INTRODUCTION
In olive trees seed development is important for overall fruit growth and persistence during the current year as well as flower abundance in the following year (Rapoport, Reference Rapoport1994). Stutte and Martin (Reference Stutte and Martin1986a) suggested an obligatory role for embryo presence in fruit development ca. 6–8 weeks post flowering. On the other hand, the effect of embryo presence on fruit survival until harvest time is not clear since a considerable amount of fruits may reach maturity without containing a viable embryo (Lavee et al., Reference Lavee, Rallo, Rapoport and Troncoso1996). It appears that the presence of the seed in olive fruit influences flowering in the following year. For example, branches of cultivars (cvs) Manzanillo and Koroneiki bearing fruit whose seed had been destroyed flowered the following spring, but those bearing seeded fruit did not (Stutte and Martin, Reference Stutte and Martin1986b).
Olive shotberries are small, commonly seedless, fruit expressing quantitative parthenocarpy. They either abscise before harvest or, if harvested, are of no commercial value. Consequently, increased incidence of shotberries has a negative impact on fruit and oil yield, and can seriously affect economic yield of olive orchards. The size of shotberries, compared to that of normal fruit, was related to reduced accumulation of assimilates while absence of the seed was proposed to play a key role in this developmental aberration (Marquez et al., Reference Marquez, Benlloch and Rallo1990).
Shotberry formation seems to be affected by a series of environmental factors. A higher number of shotberries was reported in olive trees growing in areas where the air temperature reached 41°C during the anthesis period than in areas with lower temperatures (Ayerza and Coates, Reference Ayerza and Coates2004). Low pollen viability (Lavee et al., Reference Lavee, Taryan, Levin and Haskal2002), self-pollination (Sibbett et al., Reference Sibbett, Freeman, Ferguson and Polito1992) or poor pollination in general (Ayerza and Coates, Reference Ayerza and Coates2004; Cuevas et al., Reference Cuevas, Diaz-Hermoso, Galian, Hueso, Pinillos, Sola and Polito2001; Martin et al., Reference Martin, Ferguson, Polito, Ferguson, Sibbett and Martin1994) have been proposed as factors promoting shotberry formation in olive trees. Ugrinovic and Stampar (Reference Ugrinovic and Stambar1996) observed that shotberry formation was genotype-depended, with cv. Leccino being more prone than other cvs studied. However, no significant impact on final fruit yield was reported. Increased incidence of shotberry appearance was also observed in repeated years of high fruit load.
The aim of the present study was to determine the impact of self-, cross- and free-pollination on a series of parameters describing shotberry production in cvs Koroneiki, Kalamata, Mastoidis and Amigdalolia. Additionally, the stability of shotberry formation was evaluated for all four cvs for three consecutive years, especially as it was affected by air temperature during flowering period. Anatomical observations were made to assess presence of seed in fruit. In addition, in shotberry fruits, seed presence, total fruit weight and size were recorded and compared to fully developed fruits.
MATERIALS AND METHODS
Plant materials and growth conditions
Field observations and experiments were carried out, over a three-year period (2005–2007), at the Institute of Olive Trees and Subtropical Plants, National Agricultural Research Foundation (NAGREF), Chania, Greece. Thirty- to forty-year-old irrigated trees belonging to cvs Koroneiki, Kalamata, Mastoidis and Amigdalolia were selected based on growth and flowering uniformity. Mean air temperature in this area is 18 °C, relative humidity (RH) is 64% and annual rainfall is 600–800 mm (NAGREF meteorological station, Chania). Maximum and minimum daily temperatures during April and May of the three experimentation years are presented in Figure 1.

Figure 1. Maximum and minimum daily temperature for April and May of the three years of the trials. Low temperature incidents during flowering in 2007 are circled.
Field and laboratory measurements
The percentage of shotberries/flowers was recorded in 10 replicate shoots for each treatment and year for the four cultivars following: i) self-pollination; ii–iv) cross-pollination with pollen of one of the other three cultivars; v) free multi-cultivar pollination.
Four to twelve olive trees per cultivar were used each year depending on flowering uniformity. The number of inflorescences was recorded in the selected shoots, and pollination bags were placed over shoots before flower opening to avoid undesirable cross-pollination, except for the free pollination treatment where no bags were used. A shoot of the pollinator variety carrying open and ready to open flowers was included in the bag twice: once when the specific tree was at flowering stage 63 and once at stage 65 (Sanz-Cortes et al., Reference Sanz-Cortes, Martinez-Calvo, Badenes, Bleiholder, Hack, Llacer and Meier2002). No shoots were added to the bag for the self-pollination treatments since preliminary trials showed no significant effect of including a pollinating shoot (data not shown). Free-pollination shoots were allowed to receive pollen presumably from all cultivars present in the field.
