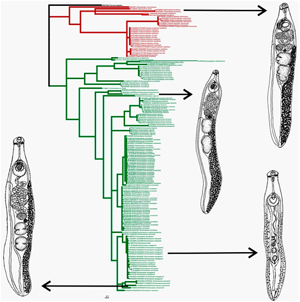
Introduction
Among the trematodes from geographically distant territories, the populations of which are isolated from each other, there are species that exhibit significant morphological similarities, both in adult and other developmental stages. As a result, many species of trematodes are considered cosmopolitan (Detwiler et al., Reference Detwiler, Bos and Minchella2010; Morley et al., Reference Morley, Adam and Lewis2010; Nugaraite et al., Reference Nugaraite, Mazeika and Paulauskas2017). The group of such worms includes some members of Echinostomatidae Looss, 1899. In particular, this applies to worms from the ‘revolutum’ group of Echinostoma Rudolphi, 1809, Isthmiophora Lühe, 1909, etc. For example, Echinostoma miyagawai Ishii, 1932 has a type locality in East Asia, but was included in the list of parasites infecting birds in Europe (Kostadinova et al., Reference Kostadinova, Gibson, Biserkov and Ivanova2000a). At the same time, the European species Echinostoma paraulum Dietz, 1909, Echinostoma revolutum (Frolich, 1802), Isthmiophora melis (Schrank, 1788) Luhe, 1909 and others are considered simultaneously as parasites of animals in East Asia (Oshmarin, Reference Oshmarin1963; Besprozvannykh et al., Reference Besprozvannykh, Ermolenko and Nadtochy2012; Nguyen et al., Reference Nguyen, Ha and Ermolenko2013). To find out whether trematodes, classified as cosmopolitans, are such or belong to different species, a comprehensive approach is necessary, including obtaining information on the life cycle and morphology of developmental stages (primarily cercariae and adults) as well as molecular data for the worms from the experiment. The greatest contribution to the study of echinostomatids, using an integrated approach, was made for worms circulating in Europe (Nasincova, Reference Nasincova1991; Kostadinova et al., Reference Kostadinova, Gibson, Biserkov and Ivanova2000a, Reference Kostadinova, Gibson, Biserkov and Chipev2000b, Reference Kostadinova, Herniou, Barrett and Littlewood2003; Kostadinova and Gibson, Reference Kostadinova and Gibson2002; Georgieva et al., Reference Georgieva, Faltynkova, Brown, Blasco-Costa, Soldanova, Sitko, Scholz and Kostadinova2014; Faltynkova et al., Reference Faltynkova, Georgieva, Soldanova and Kostadinova2015; Hildebrand et al., Reference Hildebrand, Adamczyk, Laskowski and Zalesny2015; Staneviciute et al., Reference Staneviciute, Stunzenas and Petkeviciute2015). With regard to the echinostomatids of the East Asian region, for most worms, data were obtained separately either on the morphology of developmental stages or in the form of molecular data of adult worms from naturally infected animals (e.g. Ahn et al., Reference Ahn, Ryang, Chai and Sohn1989; Lee et al., Reference Lee, Chai, Hong and Sohn1990; Chung et al., Reference Chung, Jung and Park2001a, Reference Chung, Jung, Park and Hwang2001b; Kostadinova et al., Reference Kostadinova, Herniou, Barrett and Littlewood2003; Sohn et al., Reference Sohn, Na and Shin2017). The lack of comprehensive data for East Asian echinostomatids prevents questions surrounding the taxonomy of these worms and their phylogenetic relationships with other members of Echinostomatidae from being answered.
In the Russian southern Far East, there are currently 70 Echinostomatidae species (Besprozvannykh et al., Reference Besprozvannykh, Ermolenko and Nadtochy2012) including cosmopolitan trematodes and species found only in East Asia but which have twins in Europe. Experimental studies of the life cycles were carried out and molecular data for nuclear (28S rRNA gene and ITS2 rDNA region) and mitochondrial (cox1 and nad1 genes) markers were obtained to establish the taxonomic status of four Echinostomatidae species, which were found in the first intermediate hosts in the Russian southern Far East. In addition, the phylogenetic relationships were clarified within the family Echinostomatidae.
