Introduction
The common co-occurrence of behavioral disinhibition, substance use disorders, antisocial behavior, and conduct problems reflects a broad dimension of psychopathology termed the externalizing spectrum (Krueger et al., Reference Krueger, Hicks, Patrick, Carlson, Iacono and McGue2002). Externalizing disorders are associated with poor mental health outcomes, premature death (Odgers et al., Reference Odgers, Caspi, Broadbent, Dickson, Hancox, Harrington, Poulton, Sears, Thomson and Moffitt2007; Eaton et al., Reference Eaton, Roth, Bruce, Cottler, Wu, Nestadt, Ford, Bienvenu, Crum, Rebok and Anthony2013) and are estimated to cost more than 417 billion dollars annually in the USA alone (National Institute on Drug Abuse, 2015). Twin studies have repeatedly found that externalizing phenotypes are highly heritable across different developmental periods and point to behavioral disinhibition as a common feature uniting externalizing disorders (Tarter et al., Reference Tarter, Kirisci, Mezzich, Cornelius, Pajer, Vanyukov, Gardner, Blackson and Clark2003; Krueger and Markon, Reference Krueger and Markon2006; Young et al., Reference Young, Friedman, Miyake, Willcutt, Corley, Haberstick and Hewitt2009). The goal of this study was to identify neural phenotypes of externalizing by investigating associations between measured genetic risk for externalizing disorders and brain networks. Based on growing evidence that connectivity in resting-state functional networks reflects heritable differences in brain organization and function (Smit et al., Reference Smit, Stam, Posthuma, Boomsma and De Geus2008; Glahn et al., Reference Glahn, Winkler, Kochunov, Almasy, Duggirala, Carless, Curran, Olvera, Laird, Smith and Beckmann2010), we examined patterns of resting-state connectivity as plausible heritable neural phenotypes for externalizing.
Research on the polygenic structure of psychiatric disorders is advancing rapidly through the development of polygenic scores (PSs) derived from genome-wide association studies. PSs are summary measures that weight single nucleotide polymorphism (SNP) from across the genome to provide a measured index of the genetic propensity for a given disorder. Salvatore et al. (Reference Salvatore, Aliev, Bucholz, Agrawal, Hesselbrock, Hesselbrock, Bauer, Kuperman, Schuckit, Kramer and Edenberg2015) recently developed an externalizing PS in adults with alcohol dependence and showed that it explained 6% of the variance in externalizing disorders (alcohol/substance use disorders, antisocial behavior) and 2–7% of the variance in other disinhibited phenotypes (e.g. impulsiveness). Building on these findings, we recently replicated the association between the PS and externalizing symptoms in trauma-exposed veterans and found that the PS predicted impaired performance on inhibitory control tasks (Sadeh et al., Reference Sadeh, Wolf, Logue, Lusk, Hayes, McGlinchey, Milberg, Stone, Schichman and Miller2016). Thus, initial validation studies find reliable polygenic associations with externalizing phenotypes and illustrate the promise of this approach for identifying heritable neural mechanisms associated with genetic predispositions for externalizing. An important next step in this line of research is to examine the neurobiological correlates of the externalizing PS. Research that aims to characterize the influences of genetic variations on brain structures and function has grown exponentially over the past several years, and yielded greater insight into the heritability of neurobiological risk factors for psychopathology, including emerging neurogenetics research on externalizing disorders (Shehzad et al., Reference Shehzad, DeYoung, Kang, Grigorenko and Gray2012; Karoly, et al., Reference Karoly, Harlaar and Hutchison2013; Heitzeg et al., Reference Heitzeg, Villafuerte, Weiland, Enoch, Burmeister, Zubieta and Zucker2014). To our knowledge, no research to date has examined whether the externalizing PS relates to neurobiological function or structure – potentially crucial pathways by which genes create vulnerability for externalizing disorders.
Resting-state functional connectivity provides a measure of neural activation in spatially distributed brain networks under conditions of rest (i.e. when participants are not engaged in an explicit, goal-directed task; Beckmann et al., Reference Beckmann, DeLuca, Devlin and Smith2005). Unlike task-based measures of brain activation, resting-state connectivity is relatively stable over time (Zuo and Xing, Reference Zuo and Xing2014), suggesting it indexes more trait-like brain networks and, therefore, acts as a potentially valuable medium for investigating genetic influences on brain networks. Indeed, there is growing data to support the heritability of resting-state neural activity, including aberrant connectivity in these networks in psychiatric populations (Smit et al., Reference Smit, Stam, Posthuma, Boomsma and De Geus2008; Glahn et al., Reference Glahn, Winkler, Kochunov, Almasy, Duggirala, Carless, Curran, Olvera, Laird, Smith and Beckmann2010). Recently developed graph theory tools can be applied to resting-state connectivity data to investigate brain organization and function in psychiatric populations, potentially identifying brain networks that exhibit differential features across psychiatric disorders (Fornito and Bullmore, Reference Fornito and Bullmore2015). Graph theory is an analytic tool involving the calculation of network properties that can be used to examine the organization of network connections and, thus, the functional capabilities of a network and the role that nodes (brain regions) play in global and local circuitry (Stam, Reference Stam2014). For example, the graph theory property Participation Coefficient measures the extent to which a brain region serves as a ‘hub’ that connects different modules (sub-networks). By assessing distributed functional networks, these methods capture greater complexity in the neural bases of psychiatric disorders than is possible when limiting analysis to single regions or even coupling between pairs of regions, approaches that have traditionally dominated research on the neurobiology of psychiatric disorders. This makes graph theory a potentially powerful tool for uncovering large-scale neural circuits that enable the phenotypic complexity inherent in externalizing disorders.
