INTRODUCTION
In recent decades, large-scale dinoflagellate blooms have occurred frequently in the East China Sea (ECS). Prorocentrum donghaiense Lu and Karenia mikimotoi (G. mikimotoi Miyake & Kominami ex Oda) are the most common and dominant species in the ECS. Prorocentrum donghaiense bloomed consecutively almost every spring since the 1990s (Zhou et al., Reference Zhou, Yan and Zhou2003, Reference Zhou, Shen and Yu2008, Reference Zhou2010; Lu et al., Reference Lu, Goebel, Qi, Zou, Han, Gao and Li2005), and has affected large areas (>1000–10,000 km2) for long periods of time (>30 days). Prorocentrum donghaiense does not release known phytotoxins (Glibert et al., Reference Glibert, Burkholder and Kana2012; Lu et al., Reference Lu, Qi, Gu, Dai, Wang, Gao, Shen, Zhang, Yu and Lu2014), however, the species alters the marine ecosystem (i.e. decreased zooplankton abundance in the bloom areas) by forming large-scale and dense blooms (with high biomass of 107 cells L−1). Karenia mikimotoi, a haemolytic toxin producer, was frequently associated with severe kills of farmed fish and shellfish in the ECS during blooms (Wang et al., Reference Wang, Yin, Qi, Xie and Jiang2001). For instance, the outbreak in 2012 caused massive mortality of abalone, Haliotis discus hannai, and more than $330 million in damage (State Oceanic Administration, 2012). Overall, the large spatial and temporal scales, and the detrimental impacts make the blooms worth researching.
Zooplankton are the major consumers of dinoflagellates and thus play an important role in energy transfer and material exchange in marine ecosystems. Many laboratory studies have demonstrated the deleterious effects of P. donghaiense or K. mikimotoi on certain types of zooplankton (i.e. brine shrimp, cladocerans, rotifers, copepods), including reduced survival rates and ingestion rates, depressed egg production rates and altered behaviour (Wang et al., Reference Wang, Yan, Tan and Zhou2003; Chen et al., Reference Chen, Tian, Wang, Bin and Zhou2007a, Reference Chen, Tian and Zhoub; Zou et al., Reference Zou, Yamasaki, Matsuyama, Yamaguchi, Honjo and Oda2010).
The copepod Calanus sinicus, which is an ecologically important copepod species in the ECS (Chen, Reference Chen1964), dominates in bloom areas (i.e. 68% of zooplankton abundance) (Xu et al., Reference Xu, Hong, Zhu and Chen2003). The life cycle of C. sinicus consists of the egg, nauplius, copepodite and adult stages. Due to their suitable size, all stages are a main food source of larvae of many fish species, including anchovy, small yellow croaker and mackerel (Meng, Reference Meng2002; Li et al., Reference Li, Wang and Sun2003; Guo et al., Reference Guo, Zhang and Jin2010; Liu & Xu, Reference Liu and Xu2011). In spring, C. sinicus exhibits significantly high biomass, fecundity and active population recruitment. For example, Huo et al. (Reference Huo, Wang, Sun, Li and Liu2008) found high proportions of larvae from nauplii at development stage 1 to copepodites at stage 3 (NI to CIII, >~80% of total abundance) when sampling in spring with a fine-meshed zooplankton net. Wang (Reference Wang2009) estimated a potential recruitment rate of more than 1,000 nauplii m−2 d−1 in spring. Thus, the habitat and recruitment stages of C. sinicus overlap with the location and timing of outbreaks of P. donghaiense and K. mikimotoi. Field studies have shown that copepod abundance significantly decreased in the vicinity of P. donghaiense blooms (Lin et al., Reference Lin, Yan, Zhang, Wang, Liu and Zhou2014), but the reason for this decline is poorly understood. We hypothesize that the decreased abundance of C. sinicus may be closely related to adult survival, reproduction and larval recruitment during blooms.
The objectives of this study were to evaluate the effects of the dominant harmful algal bloom (HAB) species P. donghaiense and K. mikimotoi on the reproduction and survival of individuals at each developmental stage of C. sinicus. The diatom Skeletonema costatum served as the control alga. The results will help us identify harm being done to the marine ecosystem by large-scale dinoflagellate blooms in the ECS. More specifically, the results will clarify the mechanisms by which these blooms affect recruitment of C. sinicus, which has implications for fishery resources in the ECS.