Materials and methods
Life cycle and morphology of worms
The material for the studies, which were carried out during the period 2017–2018, consisted of freshwater molluscs collected in the Russian southern Far East: Anisus centrifugops Prozorova et Starobogatov, 1997 and Helicorbis sujfunensis Starobogatov, 1957 (Planorbidae Rafinesque, 1815) from a reservoir in the Komissarovka River basin, Amuropaludina praerosa (Gersferfel, 1859) (Viviparidae Gray, 1847) from the Melgunovka River (Khasansky district) and Lymnaea auricularia (Linnaeus, 1758) (Lymnaeidae Rafinesque, 1815) from the Swan Lake (Khasansky district). Specimens shedding cercariae of Echinostomatidae were identified among the listed species of molluscs. To establish the second intermediate hosts of these trematodes, separately for each of the indicated molluscs with cercariae, five juvenile fish of the species Rhodeus sericeus (Pallas, 1776) (Cyprinidae Rafinesque, 1815), Perccottus glenii Dybowski, 1877 (Odontobutidae Hoese and Gill, 1993), tadpoles of Rana dybovskii (Guenther, 1876) (Ranidae Bonaparte, 1840) and the molluscs A. centrifugops, L. auriculaia and Boreoelona ussuriensis Ehrmann, 1927 (Bithyniidae Gray, 1857) were alternately placed in small containers (0.5–1.5 L). Fish and tadpoles were caught from the reservoir where no animals with any trematode infection were detected (50 individuals of each species of fish and tadpoles were examined for control). Molluscs were grown from clutches under laboratory conditions. The exposure time of laboratory animals in containers with infected molluscs was 24 h in each experiment. After the infection period, animals were kept in separate aquaria at a water temperature from 18 to 22°C. On the 10th day, one specimen of each animal species used in the experiments was dissected to detect infection. Metacercariae obtained in the experiment from cercariae emitted by molluscs A. centrifugops and H. sujfunensis were found both in tadpoles (6–10 metacercariae, respectively) and molluscs L. auriculaia, A. centrifugops and B. ussuriensis (3–5 metacercariae). In infection by parasites from Lymnaea (Lamarck, 1799), metacercariae were found in fish of both species (3–7 specimens) and tadpoles (8 specimens). Parasites from Amuropaludina Moskvicheva, 1979, were detected in molluscs of both species (2–11 specimens). On the 24th day after the experiment, metacercariae (by 30 specimens) obtained from cercariae from A. centrifugops were fed to two ducklings, metacercariae from H. sujfunensis were fed to two chickens, and metacercariae from L. auricularia and A. praerosa were separately fed to two rats. After that, on days 13 and 16, 6 and 11 young worms were found in the small intestines of ducks; on days 16 and 24, 8 young worms and 2 mature ones, respectively, were found in chickens. On day 20, in both cases, 9 and 7 adult worms were found in rats. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.
Euthanasia of laboratory animals was carried out in accordance with the Committee on the Ethics of Animal Experiments of Federal Scientific Center of the East Asia Terrestrial Biodiversity, Russia (Permit Number: 3 of 02.06.2011).
Rediae and metacercariae were measured on live specimens, while cercariae were fixed in hot 4% formalin before measurement. Adult flukes were fixed in 70% ethanol and stored in 96% ethanol. Whole mounts of adult flukes were prepared by staining with alum carmine, dehydrating in a graded ethanol series, clearing in clove oil and mounting in Canada balsam. All measurements were given in micrometres (μm).
In addition to experimentally obtained adult worms, whole mounts of Euparyphium amurensis Besprozvannykh, Reference Besprozvannykh2001 were used in the study: holotype No 29-Tr and paratypes No 30-32-Tr from the Zoological Museum (Federal Scientific Center of the East Asia Terrestrial Biodiversity, Far East Branch of the Russian Academy of Sciences, Vladivostok, Russia).