The goals of this study were to (i) identify genetically associated neural networks for externalizing by examining polygenic associations with resting-state functional connectivity and (ii) examine associations between the externalizing PS, neural networks, and behavioral phenotypes of externalizing (e.g. alcohol and substance use disorders). Based on our previous finding that the externalizing PS was associated with poorer performance on tasks of inhibitory control (Sadeh et al., Reference Sadeh, Wolf, Logue, Lusk, Hayes, McGlinchey, Milberg, Stone, Schichman and Miller2016), we expected this PS to predict neural network resting-state connectivity and function in regions of the brain that are central to maintaining such control [e.g. inferior frontal gyrus (IFG); dorsolateral prefrontal cortex (PFC), anterior cingulate; Aron et al., Reference Aron, Robbins and Poldrack2004; Nee et al., Reference Nee, Wager and Jonides2007; Criaud and Boulinguez, Reference Criaud and Boulinguez2013]. In light of evidence that externalizing disorders are characterized by dysfunction in mesolimbic reward and emotional-salience systems (Durazzo et al., Reference Durazzo, Tosun, Buckley, Gazdzinski, Mon, Fryer and Meyerhoff2011; Glenn and Yang, Reference Glenn and Yang2012; Gilman et al., Reference Gilman, Kuster, Lee, Lee, Kim, Makris, van der Kouwe, Blood and Breiter2014; Korponay et al., Reference Korponay, Pujara, Deming, Philippi, Decety, Kosson, Kiehl and Koenigs2017), we hypothesized that the externalizing PS may also moderate network resting-state connectivity and organization in ventral striatum and amygdala. Two graph properties were examined: Participation Coefficient indexes the extent to which a node is a ‘connector hub’ that bridges different functional modules, and Within-Module Degree Z-Score or the extent to which a node is a ‘provincial hub’ that facilitates communication within its own module (Power et al., Reference Power, Schlaggar, Lessov-Schlaggar and Petersen2013). We limited our analysis to Participation Coefficient and Within-Module Degree Z-Score, because these centrality metrics that are particularly relevant for identifying hubs that integrate information between and within functionally distinct subnetworks.
Methods and materials
Participants
Participants were military veterans of Operations Enduring Freedom and Iraqi Freedom. Exclusion criteria were a history of seizures, serious medical illness (including prior cerebrovascular accident or myocardial infarction), acute suicide risk, current psychotic disorder, bipolar disorder, or cognitive disorder due to general medical condition, pregnancy, metal implant, shrapnel, aneurysm clip, or pacemaker, and moderate or severe traumatic brain injury (TBI). To avoid potential genetic confounds related to ancestry, the sample was limited to genetically confirmed White, non-Hispanic individuals. We did not have adequate statistical power in our sample to conduct analyses with other ethnically homogenous groups. Only participants with viable functional neuroimaging data were analyzed. Study approval was obtained from all relevant Institutional Review Boards and regulatory committees. Participants provided written informed consent and were compensated financially.
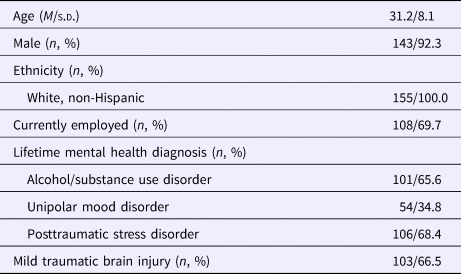
The final sample consisted of 155 male and female military veterans aged 19–57. Demographic information and clinical characteristics are presented in Table 1. The predominately male composition of this sample (~92%) is representative of other military veteran samples, but likely limits the generalizability of the findings to men.
Table 1. Descriptive characteristics (N = 155)
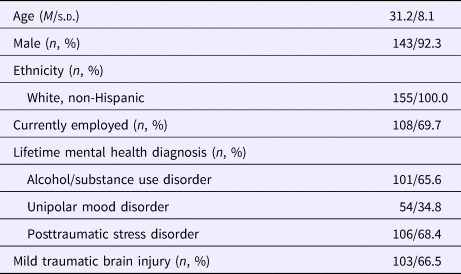
Participants with a diagnosis of current bipolar disorder, schizophrenia or psychotic disorder were ineligible to participate.
Measures
Genotyping
DNA was extracted from peripheral blood samples and genotyping details are provided in online Supplementary Methods.
PSs
To calculate the externalizing PS, we obtained a list of reference alleles and effect sizes for 587 378 SNPs from the investigators of the discovery externalizing disorders genome-wide association study (GWAS) (Salvatore et al., Reference Salvatore, Aliev, Bucholz, Agrawal, Hesselbrock, Hesselbrock, Bauer, Kuperman, Schuckit, Kramer and Edenberg2015) that reflect polygenic associations with externalizing disorders (e.g. alcohol use disorder, substance use disorder, and antisocial personality disorder). We confirmed this list had been pruned of SNPs with ambiguous coding (i.e. A/T and G/C SNPs) in our data. Of the SNPS, 480 856 were genotyped on the Illumina OMNI 2.5-8 array in our sample and available for externalizing risk-score calculations (after removal of SNPs with missing rates >0.01 and the Hardy–Weinberg equilibrium p values <0.000001). PSs were calculated with PLINK1 (Purcell et al., Reference Purcell, Neale, Todd-Brown, Thomas, Ferreira, Bender, Maller, Sklar, De Bakker, Daly and Sham2007) using the – score option, which computes a linear function of the additively coded number of reference alleles weighted by the betas from the discovery externalizing GWAS sample.
Externalizing phenotypes
Participants completed the color-word interference test (i.e. Stroop) from the Delis–Kaplan Executive Function System (Delis et al., Reference Delis, Kaplan and Kramer2001) to measure inhibitory control. The inhibition subtest measures inhibition of an automatic response (word reading) to generate a less salient incongruent response (color naming), and the inhibition/switching subtest measures flexibly switching between these response sets. We used the scaled scores from these subtests adjusted for performance on the color-naming and word-reading component tests [e.g. inhibition − (color naming + word reading)/2] (see Sadeh et al., Reference Sadeh, Wolf, Logue, Lusk, Hayes, McGlinchey, Milberg, Stone, Schichman and Miller2016 for details).