In addition to the above, fresh weight of 20 normal fruits and 40 shotberries of cv. Kalamata was also determined. Sixty fruits resulting from free-pollination of four Mastoidis trees (15 fruits per tree) were dissected to count seed number.
Statistical analysis
Data were analysed using SPSS (SPSS Inc., Chicago, USA) and were subjected to one-way analysis of variance (ANOVA). The least significant difference (LSD) test at p = 0.05 were used to distinguish treatment differences. Standard errors of each mean were calculated and presented in graphs as error bars.
RESULTS AND DISCUSSION
The influence of self-, cross- and free-pollination on shotberry production of cvs Koroneiki, Kalamata, Mastoidis and Amigdalolia for three years is depicted in Figures 2–5.
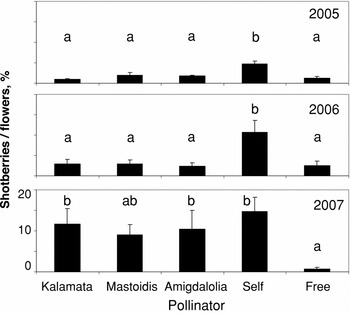
Figure 2. Percentage of shotberries/flowers in Koroneiki olives as affected by self- and cross-pollination in three consecutive years. Means within a year indicated by the same letters do not differ at p < 0.05 (LSD test, n = 10).

Figure 3. Percentage of shotberries/flowers in Kalamata olives as affected by self- and cross-pollination in three consecutive years. Means within a year indicated by the same letters do not differ at p < 0.05 (LSD test, n = 10).
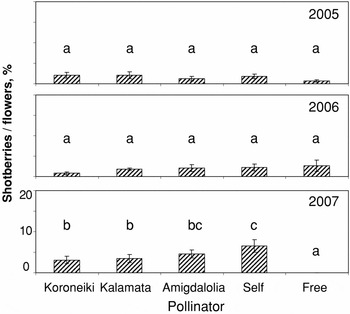
Figure 4. Percentage of shotberries/flowers in Mastoidis olives as affected by self- and cross-pollination in three consecutive years. Means within a year indicated by the same letters do not differ at p < 0.05 (LSD test, n = 10).
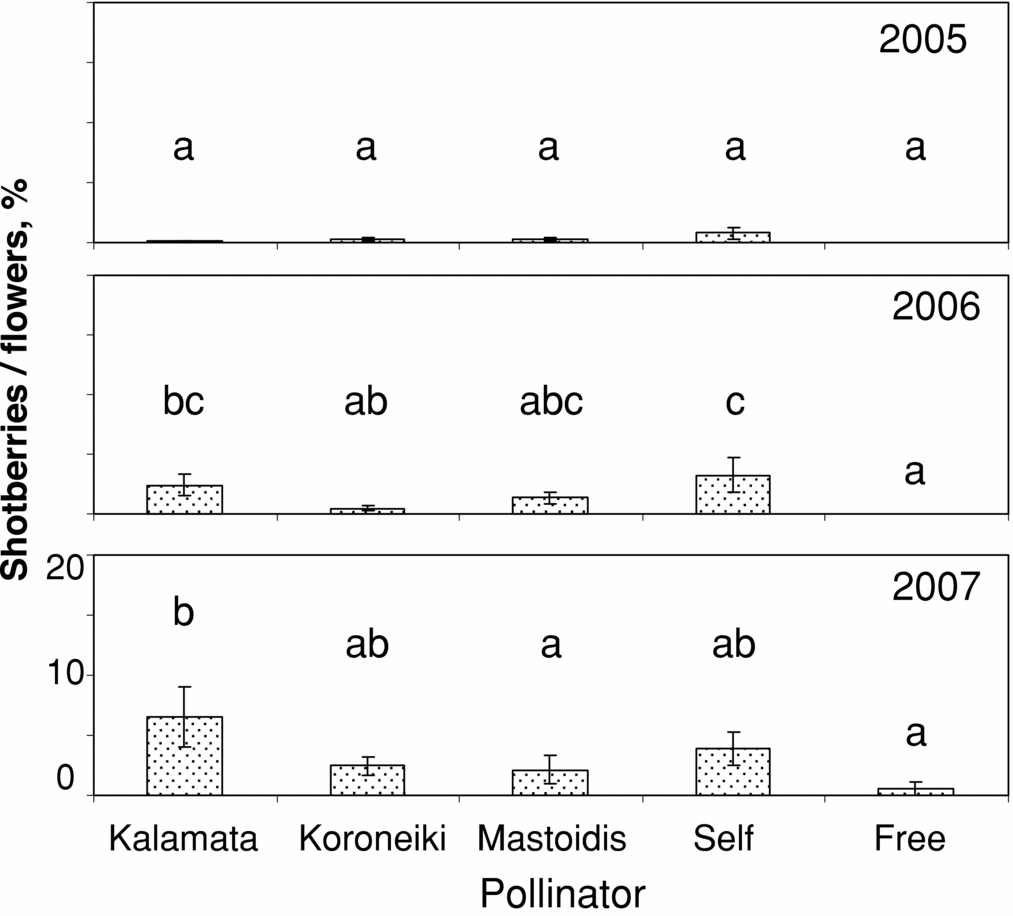
Figure 5. Percentage of shotberries/flowers in Amygdalolia olives as affected by self- and cross-pollination in three consecutive years. Means within a year indicated by the same letters do not differ at p < 0.05 (LSD test, n = 10).