Molecular data
In the analysis, two or one samples were used for each experimentally obtained adult worm (Table S1). DNA isolation was performed by the HotSHOT method (Truett et al., Reference Truett, Heeger, Mynatt, Truett, Walker and Warman2000). Partial sequences of the 28S rRNA gene (28S) and complete sequences of the ITS2 region (ITS2) of nuclear DNA, as well as partial sequences of the cox1 and nad1 genes of mitochondrial DNA, were amplified by polymerase chain reaction (PCR) using specific primers (Table S3). The annealing temperature was 55°C for 28S, ITS2 and cox1 markers, while for the nad1 marker, it was 48°C. Control of the efficiency and contamination of PCR was carried out by setting positive and negative samples, respectively. The PCR products were sequenced by the Sanger method, using the BigDye Terminator Cycle Sequencing kit (Applied Biosystems, USA). Nucleotide sequences were determined with the genetic analyser ABI3500 in the Federal Scientific Center of the East Asia Terrestrial Biodiversity, Russia. Both external and internal primers were used for sequencing (Table S3).
Processing and alignment of consensus sequences were carried out using FinchTV 1.4 and MEGA 5.0 programs (Tamura et al., Reference Tamura, Peterson, Peterson, Stecher, Nei and Kumar2011). Genetic p-distances and nucleotide composition were also determined in MEGA.
Reconstruction of phylogenetic relationships was performed using the data of other trematodes belonging to Echinostomatidae presented in the GenBank. As an outgroup, a representative from the family Fasciolidae Railliet, 1895, Fasciola hepatica Linnaeus, 1758, was chosen. This family takes basal position to branch formed from Echinostomatidae and Caballerotrematidae Tkach et al., Reference Tkach, Kudlai and Kostadinova2016 on the phylogenetic tree constructed by Tkach et al. (Reference Tkach, Kudlai and Kostadinova2016).
Phylogenetic relationships were reconstructed using the Bayesian algorithm in the program MrBayes 3.1.2. (Ronquist and Huelsenbeck, Reference Ronquist and Huelsenbeck2003), applying a model selected as optimal on the basis of the Akaike information criterion (AIC) in the program jМodeltest 2.1.7 (Darriba et al., Reference Darriba, Taboada, Doallo and Posada2012): GTR + I + G for 28S, GTR + G for ITS2, TPM1uf + G for cox1 and TVM + I + G for nad1. In the Bayesian analysis, the following number of generations of Markov chain Monte Carlo posterior part third (MCMC) was simulated: 1 000 000 for 28|S, 1 200 000 for ITS2, 900 000 for cox1 and 10 000 000 for nad1. For reconstructions based on 28S, ITS2 and cox1 sequences, the selection was performed with a frequency of every 100 generations, while for nad1, selection was performed every 1000 generations.
For all trematodes obtained in this study, the lengths of the obtained nucleotide sequences were as follows: 1121 bp for 28S, 389 bp for ITS2, 432 bp for cox1 and 459 bp for nad1. For the reconstruction of phylogenetic trees, aligned sequences of 1,074, 310, 198 and 298 residues were used for 28S, ITS2, cox1 and nad1, respectively.
Three markers, 28S, nad1 and cox1, were used in the analysis of phylogenetic relationships between taxa of this work and other members of the family. ITS2 was not used for analysis due to the low level of species separation in Echinostomatidae. However, we present this resulting tree (Fig. S4).
Results and discussion
Echinostoma chankensis Izrailskaia, Besprozvannykh, Tatonova nom. nov.
Syn. Euparyphium amurensis Besprozvannykh, Reference Besprozvannykh2001.
Definitive host: Rattus norvegicus (Berkenhout, 1769) (experimentally).
Site: lumen small intestine.
First intermediate host: Amuropaludina praerosa Gersferfel 1859.
Second intermediate host: Anisus centrifugops Prozorova et Starobogatov, 1997, Boreoelona ussuriensis Ehrmann, 1927 (experimentally).
Site: visceral muscle tissue.
Type-locality: the Melgunovka River, Primorsky Region, the Russian southern Far East; 44o35′N, 132o08′E.