Lifetime alcohol and substance use disorders was assessed via the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) (First et al., Reference First, Spitzer, Gibbon and Williams1994) based on the alcohol, cannabis, cocaine, opioids, amphetamines, polysubstance, and the other substances modules. We did not model the latent externalizing dimension, because we did not have a measure of antisocial behavior or trait constraint.
Magnetic resonance imaging (MRI) acquisition and preprocessing
Participants were instructed to remain still with eyes open while two echo-planar imaging (EPI) runs (voxel size = 3 × 3 × 3 mm, TR = 3000 ms, TE = 30 ms, scan time per run = 360 s) were acquired on a Siemens 3T TIM Trio. Two MPRAGEs (voxel size = 1 × 1 × 1 mm, T1 = 1000 ms, TR = 2530 s, TE = 3.32 ms) were acquired and averaged to create a single high contrast-to-noise image.
Individualized cortical parcellations and subcortical segmentations were created via FreeSurfer (Fischl et al., Reference Fischl, Salat, Busa, Albert, Dieterich, Haselgrove, Van Der Kouwe, Killiany, Kennedy, Klaveness and Montillo2002). Cortical surface models were manually checked slice-by-slice and edited for accuracy. The Desikan/Killiany parcellation was used (34 regions per hemisphere), along with subcortical segmentation (seven regions per hemisphere). Frontal and temporal poles were excluded due to frequent susceptibility artifact, and five visual regions (the regions of least interest) were excluded to reduce the number of multiple comparisons. The number of brain region connections that were examined, and corrected for, in the network analysis was 2278.
To ensure that our choice of particular preprocessing stream did not drive the findings, preprocessing was repeated with other options: (i) slice-timing correction, (ii) partialing of the square, autocorrelation, and first derivative of motion parameters, (iii) partialing the first derivative of global, ventricular, and white matter signals, (iv) determination on a participant-specific basis whether partialing of global signal was necessary (via the Global Negative Index), and (v) motion scrubbing. Connectivity matrices remained extremely similar (correlations >0.97), indicating that findings were not specific to our preprocessing stream.
Pairwise LiNGAM analyses
Given that the Pairwise linear non-Gaussian acyclic model (LiNGAM) method requires non-Gaussian information to be retained in the timeseries (Ramsey et al., Reference Ramsey, Sanchez-Romero and Glymour2014), preprocessing was repeated substituting in FMRIB Software Library non-linear high-pass filter. Only high-pass filtering was performed given evidence that low-pass filtering removes important non-Gaussian information (Ramsey et al., Reference Ramsey, Sanchez-Romero and Glymour2014). For each connection, a pairwise LiNGAM coefficient was first estimated for each participant. Significance of analyses was determined via permutation (5000 permutations). In order to ensure that outliers did not drive findings, analyses were repeated after winsorization of LiNGAM coefficients to 2.5 s.d., after which all findings remained significant.
Graph property computation
Resting data were preprocessed using the Graph Theory GLM (GTG) toolbox version 0.44 (http://www.nitrc.org/projects/metalab_gtg; RRID: SCR_014075) (Spielberg et al., Reference Spielberg, McGlinchey, Milberg and Salat2015a, Reference Spielberg, Miller, Heller and Banich2015b). Data were motion corrected, detrended (linear and quadratic), bandpass filtered (retaining 0.01–0.10 Hz), and the mean global, ventricular, and white matter signals were partialed out, along with estimated motion parameters. Timeseries for FreeSurfer nodes were extracted by calculating mean signal across the node for each time point, for each EPI run. Timeseries for the EPI runs were concatenated after mean-centering each timeseries within run, and a 68 × 68 Pearson correlation matrix was created for each participant. Before graph properties were computed, each participant's connectivity matrix was thresholded to include only positive weights and normalized via division by the median positive weight for each matrix (excluding zeros). Normalization was performed to remove bias due to individual differences in overall network weight. Graph theory networks, including modules, were derived from a data-driven approach based on the current sample. Details about module membership are provided in online Supplementary Methods.
Statistical analyses
An externalizing PS computed with a p value threshold cutoff of <0.50 was the primary variable used in all analyses, selected based on prior work (Sadeh et al., Reference Sadeh, Wolf, Logue, Lusk, Hayes, McGlinchey, Milberg, Stone, Schichman and Miller2016). We also report associations between the neural network connectivity measures and externalizing PSs calculated with other p value thresholds for reference. To identify network connections that varied with polygenic variation, connectivity matrices were entered as dependent variables into the Network Based Statistic (NBS) toolbox (Zalesky et al., Reference Zalesky, Fornito and Bullmore2010), with the PS as the independent variable of interest. An individual connection level threshold of t = 2.9 was used with intensity based correction for multiple comparisons (5000 permutations) and an overall corrected α < 0.05 (Pairwise LiNGAM Hyvärinen and Smith, Reference Hyvärinen and Smith2013) was used to gain initial insight into the overall direction of influence of those connections observed in NBS analyses between cortical and subcortical structures. First, we tested the significance of the mean direction (across participants) for each connection via a one-sample t test. Next, we tested whether the PS moderated the direction of influence for each connection via a regression with the same covariates used in the NBS analyses. False discovery rate was used to correct (across connections) for multiple comparisons.
We then identified graph theory properties that varied with polygenic variation. First, resting-state connectivity matrices were entered into the GTG toolbox, which computes properties for each participant using the Brain Connectivity toolbox (Rubinov and Sporns, Reference Rubinov and Sporns2010). Two graph properties were examined: Participation Coefficient or the extent to which a node is connected to nodes in different modules, and Within-Module Degree Z-Score or the extent to which a node is connected to other nodes within its own module. Graph properties were examined only for nodes that emerged in the NBS analysis. Properties were entered as dependent variables in robust regressions in the GTG toolbox. Predictor models were the same as NBS analyses. Significance was determined via permutation tests (5000 repetitions). False discovery rate was used to correct (across nodes) for multiple comparisons, and adjusted p values are in brackets.