In 2005 (first year of observations), in Koroneiki, there was a significant decrease (59–81%) in the cross- and free-pollination treatments compared to self-pollination, with similar efficiency (Figure 2). In 2006, there was more shotberry formation, but cross-pollination resulted in 72–77% fewer shotberries than self-pollination with significant differences. In 2007, percentages of shotberries drastically increased in all treatments compared to previous years, except for free-pollination which efficiently diminished shotberry formation.
For cv. Kalamata, the lowest level of shotberry formation was also recorded in 2005 (Figure 3). Self-pollination resulted in a significantly higher (3–15-fold) percentage of shotberries than the other four treatments, which were all of similar effectiveness. In 2006, free-pollination and cross-pollination with Koroneiki or Mastoidis were the most efficient treatments, with significant differences compared to self-pollination and cross-pollination with Amigdalolia. In 2007, the highest and lowest percentages of shotberries were recorded after self-pollination and cross-pollination, respectively, with intermediate values measured in cases of monovarietal cross-pollination.
In Mastoidis, the percentage of shotberries/flowers in first two years fluctuated between 0.5 and 2.7% with no significant differences between the five different treatments (Figure 4). A different pattern was observed in 2007, with drastic increase of shotberries after self-pollination and a negligible percentage after free-pollination. Cross-pollination resulted in values between 3.1 and 4.6% with Koroneiki and Kalamata being more effective than Amigdalolia in reducing shotberries.
In Amigdalolia, low levels of shotberries were observed in the first year, irrespective of the treatment (Figure 5). By contrast, increased percentages were recorded in 2006 following self-pollination while no shotberries were produced in the case of free-pollination. Cross-pollination with pollen of Koroneiki was more efficient in reducing shotberries than Mastoidis whereas Kalamata showed no positive effect. In 2007, shotberries were increased overall compared to previous years. The lowest levels of shotberries were observed in free-pollination and cross-pollination with pollen of Mastoidis.
Analysing results as three-year averages shows that a consistent observation is that the lowest values of shotberry formation were observed in free-pollination while the highest percentages were recorded in the case of self-pollination for all cultivars except for Amigdalolia (Table 1). Additionally, the intensity of shotberry production varied between cultivars. Koroneiki and Mastoidis were reciprocally the most effective pollinator varieties in reducing shotberries. Koroneiki could also be considered as the most suitable pollinator to reduce shotberries in Kalamata. In case of Amigdalolia, Koroneiki and Mastoidis diminished shotberries while Kalamata showed no positive effect.
Table 1. Influence of self- cross- and free-pollination on shotberry production of olive cultivars Koroneiki, Kalamata, Mastoidis and Amigdalolia.

Means within a cultivar (represented by values within a column) followed by the same letters do not differ at p < 0.05 (LSD test, n = 30). Three-year averages are presented. †Self-pollination results.