Etymology: The species is named at the place of first detection of the worms in Chanka lake basin.
Adult worm (material examined: five specimens)
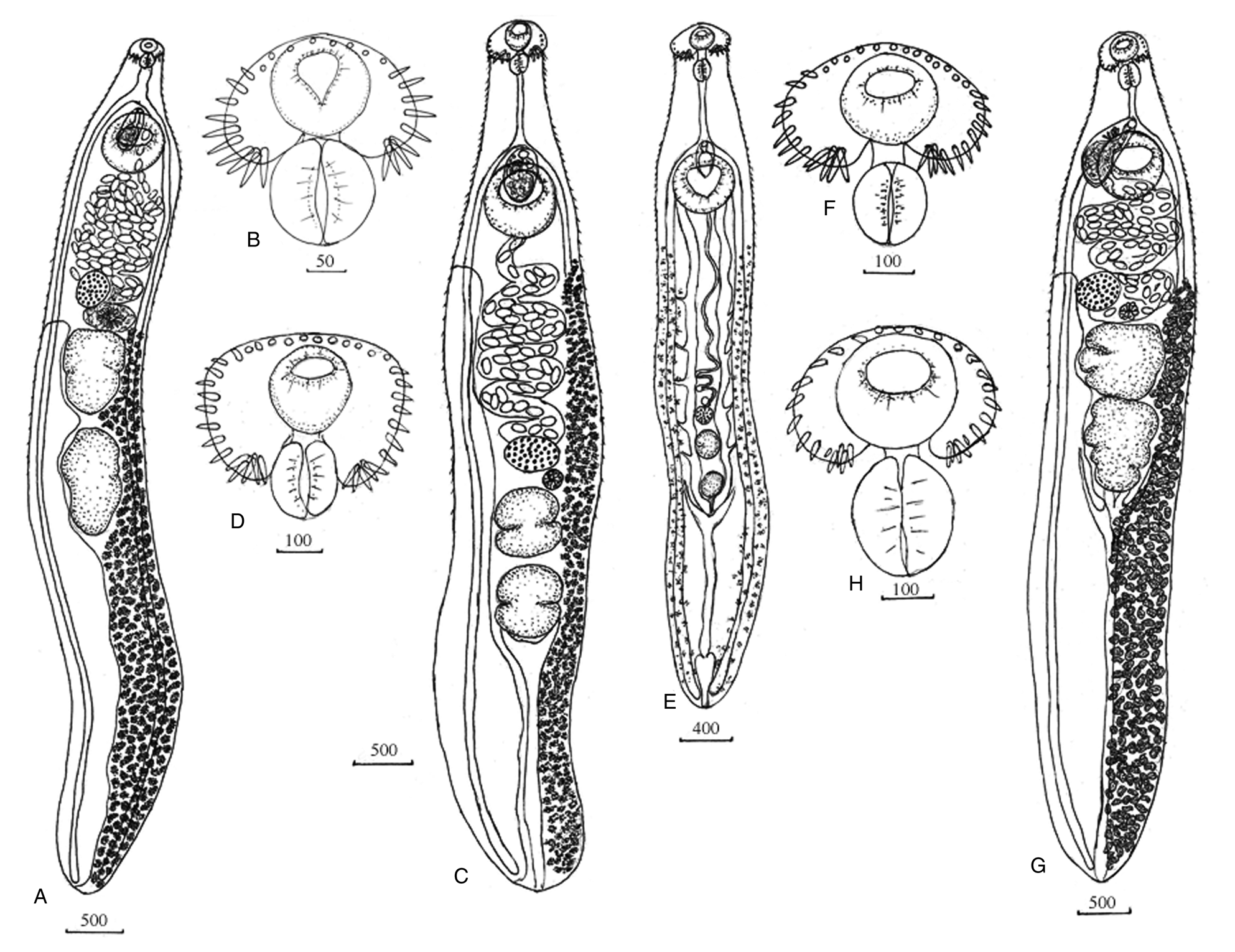
Fig. 1A, B; Table 1. Body elongate, with maximum width at level of ventral sucker, forebody short. Tegument armed with spines from anterior end to level of middle of posterior third of body. Head-collar reniform, small. Collar spines 27. Four angle spines on each ventral lappet, lateral spines in single row and dorsal spines in double row. Oral sucker round, prepharynx short, pharynx elongate-oval, equal or larger to oral sucker, oesophagus long. Intestinal bifurcation anterior to ventral sucker. Intestinal caeca blind, reach posterior extremity. Ventral sucker muscular, round, in five times larger than oral sucker. Testes two, elongated, located in the middle third of the body, tandem, separated. Surface of testes irregular with weakly expressed lobes. Cirrus-sac elongate-oval, situated between intestinal bifurcation and level of posterior third of ventral sucker. Internal seminal vesicle elongate-saccular. Prostatic cells few. Cirrus armed with very small spines. Genital pore median, between intestinal bifurcation and ventral sucker. Mehlis' gland sub-median, between testes and ovary. Ovary round, at short distance anterior to anterior testis to right of midline of body. Uterine seminal receptacle present. Uterus between ventral sucker and ovary. Eggs unembryonated, operculated. Vitellarium has two lateral fields overlapping caeca and extending from level Mehlis' gland to posterior body end; in post-testicular zone lateral fields unite or do not unite. Excretory vesicle Y-shaped, pore terminal.

Fig. 1. Echinostoma chankensis nom. nov. (Besprozvannykh, Reference Besprozvannykh2001): (A) adult worm; (B) head-collar. Echinostoma cinetorchis Ando, Ozaki, Reference Ando and Ozaki1923: (C) adult worm; (D) head-collar. Echinostoma miyagawai Ishii, 1932: (E) adult worm; (F) head-collar. Isthmiophora hortensis (Asada, 1926): (G) adult worm; (H) head-collar. Scale-bars: μm.