We included covariates in our analyses to conduct a rigorous test of our hypotheses and reduce the likelihood that these variables could account for findings. All analyses were adjusted for age, sex, the first two ancestry principal components, deployment-related blast exposure (Fortier et al., Reference Fortier, Amick, Grande, McGlynn, Kenna, Morra, Clark, Milberg and McGlinchey2014), handedness, current employment, and total cholesterol. Covariates were selected based on demonstrated associations with neural connectivity/integrity, genetic effects, and/or externalizing phenotypes in previous research (Price et al., Reference Price, Patterson, Plenge, Weinblatt, Shadick and Reich2006; Han et al., Reference Han, Mac Donald, Johnson, Barnes, Wierzechowski, Zonies, Oh, Flaherty, Fang, Raichle and Brody2014; Spielberg et al., Reference Spielberg, Sadeh, Leritz, McGlinchey, Milberg, Hayes and Salat2017). Given high rates of trauma exposure, posttraumatic stress disorder, and mild TBI in this sample, we also examined whether these variables and other comorbidities altered study findings and found that they did not (see online Supplementary Results for details). Correlations between the covariates and study variables are reported in online Supplementary Table S1.
Results
Networks related to externalizing polygenic score
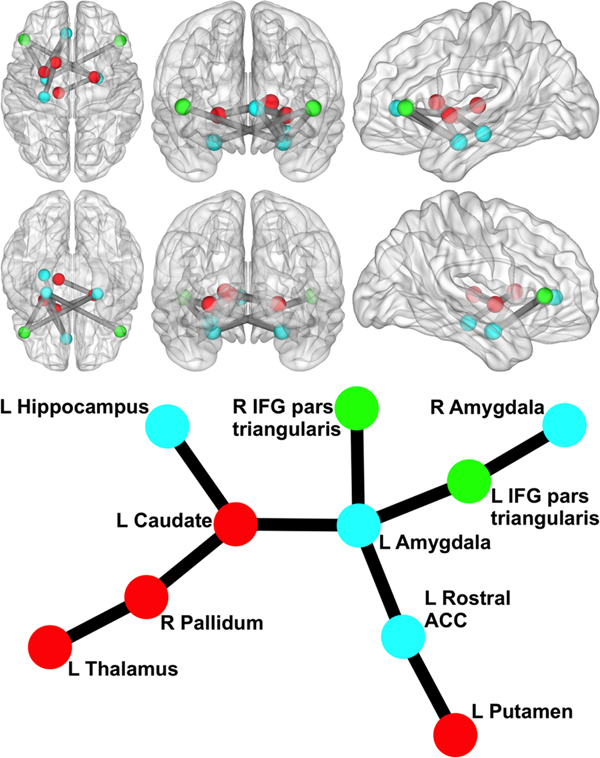
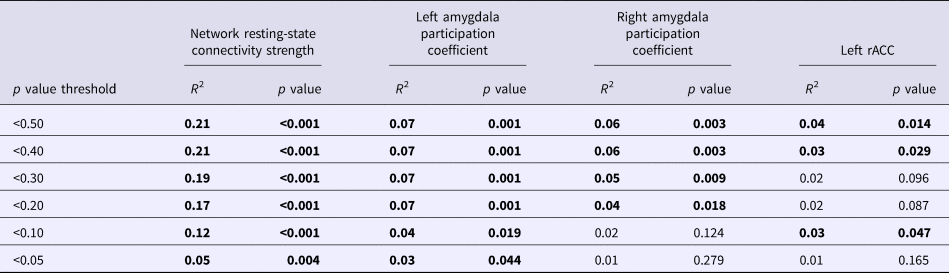
We began by examining whether polygenic variation related to externalizing predicted disturbances in functional network connectivity. The externalizing PS was associated with hyper-connectivity in a network centered on left amygdala (10 nodes, nine links, corrected p = 0.046). As displayed in Fig. 1, this network encompassed cortical [bilateral IFG pars triangularis and left rostral anterior cingulate cortex (rACC)] and subcortical regions (bilateral amygdala, hippocampus, and striatal areas) that have been implicated in emotion regulation and inhibitory control. The externalizing PS accounted for significance variance (ps < 0.005) in the strength of connectivity in this resting-state network across multiple significance p value thresholds (see Table 2).