MATERIALS AND METHODS
Phytoplankton culture
The diatom Skeletonema costatum sensu lato (Sarno et al., Reference Sarno, Kooistra, Medlin, Percopo and Zingone2005; Zingone et al., Reference Zingone, Percopo, Sims and Sarno2005; Sarno et al., Reference Sarno, Kooistra, Balzano, Hargraves and Zingone2007), which served as the control alga, was isolated from Jiaozhou Bay in the Yellow Sea of China. The dinoflagellates Prorocentrum donghaiense and Karenia mikimotoi were isolated from the ECS and provided by two National Basic Research Priority Programs of China, CEOHAB-I and -II. The diatom Phaeodactylum tricornutum, which served as the added feeds during the naupliar first feeding phase, was provided by the Algal Culture Centre of the Institute of Oceanology, Chinese Academy of Sciences (IOCAS). Prorocentrum donghaiense and K. mikimotoi were cultured in modified f/2 medium in flasks. For the diatom S. costatum and P. tricornutum, silicate was also added. All algae were cultured at 20 ± 1°C with irradiance of 56 μ Em−2 s−1 and a 12:12 h light:dark photoperiod.
The natural seawater used in this study was pumped from Taipingjiao (a clean site with no known pollution history) at Qingdao and sand filtered prior to use in the laboratory. Prior to the experiments, the seawater was also subjected to filtration through a 0.45 μm pore size cellulose nitrate membrane, boiling for sterilization and air saturation. Salinity was adjusted to 31 ± 1‰ with distilled water as determined using an ATAGO hand-held refractometer. The pH of the seawater was measured using a HI991000 pH instrument.
Zooplankton collection and culture
Samples of the copepod Calanus sinicus were collected from March to April 2012 by towing a 500 μm mesh size plankton net from Zhongyuan Wharf, Qingdao, China (120°18′36″E 36°03′36″N). Specimens were diluted into a 20 L bucket filled with surface water and delivered immediately to the laboratory, where healthy individuals that had intact appendages and were swimming actively were picked out within 2 h. Adults were placed in 1 L beakers filled with filtered seawater and acclimated for 48 h. Copepod mortality during the acclimation period was negligible. The culture was conducted in an incubator set on a 12:12 h light:dark cycle. Temperature in the incubator was set at the surface seawater temperature (12–13°C).
Experimental designs
Because the algal species differ in carbon content (Table 1), the algae used in experimental and control group were presented to copepods at the same biomass of 2.0 μg mL−1 C, to facilitate comparison between algae. For the experimental groups, P. donghaiense density of 104 cells mL−1 and K. mikimotoi density of 103 cells mL−1 were used, which were in accordance with the bloom densities occurring in the ECS. The density of the control group, S. costatum, was 3.5 × 104 cells mL−1. Algal cells were harvested at the exponential phase. A 1 mL subsample was taken and counted under the microscope after fixation in Lugol's solution. Based on this cell count, the algae were diluted to the designated density.
Table 1. List of algal species used in the experiment.

Data represent means ± SE.
a Qing Liu, unpublished data.
EGG PRODUCTION AND HATCHING SUCCESS
After acclimation, 15 fresh and healthy adult C. sinicus females were transferred into each of nine plastic bottles (with false bottoms of 220 μm mesh size to avoid cannibalism) enriched with 300 mL of one of the three algal treatments (i.e. each test treatment consisted of three replicates). The adult females in the bottles were incubated for 16 days in an incubator set on a 12:12 h light:dark cycle at the simulated field temperature (12–13°C). Numbers of live individuals and eggs in each bottle were counted under a stereomicroscope every 48 h. At each time point, the live individuals were transferred into new freshly prepared test bottles containing the algal treatments. The eggs found at each time point were carefully transferred into a 6-well tissue culture plate, with 10 eggs in each well and 10 mL algal treatment per well. Hatching success was monitored daily for 72 h. Eggs that did not hatch after 72 h were considered to be not viable. The culture conditions for hatching were the same as those used for the egg production experiment.
NAUPLIAR SURVIVAL
A second batch of healthy females was incubated to observe the hatched nauplii. During the incubation, no algal treatments were offered. Fifty newly hatched nauplii (NI) were placed in each of nine 250 mL beakers, with three replicates for each treatment. Copepod nauplii undergo three susceptible phases of development: egg–NI; first feeding phase (NII–NIII); and naupliar–copepodite (NVI–CI) (Omori & Ikeda, Reference Omori and Ikeda1984). During NII–NIII, the small-sized diatom P. tricornutum is an adequate first food source (Li et al., Reference Li, Sun, Li, Pu and Zhang2006). Therefore, the nauplii were offered mixed diets of each treatment and P. tricornutum, in order to easily go through the susceptible phase. After the NIII stage, the diets of P. tricornutum were not offered.
We estimated median development time for each stage, defined as the time when 50% of the population had moulted to that stage, based on a total count of animals in each treatment (Peterson & Painting, Reference Peterson and Painting1990). Every 48 h, individuals were counted and their larval stage was assessed under the microscope. They then were transferred to new beakers with a fresh algal treatment. After 16 days, the percentage of surviving individuals at each stage was calculated. The incubation conditions for the nauplii were the same as those used for the egg production experiment.