Table 1. Adult worms Echinostoma and Isthmiophora (Echinostomatidae) (μm)

Redia (based on five specimens)
Body elongate, saccular, conspicuous collar present, two locomotory appendages at posterior third of body. Pharynx muscular, caeca long, extending to level of locomotory appendages. Birth pore just posterior to collar-region.
Cercaria (based on 10 specimens)
Fig. 2A, B; Table 2. Body leaf-shaped, covered with small spines. Head-collar weakly expressed with spines. Oral sucker subterminal, round, prepharynx short, pharynx elongate-oval, oesophagus long. Intestinal bifurcation just anterior to ventral sucker. Intestinal branches short. Oesophagus and intestine do not have an internal lumen and consist of cells closely adjacent to each other. Ventral sucker round, larger than oral sucker. Genital primordia lies dorsal to ventral sucker. Cystogenic cells occupy entire space from posterior end of body to level of pharynx. Twelve gland cells located at oesophagus level and their ducts open at anterior end of body. Excretory vesicle saccular. Two collecting channels depart from excretory bladder, contain small granules. Caudal excretory canal opens two pores in anterior third of tail. Excretory formula [(3 + 3 + 3) + (3 × 3 + 3 + 3)] = 48. Tail longer than body, with fin-folds.

Fig. 2. Echinostoma chankensis nom. nov. (Besprozvannykh, Reference Besprozvannykh2001): (A, B), cercaria; (C), metacercaria. Echinostoma cinetorchis Ando, Ozaki, Reference Ando and Ozaki1923: (D, E), cercaria; (F), fin-folds at the tail; (G), metacercaria. Echinostoma miyagawai Ishii, 1932: (H, I), cercaria; (J), metacercaria. Isthmiophora hortensis (Asada, 1926): (K, L), cercaria; (M), metacercaria. Scale-bars: μm.
Table 2. Cercariae Echinostoma and Isthmiophora (Echinostomatidae) (μm)

*: + , finfold present; –, finfold absent; ND, no data.