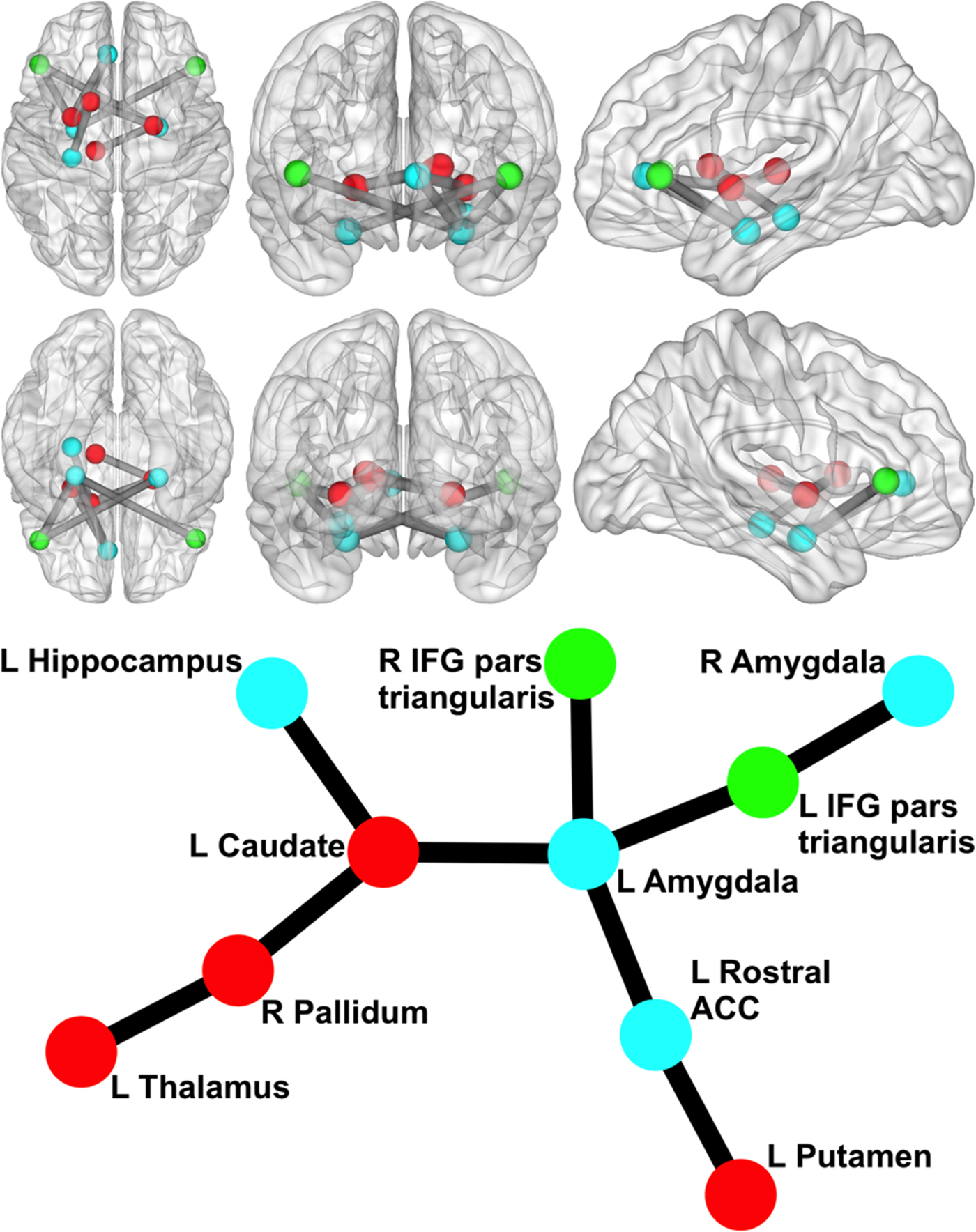
Fig. 1. Network related to externalizing PS. Circle/sphere color reflects module membership. Stick/ball figure created using the Kamada–Kawai spring embedder algorithm (SONIA; Bender-deMoll and McFarland, Reference Bender-deMoll and McFarland2006). R, right; L, left; ACC, anterior cingulate cortex; IFG, inferior frontal gyrus. Node color indicates different modules. The six 3D brain images show (clockwise from top left) an axial view from superior to the brain, a coronal view from anterior to the brain, a sagittal view from left of the brain, a sagittal view from right of the brain, a coronal view from posterior to the brain, and an axial view from inferior to the brain (created via BrainNet Viewer; Xia et al., Reference Xia, Wang and He2013).
Table 2. Variance accounted for in neural parameters across externalizing polygenic scores calculated with different p value thresholds
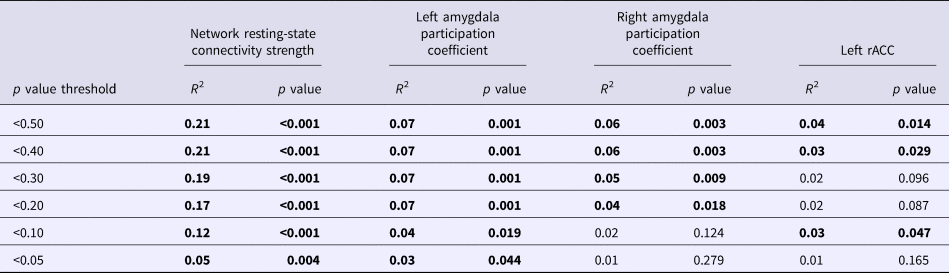
Bold significant values p < .05.
To better understand the direction of influence between nodes (i.e. A→B v. B→A), we conducted pairwise LiNGAM analyses on links observed in the NBS analyses between cortical and subcortical structures. No links evidenced a significant mean direction of influence. However, the externalizing PS significantly moderated the direction of influence for two links, and these effects survived FDR correction. Specifically, higher polygenic risk was associated with a greater direction of influence from left amygdala→left IFG pars triangularis [p = 0.007 (corrected p = 0.033)] and from right amygdala→left IFG pars triangularis [p = 0.013 (corrected p = 0.033)]. This suggests that bilateral amygdala is influencing left IFG triangularis in participants with stronger polygenic associations with externalizing, whereas the opposite is true in those with weaker polygenic associations with externalizing.
We also examined polygenic effects on the organizational properties of network nodes. Externalizing PS predicted higher Participation Coefficient for left amygdala [p < 0.001 (corrected p = 0.004)], right amygdala [p = 0.002 (corrected p = 0.012)], and left rACC [p = 0.012 (corrected p = 0.039)], suggesting that genetic variation related to externalizing modulates the importance of these nodes for communication between functional modules in the global network. The externalizing PS also nominally predicted higher Within-Module Degree Z-Score Coefficient for right pallidum, suggesting externalizing polygenic variation modulates the importance of this node for communication within its functional module, but this did not survive correction for multiple comparisons [p = 0.046 (corrected p = 0.460)]. These findings suggest that genetic variation related to externalizing modulated the extent to which these nodes functioned as communication hubs between functional modules.