COPEPODITE AND ADULT SURVIVAL
Another batch of newly hatched nauplii was incubated to obtain the copepodite (CI–CII) stages. The incubation condition and the algae used was the same as the control group used for the nauplii experiment. Fifteen newly formed copepodites were collected and placed into each of nine 250 mL beakers, with three replicates for each treatment. Over the course of 16 days, individuals were counted every 48 h under a microscope and then were transferred to new beakers containing a fresh algal treatment. The culture condition of copepodites was the same as the above.
Following the same protocol, another batch of newly captured, fresh and healthy adults was used to measure adult survival over 16 days.
Analysis
Data were analysed using the Excel 2003, Origin 8.5 and SPSS 16.0 software packages. To ensure statistical independence of the data, only the final values were compared among the treatments, in the survival rates, egg production rates and hatching success. One-way analysis of variance (ANOVA) was used for statistical evaluations. Prior to statistical analysis, percentages were arcsine square root transformed and all data were tested for normality and homogeneity (SPSS 16.0). Mean differences were considered to be significant at the 0.05 level. If the overall ANOVA results were significant, a Fisher's least significance difference (LSD) post-hoc test was performed to test among experimental combinations.
RESULTS
The survival of nauplii, copepodites and adults
We measured the survival of Calanus sinicus nauplii, copepodites and adults exposed to the two dinoflagellates Prorocentrum donghaiense and Karenia mikimotoi and the diatom Skeletonema costatum. Survival of the nauplii differed significantly among the three species (one-way ANOVA, F = 44.529, 8 df, P < 0.05, N = 9). Nauplii exposed to S. costatum for 16 days had a high survival rate (nearly 80%, Figure 1), whereas survival of those exposed to P. donghaiense (49%) and K. mikimotoi (48%) was significantly lower (LSD, P < 0.05; P < 0.05). These results suggest that the nauplii were sensitive to the dinoflagellate cultures.

Fig. 1. The survival rates of the nauplii of C. sinicus exposed to S. costatum, P. donghaiense and K. mikimotoi during 16 days.
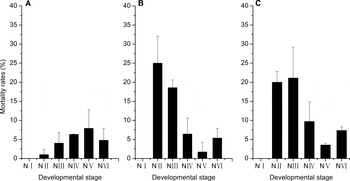
To further clarify the detrimental effects of the dinoflagellates on nauplii, the death rates of the nauplii at six stages (NI, NII, NIII, NIV, NV and NVI) were measured (Figure 2). The death rates of nauplii at NII and NIII were higher (>10%, respectively) when exposed to P. donghaiense and the K. mikimotoi treatments than when exposed to S. costatum. All six naupliar stages exposed to S. costatum had high survival rates.

Fig. 2. The mortality rates of the nauplii at six stages exposed to the three algal species during 16 days (A: S. costatum, B: P. donghaiense, C: K. mikimotoi).
Copepodites exhibited relatively high and similar survival rates (>76%, Figure 3) when exposed to all three algal species for 16 days (one-way ANOVA, F = 3.365, 8 df, P > 0.05, N = 9). Survival of Calanus sinicus adults differed significantly among the three species (one-way ANOVA, F = 6.612, 8 df, P < 0.05, N = 9). The adults showed a high survival rate (96%) after 16 days of exposure to S. costatum (Figure 4). In contrast, survival of adults exposed to P. donghaiense and K. mikimotoi decreased significantly (LSD, P < 0.05; P < 0.05), although survival remained relatively high (>78%). Overall, these results indicate that the nauplii, especially those at the early stages (NII and NIII), are more susceptible to P. donghaiense and K. mikimotoi blooms than adults and copepodites.