Metacercaria (based on 5 specimens)
Fig. 2C. Cyst 142–160 in diameter. Cyst shell multi-layer, 2,4–3,0. Body covered with fine spines. Head-collar with spines. Oral sucker 39–58 × 54–62, pharynx oval, 31–35 × 21–25. Ventral sucker 50–62 × 58–62. Caecal cavity filled with stick-shaped granules. Excretory bladder V-shaped. Excretory channels with granules.
Remark
The adult worms, as well as cercariae and metacercariae obtained in this study, correspond to E. amurensis Besprozvannykh, Reference Besprozvannykh2001 according to all morphological and metric measurements (Tables 1, 2). Their life cycles are also identical. For the first time, the adult worms described above were experimentally obtained by Besprozvannykh (Reference Besprozvannykh2001), as well as in the present study, from cercariae emitted from A. praerosa molluscs in the Nesterovka River, a tributary of the Melgunovka River in the Russian southern Far East. The suggestion that this trematode belongs to the genus of Euparyphium Dietz, 1909 was based on the correspondence of the diagnostic characters of adult worms to the genus and on the life cycle data by Besprozvannykh (Reference Besprozvannykh2001). Kostadinova and Gibson (Reference Kostadinova and Gibson2002) suggested that E. amurensis may be synonymized with Isthmiophora hortensis. However, the authors did not take into account that the first intermediate host of E. amurensis is the caenogastropod mollusc (Viviparidae), while I. hortensis infects the pulmonate mollusc (Lymnaeidae). Usually, trematodes are specific to their first intermediate hosts, and circulation using molluscs from different families would indicate that the worms designated as E. amurensis and I. hortensis cannot belong to the same species.
Phylogenetic reconstruction based on cox1 showed that worms in this study do not form a common clade with representatives of both Isthiomorpha and Echinostoma (Fig. 3). However, this configuration of the tree apparently resulted from the limited data on cox1 for Echinostomatidae. Results based on 28S and nad1 showed that our worms, previously assigned to the genus Euparyphium, cluster together with members of Echinostoma (Figs. 4, S5, 6). Among the Echinostoma representatives, worms of the European species Echinostoma bolschewense (Kotova, 1939), as well as worms in this study, circulate using Viviparidae molluscs as the first intermediate hosts. Based on the phylogenetic reconstructions using the nad1 marker, our samples and E. bolschewense form a separate branch (Subcluster 6) within group 2 (Figs. S5, 6). This clustering may be connected by the first intermediate hosts of both parasites, caenogastropod molluscs, in contrast to the vast majority of echinostomes presented in the molecular analysis, which employ pulmonate molluscs as hosts. Despite the fact that the genetic distance between these species based on 28S is not statistically significant (0.1%), there are morphological differences between the worms in this study and E. bolschewense. The adult stage of these worms differs in the number of collar spines (27 vs 37, respectively), and the size of the cercariae of E. bolschewense is more than twice the size of the cercariae in this study (Tables 1, 2). The differences between these species are also reaffirmed by the presence of genetic distances for nad1 of 13.3%, which correspond to the level of interspecific values for representatives of this genus. The similarity between our samples and E. bolschewense, according to 28S data, is due to the conservatism of the nuclear marker in comparison with the mitochondrial one (nad1), indicating a close relationship between these species and their relatively recent divergence. Morphological information and data on the mitochondrial marker nad1 may lead to conclusion that East Asian worms (this study) and the European E. bolschewense worms belong to different species. Given that the species name ‘amurensis’ has been used for Echinostoma amurensis (Petrochenko & Khrustaleva, 1963), we named the worms formerly known as Euparyphium amurensis as Echinostoma chankensis nom. nov.

Fig. 3. The phylogeny based on cox1 sequences using the Bayesian algorithm. The outgroup species are listed in Table S1.
Echinostoma cinetorchis Ando and Ozaki, Reference Ando and Ozaki1923
Definitive host: Gallus gallus dom. (experimentally).
Site: lumen small intestine.
First intermediate host: Helicorbis sujfunensis Starobogatov, 1957.
Second intermediate host: Rana dybowskii (Guenther, 1876), Boreoelona ussuriensis Ehrmann, 1927 (experimentally).
