with the arbitrary value of 0.5%. This paper provides the means of cover data recorded for the October (autumn) and May (spring) surveys of each year. Taxonomic nomenclature refers to Conti et al. (Reference Conti, Abbate, Alessandrini and Blasi2005).
Numerical analysis
For each treatment, the floristic richness (S) of the seed bank and autumn and spring weed flora was calculated as the mean number of vascular plant species and families. The richness was expressed as the mean number of taxa found in the sampling area (56 m2) of each replication. The diversity of each treatment was estimated by the Shannon–Wiener index (H) which combines evenness and richness of species. The index was calculated as follows: H = −∑p i ln p i; where p i is the proportion of individuals (for seed bank) or cover (for field surveys) of the ith species.
For each treatment, the mean sum of the plant cover values in each replicate was indicated as the ‘total weed cover’. This index was used to roughly estimate the structure (biomass, stratification, coverage) of weed communities. Occasionally, the total weed cover value exceeded 100% because individual plants can overlap one to each other.
All the values were square root transformed to ensure the homogeneity of variances and submitted to the analysis of variance. The means were compared using the Duncan's test. Tables show the mean data, non-transformed, of replicates.
The indicator species analysis (ISA) (Dufrêne and Legendre, Reference Dufrêne and Legendre1997) was used to find the species characterizing each treatment and some of their combinations.
The ISA recently arranged within a statistical framework of methods for assessing the association between species and site groups (De Cáceres and Legendre, Reference De Cáceres and Legendre2009), has been widely used in ecology. The strength of the association between a species and a group of sites is measured by the indicator value index (IndVal). The highest IndVal of a species along a site typology identifies the group of sites for which that species can be considered as an indicator species (IS) (Dufrêne and Legendre, Reference Dufrêne and Legendre1997). The IndVal of species i for the group of sites j are calculated as follows: IndVal ij = A ij × B ij × 100; where A ij is the ratio between the mean abundance of species i in the sites of group j and the mean abundance of species i along all the groups of sites. B ij is the ratio between the number of sites of cluster j where the species i is present and the total number of sites in that group. The maximum value of the index (IndVal = 100) is reached for species that are present in only one group and in all of its sites.
The method can be adapted for a randomized block experimental design, as in our case, by a pre-relativization by species within each replicate (i.e., blocked ISA) (McCune and Grace, Reference McCune and Grace2002; McCune and Mefford, Reference McCune and Mefford2011). The IndVal were tested for statistical significance (P ≤ 0.05) by using a Monte Carlo test, with 5000 permutations.
To find the IS associated with more than one treatment, the treatments were hierarchically clustered (flexible-beta method, β = −0.25, with Bray–Curtis distance measure) on the basis of both autumn and spring species mean cover. The cluster analyses for autumn and spring data gave very similar results; only the dendrogram for the autumn survey was shown. Significant IS were calculated for the clusters of treatments obtained for every level of the dendrogram. Each significant IS was then assigned to the treatment or group of treatments for which it yielded the highest IndVal (Dufrêne and Legendre, Reference Dufrêne and Legendre1997; McCune and Grace, Reference McCune and Grace2002).
All the multivariate analyses described above were carried out by using the software pc-ord v.6.11 (McCune and Mefford, Reference McCune and Mefford2011).
Results
The cluster analysis (Fig. 1) clearly differentiated the residual herbicide from the other treatments. The second partitioning level of the dendrogram separated the foliar herbicide and mowing treatments from the crop covering and conventional tillage. The last two treatments were separated at the third partitioning level, whereas foliar herbicide and mowing were the most floristically similar treatments.

Figure 1. Cluster analysis [flexible beta (β = −0.25), Bray–Curtis distance measure] of the treatments based on the autumn weed surveys. ReH, residual herbicide; FoH, foliar herbicide; Mow, mowing; Cov, cover crop; Til, conventional tillage.