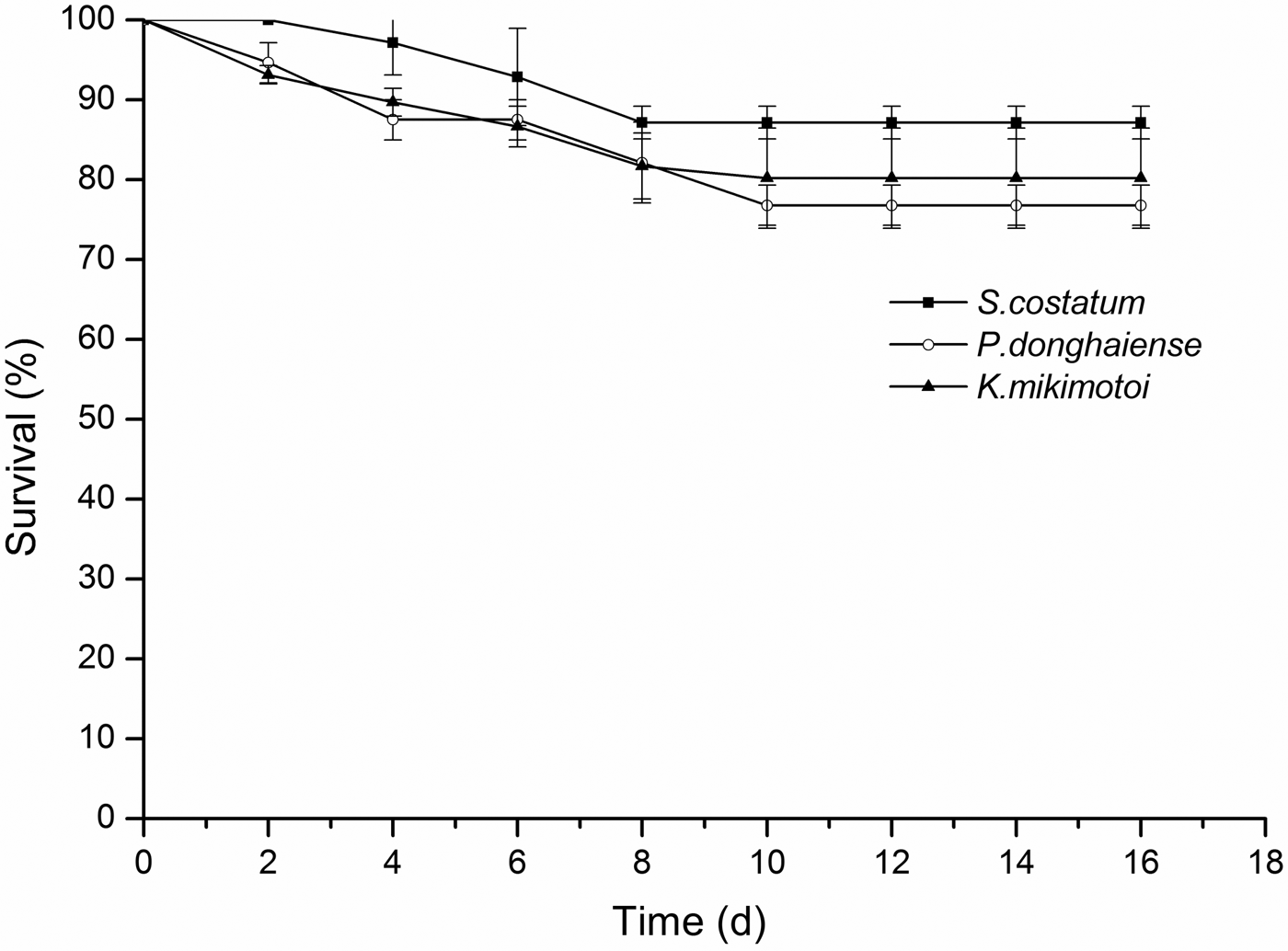
Fig. 3. The survival rates of the copepodites of C. sinicus exposed to S. costatum, P. donghaiense, K. mikimotoi during 16 days.
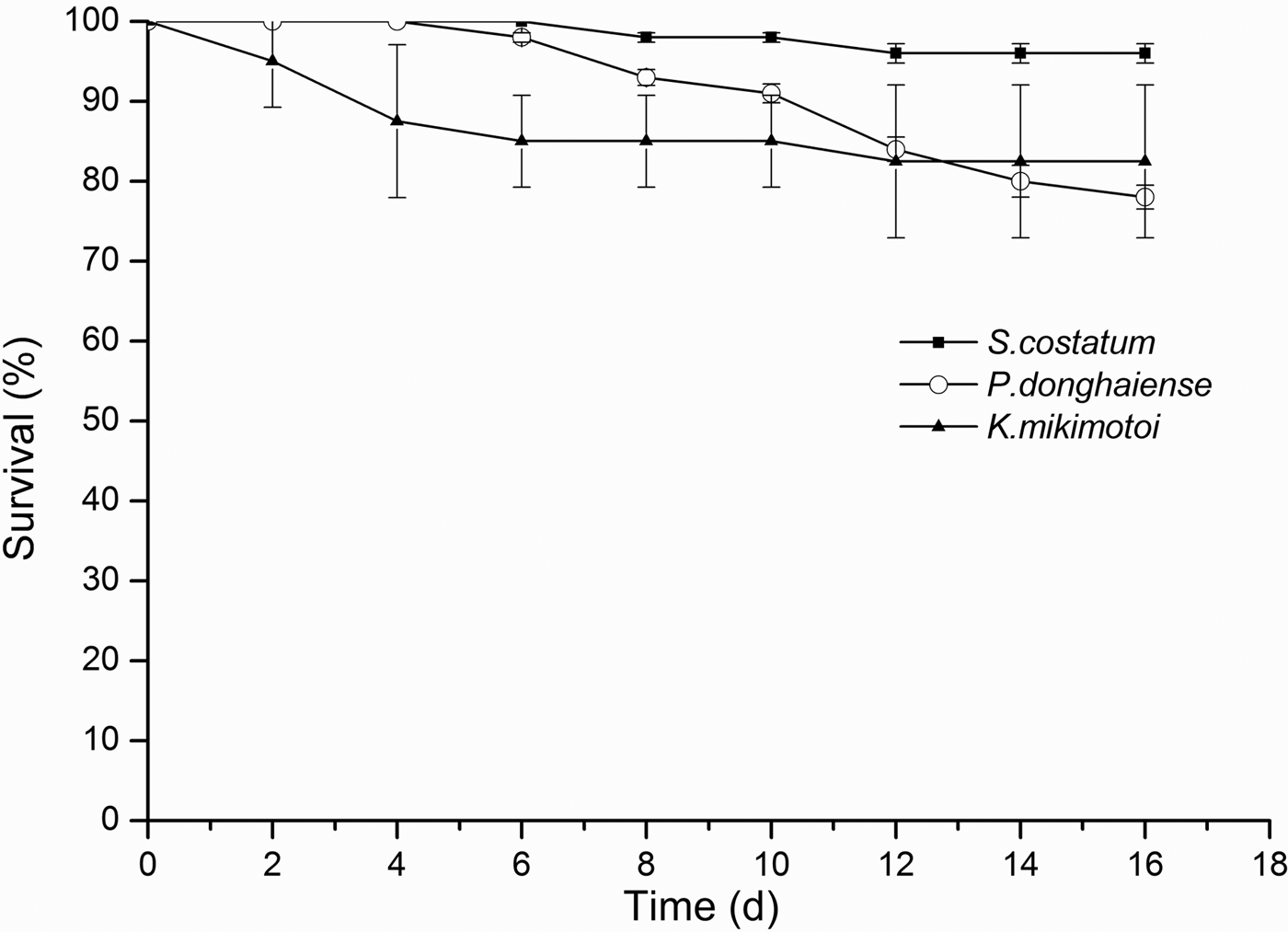
Fig. 4. The survival rates of the adults of C. sinicus exposed to S. costatum, P. donghaiense, K. mikimotoi during 16 days.
Reproduction – egg production rates and hatching success
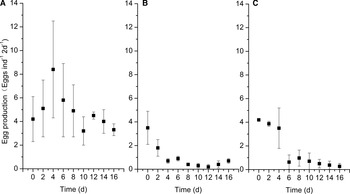
The egg production rate (EPR) of C. sinicus differed significantly among the exposure to three algal species (one-way ANOVA, F = 65.148, 8 df, P < 0.01, N = 9), which was lower in the P. donghaiense and K. mikimotoi treatments (average values: 0.65 and 1.67 eggs female−1 2 d−1, LSD, P < 0.05, respectively), compared with the control treatment (3.94 eggs female−1 2 d−1). However, no significant difference in the rate was found between the P. donghaiense and K. mikimotoi treatments (LSD, P > 0.05). Exposure to P. donghaiense and K. mikimotoi also induced a significant decrease in EPR with time (Figure 5). The results suggested that the dinoflagellate P. donghaiense and K. mikimotoi were unfavourable for EPR of C. sinicus.

Fig. 5. The egg production rates of C. sinicus exposed to the three algal species during 16 days (A: S. costatum, B: P. donghaiense, C: K. mikimotoi).
Hatching success also differed among the three algal species (one-way ANOVA, F = 18.947, 8 df, P < 0.05, N = 9), which was significantly lower for the P. donghaiense treatment (average, 49.22%, LSD, P < 0.05) compared with the control treatment (average, 76.44%), and it decreased with time (Figure 6). In contrast, hatching success was relatively high in the K. mikimotoi treatment (average, 79.08%), and it did not differ significantly from that of the control (LSD, P > 0.05).