An increased incidence of shotberry formation after self-pollination was also observed in Manzanillo by Sibbett et al. (Reference Sibbett, Freeman, Ferguson and Polito1992) while Mehri and Mehri-Kamoun (Reference Mehri and Mehri-Kamoun2007) reported that 30% of Tunisian cultivar Gerboui fruit following self-pollination were parthenocarpic. Cross-pollination was also found to reduce shotberries of Gordal Sevillana olives (Fernandez-Escobar and Gomez-Valedor, Reference Fernandez-Escobar and Gomez-Valedor1985). Castillo-Lianque et al. (Reference Castillo-Llanque, Casilla and Baumann2008) reported a high incidence of shotberries during harvest only in self-pollinated Ascolana trees confirming the impact of pollen source on successful flower fertilization of olive. Additionally, intensity of shotberry production varied between cultivars in our work, in agreement with previous studies of other olive cultivars (Gozel et al., Reference Gozel, Karadag, Tahtacı and Dogruer2008; Ugrinovic and Stampar, Reference Ugrinovic and Stambar1996).
Previous studies associated temperature with olive bud induction and differentiation (see review paper by Fabbri and Benelli, Reference Fabbri and Benelli2000), bud development (Bignami et al., Reference Bignami, Natali and Amadei1999), timing of flowering (Pérez-López et al., Reference Pérez-López, Ribas, Moriana, Rapoport and De Juan2008), pollen germination (Cuevas et al., Reference Cuevas, Rallo and Rapoport1994; Fernandez-Escobar et al., Reference Fernandez-Escobar, Gomez-Valledor and Rallo1983; Koubouris et al., Reference Koubouris, Metzidakis and Vasilakakis2009) and fruit set (Ayerza and Coates, Reference Ayerza and Coates2004). The higher overall incidence of shotberries observed in 2007 could be related to climatic factors such as low temperature during anthesis. In 2005, the lowest temperature values during anthesis were recorded on 24–25 April (4 °C). In 2006, similarly low temperatures were observed on 6 May (4 °C). Strikingly, in 2007, the lowest temperature values were 2 °C (19 and 24 April) and 3 °C (25 April). Denney et al. (Reference Denney, Martin, Kammereck, Ketchie, Connell, Krueger, Osgood, Sibbeft and Nour1993) also observed that unusually low temperature during flowering resulted in serious harm in flowering, pollination and fruit set as well as abnormally developed olive fruits in California. Ayerza and Coates (Reference Ayerza and Coates2004) reported that shotberries were increased following extreme high temperature values during anthesis. Relevant experimental data are scarce and most observations have been based on random incidents of extreme temperatures during field studies.
The fresh weight of shotberries was significantly lower (44-fold) than that of normal fruit. Significant variation observed in shotberry fresh weight in the present study indicates different levels of stimulus expression even in flowers of the same inflorescence (Figure 6), although more research is necessary in that aspect. Shotberry size was also markedly lower (Figure 6). Our results indicate that parthenocarpic fruit have a competitive disadvantage in accumulation of assimilates as proposed by Marquez et al. (Reference Marquez, Benlloch and Rallo1990). Furthermore, Farinelli et al. (Reference Farinelli, Tombesi and Hassani2008) also reported that absence of seed in Carolea and Kalamata led to smaller fruit due to a smaller endocarp.

Figure 6. (A) Normal (top) and parthenocarpic (bottom) olive fruits after cross- and self-pollination, respectively. (B) Size comparison between normal (right) and parthenocarpic (left) olive fruits. (C) Parthenocarpic fruits of several sizes (1, 2) and normal fruits (3) co-exist in the same panicle indicating different levels of expression of the stimulus responsible. (D) Contrasting to seedlessness of shotberries, some fruits resulting from cross-pollination included two seeds.
In the present study, shotberries usually lacked seed while up to 3.3% of Mastoidis fruit resulting from free-pollination included two seeds (Figure 6). In a previous study, Cuevas and Oller (Reference Cuevas and Oller2002) reported that up to 14% of Hojiblanca fruit from open-pollination were two-seeded while only 4% and 1% were found in Arbequina and Sevillano, respectively. Similarly, up to 5.1% of fruit of Carolea derived from open-pollination was two-seeded while in Kalamata it was only 3% (Farinelli et al., Reference Farinelli, Tombesi and Hassani2008).
The results of the present study suggest that the incidence of parthenocarpic fruit is influenced by genetic factors and varies between different cultivars. Cultivars Koroneiki and Kalamata were more prone to produce shotberries than Mastoidis and Amigdalolia. Intra-varietal fluctuation observed between consecutive years implies that other factors might also be involved in the level of stimulus expression leading to production of parthenocarpic olive fruit.
Acknowledgements
We are grateful to Drs A. Doulis and G. Psarras at NAGREF and anonymous reviewers whose comments led to substantial improvements in this paper.