Associations with cognitive/psychiatric phenotypes
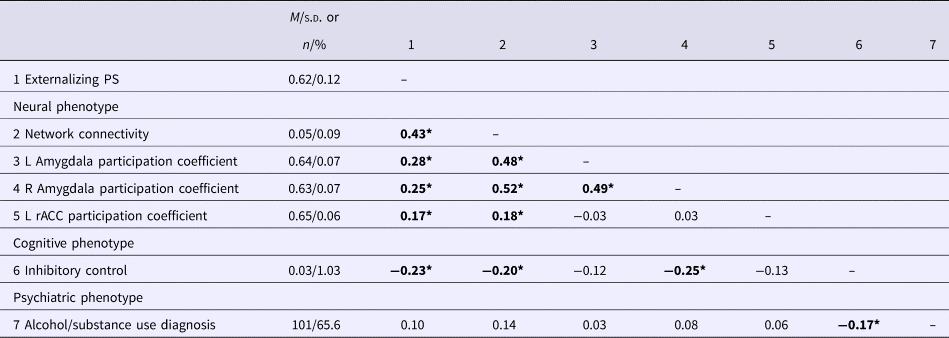
Bivariate associations between the study variables are presented in Table 3. We assessed the relevance of the genetic and network metrics for behavioral outcomes by examining relationships between these variables and externalizing phenotypes, specifically inhibitory control and lifetime alcohol/substance use using hierarchical regressions. Covariates were entered in block 1 of the regression and explanatory variables were entered in block 2.
Table 3. Descriptive statistics and bivariate correlations among study variables
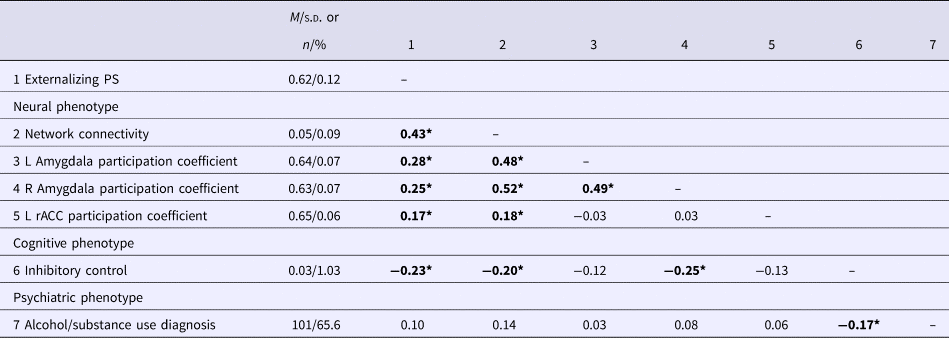
Bold significant values p < .05. PS, Polygenic Score; Network Connectivity, average resting-state connectivity in the neural network associated with the externalizing PS (see Fig. 1); L, left hemisphere; rACC, rostral anterior cingulate cortex.
*p < 0.05.
At the genomic level, the externalizing PS was not related to a diagnosis of alcohol/substance use disorders (p > 0.23, ΔR 2 = 0.01). As reported in this sample in Sadeh et al. (Reference Sadeh, Wolf, Logue, Lusk, Hayes, McGlinchey, Milberg, Stone, Schichman and Miller2016), the externalizing PS was associated with poorer performance on inhibitory control tasks (p = 0.01, ΔR 2 = 0.05).
Mean connectivity in the network was negatively associated with performance on inhibitory control tasks (β = −0.21, p = 0.015, ΔR 2 = 0.04) and with a greater likelihood of a lifetime alcohol or substance use diagnosis (Wald χ2 = 4.9, p = 0.026, ΔR 2 = 0.03), indicating that hyper-connectivity in the network confers risk for externalizing phenotypes. Examination of the graph properties revealed an inverse association between right amygdala Participation Coefficient and inhibitory control (β = −0.24, p = 0.005, ΔR 2 = 0.05), but no associations emerged for alcohol/substance use diagnosis.
Overall, the externalizing PS explained approximately 5% of the variance in inhibitory control (p = 0.016), and the neural network parameters (combined) explained approximately 8% of the variance in inhibitory control (p = 0.023). The combined variance explained across the genomic and neural levels of analysis was also significant (R 2 = 0.10, p = 0.016). In contrast, the variance explained by the externalizing PS (1%), neural network parameters (4%), and combined genomic and neural levels of analysis (4%) was not significant for alcohol/substance use disorders.
Discussion
The identification of heritable mechanisms that link genes to complex clinical phenotypes is a critical step in mapping the developmental course of psychiatric disorders. We leveraged recent methodological advances in modeling the polygenic architecture of externalizing to uncover novel neural phenotypes for this spectrum of disorders. The externalizing PS predicted hyper-connectivity in a distributed brain network that included PFC and subcortical regions critical for salience processing, emotion regulation, reinforcement learning, and impulse control. Directional analyses revealed that subcortical regions influenced PFC to a greater extent in participants with stronger polygenic associations with externalizing, whereas the opposite direction of influence was observed for those with weaker polygenic associations. The externalizing PS was associated with the organization of network nodes, specifically higher Participation Coefficient for bilateral amygdala and left rACC. Findings provide preliminary support that disturbed resting-state connectivity in this brain circuit is a genetically influenced mechanism associated with the manifestation of disinhibited behaviors and alcohol/substance disorders.