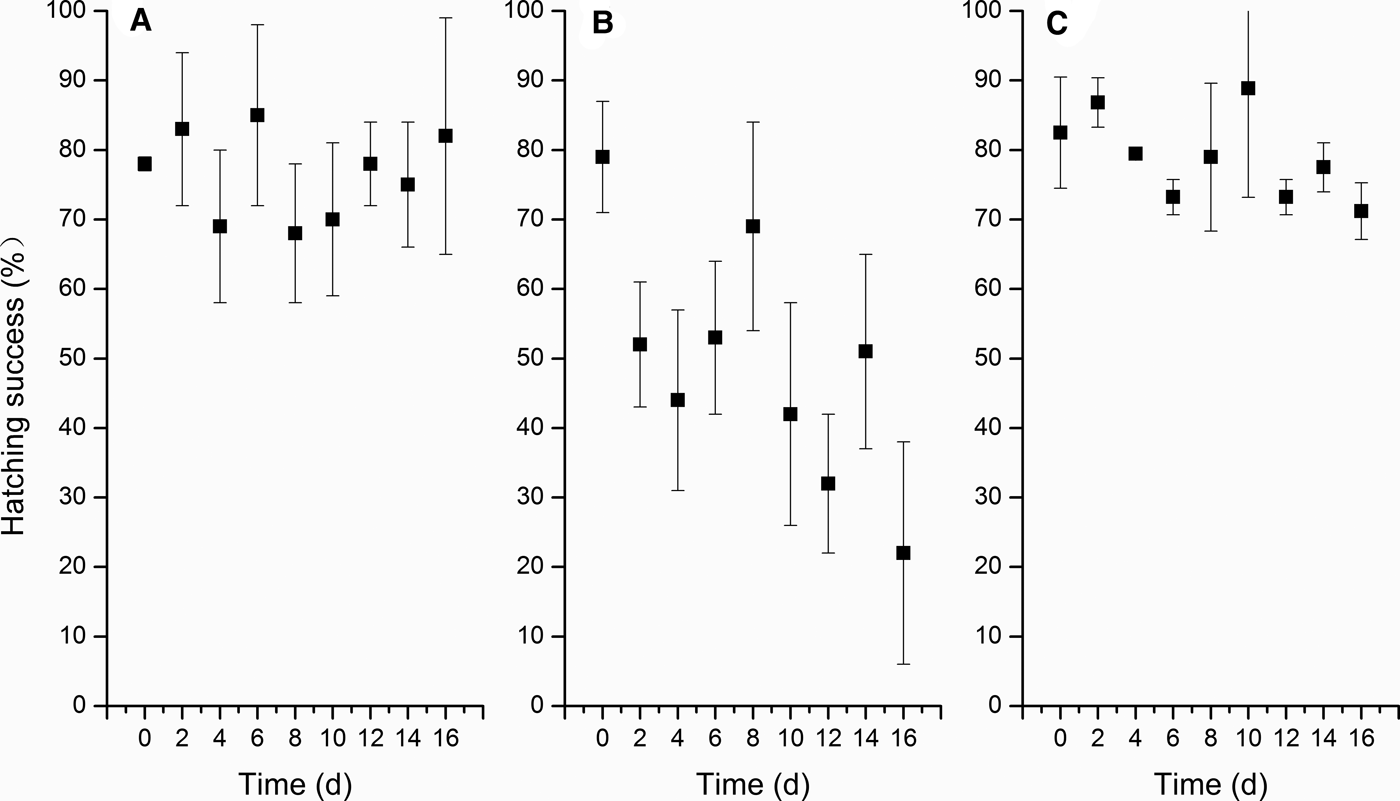
Fig. 6. The egg hatching success of C. sinicus exposed to the three algal species during 16 days (A: S. costatum, B: P. donghaiense, C: K. mikimotoi).
DISCUSSION
The impacts of Prorocentrum donghaiense and Karenia mikimotoi on Calanus sinicus
Field observations have shown that some blooms of Prorocentrum donghaiense significantly decreased the total zooplankton, or some dominant copepod abundance (Lin et al., Reference Lin, Yan, Zhang, Wang, Liu and Zhou2014). In general, the decreased abundance of zooplankton is closely related with the adult survival, reproduction and larval recruitments.
In our study, exposure to P. donghaiense (~104 cell mL−1, the approximate algal density in blooms in the field) and K. mikimotoi (~103 cell mL−1) significantly decreased survival rates of C. sinicus adults (80%). In an in situ study, Lin et al. (Reference Lin, Yan, Zhang, Wang, Liu and Zhou2014) also found that nearly 20% of C. sinicus adults died during peak phases of the P. donghaiense bloom in the ECS. However, our results indicated that nauplii were more vulnerable (>50% mortality rate) to P. donghaiense and K. mikimotoi than adults and copepodites during the 16 days of the experiment. In other studies, Prorocentrum minimum and P. lima caused decreased naupliar survival and impaired developmental speeds (Galvão, Reference Galvão2004; Ismar et al., Reference Ismar, Hansen and Sommer2008), although they also were shown to be a good food source to fuel reproduction in some copepod species (Turner et al., Reference Turner, Ianora, Miralto, Laabir and Esposito2001; Carotenuto et al., Reference Carotenuto, Ianora, Buttino, Romano and Miralto2002; Ianora et al., Reference Ianora, Miralto, Poulet, Carotenuto, Buttino, Romano, Casotti, Pohnert, Wichard and Colucci-D'Amato2004a). Gymnodinium sanguineum and Karenia brevis also adversely affected naupliar survival (Murray & Marcus, Reference Murray and Marcus2002; Turner et al., Reference Turner, Roncalli, Ciminiello, Dell'Aversano, Fattorusso, Tartaglione, Carotenuto, Romano, Esposito and Miralto2012), and Silva et al. (Reference Silva, Tang and Lopes2013) reported that Alexandrium tamiyavanichii and Gonyaulax sp. caused 88–100% mortality of Acartia lilljeborgii nauplii within a short time. Thus, the mortality of nauplii may be the main reason for decreased copepod abundance during the peak bloom periods of P. donghaiense and K. mikimotoi.