The residual herbicide was characterized by the lowest diversity, richness and total weed cover, both in autumn and spring surveys (Tables 1 and 2). Also for the seed bank, the values of the Shannon–Wiener and richness indexes were statistically different and lower than in all the other treatments (Table 3). The number of seeds in the seed bank for the residual herbicide was the lowest one, significantly different from all the others with the exception of the foliar herbicide (Table 3).
Table 1. Autumn surveys: effects of different systems of treatments on total weed cover, richness and diversity 1 .

1 Values followed by different letter in each row are significantly different from each other at P < 0.05 (Duncan test).
2 ReH, residual herbicide; FoH, foliar herbicide; Mow, mowing; Cov, cover crop; Til, conventional tillage.
Table 2. Spring surveys: effects of different systems of treatments on total weed cover, richness and diversity 1 .

1 Values followed by different letter in each row are significantly different from each other at P < 0.05 (Duncan test).
2 ReH, residual herbicide; FoH, foliar herbicide; Mow, mowing; Cov, cover crop; Til, conventional tillage.
Table 3. Seed bank: effects of different systems of treatments on total number of seeds, richness and diversity 1 .

1 Values followed by different letter in each row are significantly different from each other at P < 0.05 (Duncan test).
2 ReH, residual herbicide; FoH, foliar herbicide; Mow, mowing; Cov, cover crop; Til, conventional tillage.
Regarding the other treatments, in the autumn survey, the lowest total weed cover was recorded for the conventional tillage, whereas the lowest richness value was calculated for the foliar herbicide (Table 1). During spring surveys (Table 2), the highest mean total weed cover was found for the foliar herbicide and mowing. The lowest mean numbers of botanical species and families (richness) were found in the mowing. The Shannon–Wiener index values calculated for foliar herbicide, mowing, conventional tillage and cover cropping (Table 2), were not statistically different from each other.
Regarding the seed bank, except for the residual herbicide, all the other treatments showed no statistically different values of richness, diversity and number of seeds (Table 3).
IS analysis
In the autumn surveys, the residual herbicide was not associated with any IS, whereas Avena sterilis, Bromus sterilis and Calendula arvensis characterized the other treatments. The number of species associated with the clusters Cov + Til and FoH + Mow were eight and five, respectively, with seven IS of single treatments (Fig. 1, Table 4). The highest IndVal were recorded for Convolvulus arvensis, Cynodon dactylon, Diplotaxis muralis and Hordeum murinum (associated with the cluster FoH + Mow) and Amaranthus retroflexus (Cov + Til), Lathyrus sp. (Cov) and Heliotropium europaeum (Til).
Table 4. Autumn surveys: mean cover percentage and the highest indicator value (IndVal) of weed species significantly (P ≤ 0.05) associated with a treatment or a group of treatments.

1 ReH, residual herbicide; FoH, foliar herbicide; Mow, mowing; Cov, cover crop; Til, conventional tillage.
In the spring surveys, only two significant IS (Arenaria leptoclados and Erigeron Canadensis) were recorded for the residual herbicide. A. sterilis and B. sterilis were associated with the other four treatments (Table 5). The clusters Cov + Til and FoH + Mow were characterized by an equal number of IS. The highest IndVal were recorded for H. murinum, Galium aparine and Sherardia arvensis (FoH + Mow) and Fumaria capreolata, Lamium amplexicaule and Veronica hederifolia (Cov + Til). Among the single treatments, the conventional tillage was floristically differentiated by four significant IS (C. arvensis, Diplotaxis erucoides, Lolium rigidum, Senecio vulgaris); two IS were recorded for mowing and foliar herbicide.
Table 5. Spring surveys: mean cover percentage and the highest indicator value (IndVal) of weed species significantly (P ≤ 0.05) associated with a treatment or a group of treatments.

1 ReH, residual herbicide; FoH, foliar herbicide; Mow, mowing; Cov, cover crop; Til, conventional tillage.
The data concerning the seed bank (Table 6) showed that only A. leptoclados was significantly associated with the residual herbicide, whereas the other treatments had five significant IS, among which C. arvensis (IndVal = 93.8) and Portulaca oleracea (93.8) with the highest IndVal. The significant IS associated with Fow + Mow were Euphorbia helioscopia, B. sterilis and Sonchus oleraceus, whereas Capsella bursa-pastoris characterized Cov + Til. Few other significant IS resulted associated with single treatments. However, we cannot exclude that the lower number of species with significant treatment associations in the seedbank is due to a greater variability observed in data recorded in the soil sampling than in those of aboveground visual surveys.