To our knowledge, this is the first study to use a polygenic approach to isolate brain circuits related to externalizing. We found that an aggregate measure of genetic risk for externalizing was associated with functional connectivity in a circuit centered on left amygdala that included several cortical (bilateral IFG pars triangularis and left rACC) and subcortical (bilateral amygdala, hippocampus, and striatum) regions. These regions are central to psychological processes previously implicated in externalizing, including emotion regulation (amygdala and ACC), impulse control (IFG), and reward learning (caudate and putamen) (Baskin-Sommers et al., Reference Baskin-Sommers, Curtin, Larson, Stout, Kiehl and Newman2012; Glenn and Yang, Reference Glenn and Yang2012; DeVito et al., Reference DeVito, Meda, Jiantonio, Potenza, Krystal and Pearlson2013; Sadeh et al., Reference Sadeh, Spielberg, Miller, Milberg, Salat, Amick, Fortier and McGlinchey2015). Although connectivity studies on the latent externalizing spectrum are sparse, there is evidence from within-region activation studies of increased amygdala reactivity and heightened engagement of executive control regions in impulsive and externalizing individuals (Sadeh et al., Reference Sadeh, Spielberg, Heller, Herrington, Engels, Warren, Crocker, Sutton and Miller2013; Foell et al., Reference Foell, Brislin, Strickland, Seo, Sabatinelli and Patrick2016). Our findings extend this work by showing that externalizing is also associated with increased amygdala connectivity at rest, primarily to PFC (see Fig. 1). These findings also converge with neuroimaging studies conducted with specific externalizing disorders, which tend to implicate abnormalities in the structure and function of prefrontal regions important for cognitive and behavioral control (e.g. ACC) and subcortical reward and salience systems (amygdala and ventral striatum) (Dom et al., Reference Dom, Sabbe, Hulstijn and Van Den Brink2005; Yang and Raine, Reference Yang and Raine2009; Cardenas et al., Reference Cardenas, Durazzo, Gazdzinski, Mon, Studholme and Meyerhoff2011; Nikolova et al., Reference Nikolova, Knodt, Radtke and Hariri2016). Unlike much of the previous research, this study linked resting-state functional connectivity in these key regions to genetic variation for externalizing disorders without relying on the selection of a few brain regions of interest. While this more unbiased approach to identifying an externalizing PS-related neural network is a strength of the current study, its exploratory nature highlights the need for replication in an independent sample.
Although PFC is typically thought to exert top-down control over subcortical structures, our analyses revealed that this was not the case for individuals with higher polygenic externalizing scores. Bilateral amygdala was found to influence left IFG in high polygenic scorers, whereas the opposite direction of influence was observed for low scorers. This connectivity pattern with PFC is in contrast to what has been observed in healthy individuals. For example, Etkin et al. (Reference Etkin, Egner, Peraza, Kandel and Hirsch2006) examined interregional connectivity during an emotional conflict task in a sample of healthy subjects and found a significant inverse relationship between activity in rostral cingulate and right amygdala when emotional conflict resolution demand was high. This finding suggests that rostral cingulate may help regulate emotional reactivity in part by decreasing engagement of the amygdala in response to emotional distractors in healthy individuals. Our findings suggest that this pattern may be reversed in individuals with higher polygenic associations with externalizing disorders, such that amygdala has greater influence on prefrontal cognitive control structures, at least during the resting state.
Polygenic burden was also associated with greater bilateral amygdala and left rACC Participation Coefficient, suggesting that these regions are more likely to serve as ‘connector hubs’ as genetic risk increases. Connector hubs provide ‘shortcuts’ to communicate between segregated modules, and such module segregation is necessary for a network to perform multiple types of specialized processes. Thus, our findings suggest that amygdala and rACC influence a wider range of specialized processes as externalizing PSs increase. This may lead to greater influence of amygdala on, not only bilateral IFG as the resting-state connectivity analyses suggested, but on a diverse range of processes. These findings extend previous work on externalizing by suggesting that abnormalities in neural network organization may be a heritable mechanism by which genetic risk for externalizing is conferred.
This study identified genetically associated circuitry via resting-state coupling in order to examine genomic relations with stable functional networks. Thus, an important next step in this line of research will be to investigate the functional and structural features of externalizing PS-related brain networks. First, examining how connectivity in this network shifts in response to cognitive/emotional challenges, like inhibitory control tasks, will be important to address in future research. Connectivity between PFC executive-control regions (rACC and IFG) and subcortical regions crucial for emotion/salience (amygdala) increases with the demand for inhibitory control (Spielberg et al., Reference Spielberg, McGlinchey, Milberg and Salat2015a, Reference Spielberg, Miller, Heller and Banich2015b). Based on limited research on functional connectivity and impulsivity (Shannon et al., Reference Shannon, Raichle, Snyder, Fair, Mills, Zhang, Bache, Calhoun, Nigg, Nagel and Stevens2011; Farr et al., Reference Farr, Hu, Zhang and Chiang-shan2012) we would not expect high externalizing individuals to show this increase in PFC-subcortical connectivity with increasing inhibitory control demands in functional tasks. An alternative hypothesis, based on the present findings, is that bottom-up (e.g. affective) processes would influence inhibitory control to a greater extent in high externalizing individuals, rather than top-down control simply being deficient. Second, it will be important to isolate the anatomical pathways that enable the identified functional network, given that resting-state functional MRI (fMRI) coupling does not imply direct anatomic connection and these analyses are not bounded by such connections.
Given that brain circuits, behavioral phenotypes, and environmental mechanisms are likely reciprocally influential (Miller and Rockstroh, Reference Miller and Rockstroh2013), studying how these dynamic relationships evolve across development will be important for establishing developmental pathways to behavioral manifestations of externalizing. The cross-sectional nature of our data prohibits strong conclusions regarding the direction of the proposed effects, and prospective research is needed to ascertain the mechanism(s) by which neural and behavioral phenotypes interact overtime. The observed associations across genetic, neural, and behavioral levels of analysis provide compelling evidence that resting-state connectivity in the identified network is a heritable mechanism that confers risk for externalizing. At the same time, more research is needed to validate this network as an endophenotype, including examining the generalizability of the polygenic associations with network connectivity. Additionally, although this study focused on identifying neurobiological mechanisms related to genetic risk for externalizing, understanding the role environmental factors play in the etiology of externalizing disorders is equally important. There is extensive evidence to suggest that environmental influences exert both direct and interactive effects on externalizing outcomes. For example, a large literature points to exposure to risky contexts (e.g. deviant peers) and stressful events (e.g. trauma) as powerful environments that interact with genes to confer risk for externalizing outcomes (Hicks et al., Reference Hicks, Krueger, Iacono, McGue and Patrick2004; Enoch, Reference Enoch2012), including in studies of polygenic risk for externalizing (Salvatore et al., Reference Salvatore, Aliev, Bucholz, Agrawal, Hesselbrock, Hesselbrock, Bauer, Kuperman, Schuckit, Kramer and Edenberg2015; Sadeh et al., Reference Sadeh, Wolf, Logue, Lusk, Hayes, McGlinchey, Milberg, Stone, Schichman and Miller2016). Based on these findings, incorporating the environment as a level of analysis in future polygenic-neuroimaging studies will be important for developing comprehensive etiological models of externalizing disorders.