Exposure to either P. donghaiense or K. mikimotoi also resulted in sharply diminished egg production rates of C. sinicus, which agreed with the adverse effects reported from field studies (Irigoien et al., Reference Irigoien, Harris, Head and Harbour2000; Lin et al., Reference Lin, Yan, Zhang, Wang, Liu and Zhou2014). Reduced egg production was previously recorded for copepods feeding on certain dinoflagellate bloom species, including K. brevis, P. minimum and Alexandrium minutum (Frangópulos et al., Reference Frangópulos, Guisande, Maneiro, Riveiro and Franco2000; Dam & Colin, Reference Dam and Colin2005; Speekmann et al., Reference Speekmann, Hyatt and Buskey2006; Waggett et al., Reference Waggett, Hardison and Tester2012). In our study, hatching success decreased when C. sinicus was exposed to P. donghaiense but not K. mikimotoi. In contrast, other studies reported that copepod egg hatching success was highest (>80%) when exposed to P. minimum (Colin & Dam, Reference Colin and Dam2002; Ianora et al., Reference Ianora, Turner, Esposito, Carotenuto, d'Ippolito, Romano, Fontana, Guisande and Miralto2004b; Calliari & Tiselius, Reference Calliari and Tiselius2005; Dam & Colin, Reference Dam and Colin2005). The differing results for the Prorocentrum sp.–copepod interaction may be due to species-specific responses.
Copepod recruitment involves three distinct functions: egg production, egg hatching success and larval somatic growth (Poulet et al., Reference Poulet, Escribano, Hidalgo, Cueff, Wichard, Aguilera, Vargas and Pohnert2007). According to our results, exposure to P. donghaiense and K. mikimotoi caused decreased egg production, which may be another explanation for the lower copepod abundance in the peak bloom areas.
Possible causes of the adverse effects from P. donghaiense and K. mikimotoi
Previous studies have suggested that the adverse effects of dinoflagellates on zooplankton may be related to unsuitable prey size (Colin & Dam, Reference Colin and Dam2002, Reference Colin and Dam2003), prey nutrient deficiencies (Dam & Colin, Reference Dam and Colin2005; Zheng et al., Reference Zheng, Dam and Avery2011) and prey toxicity (Kim et al., Reference Kim, Sato, Oda, Muramatsy, Maysuyama and Honjo2000; Da Costa et al., Reference Da Costa, Franco, Cacho and Fernández2005; Wang et al., Reference Wang, Yan, Yu and Zhou2005; Zhou et al., Reference Zhou, Fernandez, Chen, You and Yan2011).
For nauplii, survival and development seem to be closely correlated to prey size (Berggreen et al., Reference Berggreen, Hansen and Kiørboe1988; Harris et al., Reference Harris, Irigoien, Head, Rey, Hygum, Hansen, Niehoff, Meyer-Harms and Carlotti2000; Li et al., Reference Li, Sun, Li, Zhang and Pu2008). During the naupliar first feeding phase (NII–NIII), P. tricornutum has been reported to be an adequate first food due to its small size (Li et al., Reference Li, Sun, Li, Pu and Zhang2006). Therefore, the early nauplii were offered a mixed diet of each algal treatment and Phaeodactylum tricornutum. Berggreen et al. (Reference Berggreen, Hansen and Kiørboe1988) found the optimum particle size for copepods was 2–5% of the prosome length, thus the medium-sized P. donghaiense and K. mikimotoi should be the ideal size for C. sinicus late nauplii and early copepodites (i.e. neither too small nor too large). Adult C. sinicus reportedly ingest P. donghaiense and K. mikimotoi (Xie, Reference Xie2009; Song, Reference Song2014). All of these results rule out the possibility that unsuitable prey size or prey deterrence led to the adverse effects observed in this study.