Table 6. Seed bank: average number of seeds per square meter and indicator value (IndVal) of weed species significantly (P ≤ 0.05) associated with a treatment or a group of treatments.
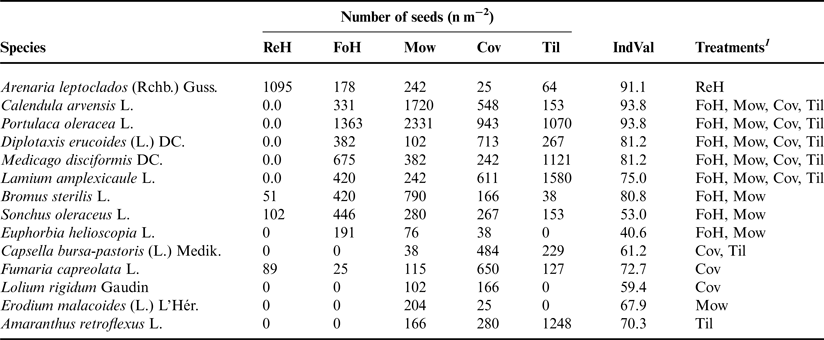
1 ReH, residual herbicide; FoH, foliar herbicide; Mow, mowing; cov, cover crop; Til, conventional tillage.
Discussion and Conclusions
Among the five treatments compared in this paper, the residual herbicide was the most differentiated for low levels of total weed cover, richness and diversity. The ISA selected very few species significantly associated with this treatment. Moreover, the seed bank, which showed slight differences among the other treatments, was clearly impoverished after the long-term applications of residual herbicides. On the other hand, another study (De Giorgio and Lamascese, Reference De Giorgio, Lamascese, Oliveira and Cordeirov2005) on the almond tree productivity in the same experimental plots, recorded the highest yield for this treatment (4.86 kg per tree). For the foliar herbicide, cover cropping and conventional tillage, the yields per tree ranged between 4.05 and 3.56 kg; whereas the lowest yield was recorded for the mowing (2.72 kg per tree) (De Giorgio and Lamascese, Reference De Giorgio, Lamascese, Oliveira and Cordeirov2005). Although these data referred to are the means of the period 1988–2000 (De Giorgio and Lamascese, Reference De Giorgio, Lamascese, Oliveira and Cordeirov2005) and need to be updated, we might conclude that the treatment providing the lowest level of diversity (i.e., the less sustainable practice) is the most productive. However, emphasis should be placed on the off-farm environmental benefits of adopting conservative agriculture techniques that provide adequate level of biodiversity and low impact to the soil (Holland, Reference Holland2004). From this point of view, the agroecological role of weed flora communities (Buhler, Reference Buhler2002; Chauhan et al., Reference Chauhan, Singh and Mahajan2012) should be prioritized and weed management should be integrated with other cultural practices in order to optimize the whole cropping system rather than solely weed control (Elmore, Reference Elmore1996; Bàrberi, Reference Bàrberi2002).
During autumn months, the residual herbicide and conventional tillage were the less infested (cf. Table 1: total weed cover). This effect does not constitute a benefit (Montemurro and Fracchiolla, Reference Montemurro, Fracchiolla and Pisante2013); as a matter of fact, the presence of the sward in the autumn-winter period does not cause any damage and is rather desirable because it prevents soil erosion processes that are particularly common in many Mediterranean areas (García-Ruiz, Reference García-Ruiz2010; Novara et al., Reference Novara, Gristina, Saladino, Santoro and Cerda2011). Moreover, it is important to consider the high capacity of the natural flora to takeup soil nitrogen (Blackshaw et al., Reference Blackshaw, Brandt, Janzen, Entz, Grant and Derksen2003) thus preventing in the rainy season leaching processes that might cause pollution (Constantin et al., Reference Constantin, Mary, Laurent, Aubrion, Fontaine, Kerveillant and Beaudoin2010).