This study has several strengths including analysis of multiple levels of analysis (genes, brain circuits, and behavior), a relatively large neuroimaging-genetics sample, and use of thousands of loci to identify genetically associated brain circuits. Several limitations should also be considered. First, we were unable to model the latent externalizing spectrum because a measure of antisocial behavior and/or trait constraint was not available, a limitation that may have impacted associations between the externalizing PS and externalizing psychopathology (i.e. alcohol and substance use disorders) in this sample. Given that the externalizing PS was developed to capture genetic risk for comorbidity among externalizing disorders, assessing the covariance among alcohol/substance use disorders and antisocial behavior may be necessary to detect a robust polygenic association with these phenotypes. Second, although our ability to detect small effects was limited by the modest sample size, the sample was comparable to other recent neuroimaging-genetic studies (Little et al., Reference Little, Olsson, Youssef, Whittle, Simmons, Yücel, Sheeber, Foley and Allen2015; Pagliaccio et al., Reference Pagliaccio, Luby, Bogdan, Agrawal, Gaffrey, Belden, Botteron, Harms and Barch2015). Further, we cannot say definitively that the current results will generalize to the latent externalizing spectrum. Third, we did not have an independent sample to examine the replicability of our findings. Investigating the replicability of our findings in a larger independent sample with more diverse sample characteristics (e.g. greater representation of women, ethnic minorities, and civilians) is needed before strong conclusions can be drawn. Fourth, the cross-sectional nature of our data prohibits conclusions regarding the direction of the proposed effects, and prospective research is needed to ascertain the mechanism(s) by which neural and behavioral phenotypes interact overtime. For example, it is possible that the externalizing PS causes an externalizing phenotype (clinical symptoms) that then leads to variability in the neural phenotypes rather than the genes relating directly to neural network functioning. Only longitudinal studies that assess the interactive effects of multiple levels of analysis over time are well positioned to clarify the direction of these effects. Finally, although the use of an independently derived PS represents a less biased approach to genetic analysis than selecting candidate genes, there are limitations to the polygenic approach. For example, the predictive power of the PS is limited by the robustness of the original GWAS, and the PS assumes an additive genetic model without interactions, which might not optimally reflect the underlying genetic architecture of externalizing. Additionally, the PS does not take into account environmental effects. Given the high level of stress exposure in the current sample and externalizing prone samples in general (Luntz and Widom, Reference Luntz and Widom1994; Douglas et al., Reference Douglas, Chan, Gelernter, Arias, Anton, Weiss, Brady, Poling, Farrer and Kranzler2010), examining the influence of potential epigenetic effects in relation to externalizing disorders will be important to explore in future research, such as whether differentially methylated loci associated with externalizing overlap with the externalizing PS.
In summary, findings suggest that disturbed resting-state connectivity in a circuit implicated in emotion regulation, impulse control, and reinforcement learning is a putative biological pathway linking polygenic risk for externalizing with psychiatric outcomes. Preliminary data provide compelling evidence that resting-state connectivity in this network represents an inherited neurobiological vulnerability for externalizing disorders.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291718002672.
Acknowledgements
This research was supported in part by NIGMS grant 2P20GM103653-06-6527, NIMH grant R21MH102834, and the Translational Research Center for TBI and Stress Disorders (National Network Research Center B9254-C) and the Cooperative Studies Program, Department of Veterans Affairs. This research is the result of work supported with resources and the use of facilities at the Pharmacogenomics Analysis Laboratory, Research and Development Service, Central Arkansas Veterans Healthcare System, Little Rock, Arkansas. This work was also supported by a Career Development Award to EJW from the United States Department of Veterans Affairs, Clinical Sciences Research and Development Program. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States Government. We would like to thank the Collaborative Study on the Genetics of Alcoholism (COGA) who supplied data for calculating the polygenic risk scores used in this study. COGA Principal Investigators B. Porjesz, V. Hesselbrock, H. Edenberg, and L. Bierut, includes 11 different centers: University of Connecticut (V. Hesselbrock); Indiana University (H.J. Edenberg, J. Nurnberger Jr., and T. Foroud); University of Iowa (S. Kuperman and J. Kramer); SUNY Downstate (B. Porjesz); Washington University in St. Louis (L. Bierut, J. Rice, K. Bucholz, and A. Agrawal); University of California at San Diego (M. Schuckit); Rutgers University (J. Tischfield and A. Brooks); University of Texas Medical Center in San Antonio (L. Almasy), Virginia Commonwealth University (D. Dick), Mount Sinai School of Medicine (A. Goate), and Howard University (R. Taylor). Other COGA collaborators include: L. Bauer (University of Connecticut); D. Koller, S. O'Connor, L. Wetherill, and X. Xuei (Indiana University); Grace Chan (University of Iowa); D. Chorlian, N. Manz, K. Chella, and A. Pandey (SUNY Downstate); J.-C. Wang (Mount Sinai School of Medicine); and F. Aliev (Virginia Commonwealth University). A. Parsian and M. Reilly are the NIAAA Staff Collaborators. COGA continues to be inspired by memories of Henri Begleiter and Theodore Reich, founding PI and Co-PI of COGA, and also owes a debt of gratitude to other past organizers of COGA, including Ting-Kai Li, currently a consultant with COGA, P. Michael Conneally, Raymond Crowe, and Wendy Reich, for their critical contributions. This national collaborative study is supported by NIH Grant U10AA008401 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the National Institute on Drug Abuse (NIDA).
Conflicts of interest
Drs Sadeh, Spielberg, Logue, Hayes, Wolf, McGlinchey, Milberg, Schichman, and Miller and Ms. Stone have no conflicts of interests or financial disclosures to declare.