Prorocentrum donghaiense does not release any known phytotoxins (Glibert et al., Reference Glibert, Burkholder and Kana2012), so it is unlikely that any known toxins led to the negative effects observed in this study. However, P. donghaiense might produce some other substances, and whether the detrimental effect is related to the unknown substances requires further investigation. The strain of K. mikimotoi cultured in our lab has been shown to produce haemolytic compounds (Qing-Chun Zhang, unpublished), with high contents in extra- and whole-cell extracts. There is ample evidence that K. mikimotoi produces multiple compounds with a range of toxicities (i.e. haemolytic activity, ichthyotoxicity, rheotoxicity, cytoxicity and embryotoxicity) (Yasumoto et al., Reference Yasumoto, Underdal, Aune, Hormazabal, Skulberg, Oshima and Granell1990; Jenkinson & Arzul, Reference Jenkinson, Arzul, Hallegraeff, Blackburn, Bolch and Lewis2002; Satake et al., Reference Satake, Shoji, Oshima, Naoki, Fujita and Yasumoto2002, Reference Satake, Tanaka, Ishikura, Oshima, Naoki and Yasumoto2005; Silke et al., Reference Silke, O'Beirn and Cronin2005; Chen et al., Reference Chen, Yan, Yu and Zhou2011), which are the suspected causes of its adverse effects on zooplankton or other marine organisms. For example, the toxic exudates by Gymnodinium sp. decreased survivorship of planktonic copepod nauplii (Silva et al., Reference Silva, Tang and Lopes2013). Zou et al. (Reference Zou, Yamasaki, Matsuyama, Yamaguchi, Honjo and Oda2010) suggested that the live cell-mediated haemolytic activity of K. mikimotoi might be linked to its toxic effects on rotifers. Sellem et al. (Reference Sellem, Pesando, Bodennec, El Abed and Girard2000) demonstrated that the 18:5n3fatty acid produced by G. cf. mikimotoi led to delayed first cleavage of sea urchin (Paracentrotus lividus) eggs. Despite these studies, more data are needed to clarify the relationships between the toxicity by K. mikimotoi and the adverse effects observed in our study.
Many studies have demonstrated that nutritional components are vital for growth of nauplii and high fecundity in marine copepods. Studies of P. donghaiense and K. mikimotoi in our laboratory demonstrated that the concentrations of some essential fatty acids, such as arachidonic acid [20:4(n-6), ARA], eicosapentaenoic fatty acid [20:5(n-3), EPA] and 22:5(n-3)/20:6(n-3) (EPA/DHA), were lower compared with S. costatum (Song, Reference Song2014). In addition, the two algal species reportedly contain low amounts of phenylalanine, lysine and histidine (Chen et al., Reference Chen, Tian and Zhou2007b; Song, Reference Song2014). The levels of some nutrients (ingested and stored in female reserves), such as EPA, EPA/DHA, arginine and histidine, are related to the fecundity of copepods (Jónasdóttir, Reference Jónasdóttir1994; Jónasdóttir & Kiørboe, Reference Jónasdóttir and Kiørboe1996; Kleppel et al., Reference Kleppel, Burkart and Tomas1998; Arendt et al., Reference Arendt, Jónasdóttir, Hansen and Gärtner2005; Jónasdóttir et al., Reference Jónasdóttir, Visser and Jespersen2009). Copepods cannot synthesize many of the essential components needed for reproduction and growth, and they rely on dietary sources for these compounds (Pond et al., Reference Pond, Harris, Head and Harbour1996). Therefore, the nutrient deficiency in uni-algal diets of P. donghaiense or K. mikimotoi may result in the adverse effects observed in this study.
Potential threats to the marine ecosystem posed by P. donghaiense and K. mikimotoi blooms
Large-scale P. donghaiense and K. mikimotoi blooms have occurred most often in April and May in the Changjiang River Estuary and the adjacent sea area. Coincidentally, C. sinicus exhibits active population recruitment during the same time period, with more than 1,000 nauplii m−2 d−1 (Wang, Reference Wang2009). Thus, the temporal and spatial overlaps probably make the reproductive process and nauplii of C. sinicus more susceptible to dinoflagellate blooms. The decreased egg production, egg viability and survival of the early stages of C. sinicus is likely to affect future generations and thereby negatively impact population growth and recruitment.
Spawning grounds for some commercially important fish species, such as the anchovy Engraulis japonicas, are distributed in coastal areas of the ECS, and these areas have been reported to overlap with the high density areas of C. sinicus, which serve as prey for fish larvae, fry, juveniles and adults. Accordingly, changes in the abundance of C. sinicus are closely associated with the dynamics of fishery resources and the marine food web. In the ECS, large-scale P. donghaiense and K. mikimotoi blooms have lasted for more than 1 month and have affected areas as large as 104 km2. Prorocentrum donghaiense blooms have occurred consecutively almost every year since 2000. In this scenario, blooms with such large spatial and temporal scales may ultimately result in a decline in the C. sinicus population and degradation of fishery resources.
CONCLUSION
Blooms of the two abundant dinoflagellates, Prorocentrum donghaiense and Karenia mikimotoi, in the ECS have adverse effects on the survival (especially for nauplii) and reproduction of Calanus sinicus, which could pose a major threat to the population recruitment of this copepod. In the long term, the annual springtime occurrence of blooms with large spatial and temporal scales may negatively impact the fishery resources and marine ecosystem balance of the ECS.
ACKNOWLEDGEMENTS
We would like to thank Dr Song Feng for the help in the running of the experiments.
FINANCIAL SUPPORT
This study was supported by the National Natural Science Foundation of China (No. 41476102), Strategic Priority Research Program (XDA01020304) of the Chinese Academy of Sciences, and the Innovation Research Group Program of Natural Science Foundation of China (No. 41121064).