In springtime, prior to sward control operations, the plots treated by foliar herbicide and mowing showed the highest total weed cover, thus supplying a substantial amount of biomass to the soil, after sward had been destroyed. Among the species associated with these treatments, a large contribution to the total weed cover come from three Poaceae: B. sterilis, A. sterilis and H. murinum.
These species produce a substantial amount of biomass and have bunched roots; consequently they supply beneficial effects by improving porosity and structure of the soil and reducing erosion hazard. However, they can strongly compete with almond trees for soil water, particularly in rainfed orchards placed in Mediterranean conditions with pronounced summer drought. The lowest yield, recorded for the mowing treatment by De Giorgio and Lamascese (Reference De Giorgio, Lamascese, Oliveira and Cordeirov2005), is explained as a consequence of a greater competition for water. In this respect, a realistic proposal that could be verified by further studies would be to keep the orchard fully free of weeds during the drought period to minimize the negative effects of water competition, while benefiting from the positive effects of the sward (Ramos et al., Reference Ramos, Benitez, Garcia and Robles2010; Fracchiolla et al., Reference Fracchiolla, Caramia, Lasorella and Montemurro2013).
Besides the ‘timing’, another core concern is the techniques of weed removal. Post-emergence weed control by mowing or using chemical herbicides or the green manure of the cover crop may be proposed to reduce impact to the soil and to promote the growth of abundant and sufficiently diversified and balanced flora. Although the research has not regarded the effects on the agro-ecological system and on the physico-chemical soil properties, this flora can definitely have beneficial effects, following the indications provided by the literature.
This paper reports data of a trial that has merely involved the comparison of single weed control practices repeated for several years. On the other hand, several studies support the idea that each single practice should be considered only as a component of a more general system of diversified strategies of integrated weed management (Buhler, Reference Buhler2002; Chauhan et al., Reference Chauhan, Singh and Mahajan2012). For example, mowing could be adopted to quickly suppress groundcover at the beginning of the drought period and chemical weeding can be used to cheaply control subsequent regrowing. The sowing of the cover crop could be performed to promote natural soil fertility and to introduce a further different management technique in the whole cropping system. One remaining question is the effects, both on- and off-farm, of adopting a combination of management practices on weed flora, productivity and biodiversity for a sustainable rural land use.
Acknowledgements
We would like to thank Prof. F.S. D'Amico (University of Bari) and the anonymous referees for their useful suggestions and comments.
Appendix
List of herbicides (active ingredients) used for the residual herbicides and foliar herbicides treatments. For each year, applications are indicated by the active ingredient [month, dose (kg ha−1)].
Residual herbicide treatment: 1976 – Bromacil (January, 2.0); Propyzamide + Simazine (November, 1.0 + 1.5). 1977 – Diclhobenil (October, 3.375). Paraquat (February, 0.47). 1979 – Paraquat (February, 0.47); Chlopropham + Diuron (February, 2.45 + 2.45); Dalapon (May, 0.04). 1980 – Bromacil (January, 2.0); Propyzamide + Simazine (1.0 + 1.5). 1981 – Diclhobenil (October, 3.375). 1983 – Paraquat (February, 0.47); Chlopropham + Diuron (March, 2.45 + 2.45). 1984 – Bromacil (January, 2.0); Paraquat (May, 0.925). 1985 – Propyzamide + Simazine (February, 1.0 + 1.5); Paraquat (May, 0.925); Diclhobenil (October, 3.375). 1986 – Paraquat (April, 0.925). 1987 – Paraquat (March, 0.47); Chlopropham + Diuron (April, 0.245 + 0.245); Paraquat (May, 0.47). 1988 – Bromacil (February, 2.0); Propyzamide + Simazine (November 1.0 + 1.5). 1989 – Diclhobenil (October, 3.375). 1990 – Chlopropham + Diuron (October, 2.45 + 2.45). 1992 – Glyphosate + Simazine (February, 1.08 + 3.0). 1993 – Glyphosate + Simazine (January, 1.08 + 3.0). 1994 – (February, Glyphosate + Simazine, 1.08 + 3.0). 1995 – Glyphosate + Simazine (May, 1.08 + 3.0). 1996 – Glyphosate + Simazine (November 1.08 + 3.0). 1997 – Paraquat (April, 0.925). 1998 – Glyphosate (October, 1.54). 1999 – Glyphosate (April, 1.23). Glyphosate (October, 1.5). Oxadiazon + Glyphosate (November, 0.5 + 0.25). 2000 – Glyphosate (May, 1.23); Oxadiazon + Glyphosate (October, 2.0 + 1.0). 2001 – Oxadiazon + Glyphosate (January, 2.0 + 1.0); Oxadiazon (May, 1.7); Diquat + Paraquat (May, 0.35 + 0.7); Oxadiazon (November, 1.7). 2002 – Diquat + Paraquat (May, 0.35 + 0.7); Diquat + Paraquat (October, 0.13 + 0.33). 2003 – Glyphosate (April, 1.53); Diquat + Paraquat (July, 0.04 + 0.12); Oxadiazon + Glyphosate (September, 2.0 + 1.0). 2004 – Diquat + Paraquat (April, 0.32 + 0.67). 2005 – Oxadiazon + Glyphosate (February, 2.0 + 1.0); Diquat + Paraquat (April, 0.13 + 0.33). 2006 – Oxadiazon + Glyphosate (April, 2.0 + 1.0); Glyphosate + Oxyfluorfen (October, 0.9 + 0.07). 2007 – Glyphosate + Oxyfluorfen (April, 0.9 + 0.07); Glyphosate (October, 0.108). 2008 – Glyphosate + Oxyfluorfen (May, 0.9 + 0.07); Glyphosate (November, 0.108). 2009 – Glyphosate + Flazasulfuron (May, 0.108 + 0.06). 2010 – Glyphosate + Flazasulfuron (May, 0.108 + 0.06).
Foliar herbicide treatment: 1976 – Paraquat (May, 0.925). 1977 – Paraquat (May, 0.925). 1978 – Paraquat (May, 0.925). 1979 – Paraquat (May, 0.925). 1980 – Paraquat (May, 0.925). 1981 – Paraquat (April, 0.925). 1982 – Paraquat (May, 0.925). 1983 – Paraquat (April, 0.925). 1984 – Paraquat (May, 0.925). 1985 – Paraquat (May, 0.925). 1986 – Paraquat (May, 0.925). 1987 – Paraquat (May, 0.925). 1988 – Paraquat (February, 0.925). 1989 – Paraquat (May, 0.925). 1990 – Paraquat (April, 0.925). 1991 – Paraquat (May, 0.925). 1992 – Paraquat (May, 0.925). 1993 – Paraquat (May, 0.925). 1994 – Paraquat (January, 0.925). 1995 – Diquat + Paraquat (May, 0.35 + 0.7). 1996 – Diquat + Paraquat (November, 0.35 + 0.7). 1997 – Paraquat (April, 0.925). 1998 – Diquat + Paraquat (May, 0.35 + 0.7). 1999 – Paraquat (May, 0.925). 2000 – Paraquat (May, 0.925). 2001 – Paraquat (May, 0.925). 2002 – Diquat + Paraquat (May, 0.29 + 0.6). 2003 – Diquat + Paraquat (November, 0.29 + 0.6). 2004 – Diquat + Paraquat (May, 0.29 + 0.6). 2005 – Diquat + Paraquat (May, 0.29 + 0.6). 2006 – Diquat + Paraquat (April, 0.29 + 0.6); Glufosinate ammonium (December, 0.9). 2007 – Glufosinate ammonium (October, 0.9). 2008 – Glufosinate ammonium (May, 0.9); Glufosinate ammonium (September, 0.9). 2009 – Glufosinate ammonium (May, 0.9). 2010 – Carfentrazone ethyl (May, 0.19).