Site: visceral muscle tissue.
Type-locality: the backwater in basin of the Komissarovka River, Primorsky Region, the Russian southern Far East; 44°49′N, 131°33′E.
Adult worm (material examined: six specimens)
Fig. 1C, D; Table 1. Body elongate, with maximum width at level of ovary, forebody short. Tegument armed with spines from anterior end to level of posterior testis, distributed densely in forebody, but more sparsely in hindbody. Head-collar reniform, small. Collar spines 37. Five angle spines on each ventral lappet, lateral spines in single row, dorsal spines in double row. Oral sucker round or elongate-oval, prepharynx short, pharynx elongate-oval, equal in size to oral sucker, oesophagus long. Intestinal bifurcation anterior to ventral sucker. Intestinal caeca blind, reach close to posterior extremity. Ventral sucker muscular, round, with deep cavity. Testes two, elongated, with median constriction, located in third quarter of body, tandem, separated. Cirrus-sac elongate-oval, between intestinal bifurcation and mid-level of ventral sucker. Internal seminal vesicle elongate-saccular. Prostatic cells few. Genital pore median, between intestinal bifurcation and ventral sucker. Mehlis' gland sub-median, between testes and ovary. Ovary transversally oval, at short distance anterior to anterior testis. Uterine seminal receptacle present. Uterus between ventral sucker and ovary. Eggs unembryonated, operculated. Vitellarium, formes two lateral fields overlapping caeca and extending from short distance posterior to ventral sucker to the posterior body end, lateral fields do not unite in post-testicular zone. Excretory vesicle Y-shaped, pore terminal.
Redia (based on five specimens)
Body elongate, saccular, conspicuous collar present, two locomotory appendages at posterior third of body. Pharynx muscular, caeca long, extending to level of locomotory appendages. Birth pore just posterior to collar-region.
Cercaria (based on 10 specimens)
Fig. 2 D–F; Table 2. Body leaf-shaped, covered with small spines. Head-collar weakly expressed with spines. Oral sucker subterminal, round or elongate-oval, prepharynx short, pharynx elongate-oval, oesophagus long. Intestinal bifurcation just anterior to ventral sucker. Intestinal branches extending to level of middle of ventral sucker. Oesophagus and intestines do not have an internal lumen and consist of cells closely adjacent to each other. Ventral sucker round or transversally oval, larger than oral sucker. Genital primordia lies dorsal to ventral sucker. Cystogenic cells occupy entire space from posterior end of body to level of pharynx. Twelve gland cells located at oesophagus level and their ducts open at anterior end of body. Excretory vesicle saccular. Two collecting channels depart from excretory bladder, contain small granules. Caudal excretory canal opens two pores in anterior third of tail. Excretory formula [(3 + 3 + 3) + (3 × 3 + 3 + 3)] = 48. Tail longer than body, with fin-folds.
Metacercaria (based on 5 specimens)
Fig. 2G. Cyst 120–160 in diameter. Cyst shell multi-layer, 1,2–1,5. Body covered with fine spines. Head-collar with spines. Oral sucker 39–42 × 42–56, pharynx oval, 22–39 × 17–25. Ventral sucker 50–0,057 × 65–75. Excretory bladder V-shaped. Excretory channels with granules.
Remark
The type locality for E. cinetorchis is Japan, where adult worms of this species were found in Rattus norvegicus (Ando and Ozaki, Reference Ando and Ozaki1923). Subsequently, worms of this species were repeatedly recorded in the East Asian region (Ahn et al., Reference Ahn, Ryang, Chai and Sohn1989; Lee et al., Reference Lee, Chai, Hong and Sohn1990; Chung et al., Reference Chung, Jung and Park2001a, Reference Chung, Jung, Park and Hwang2001b). In 1927, Takahashi (Takahashi 1927 citation of Ito, Reference Ito1964) established that the freshwater mollusc Segmentina nitida (Müller, 1774) (Planorbidae) plays the role of the first intermediate host for this trematode, and the second intermediate hosts are amphibians, molluscs and fishes. Later, under experimental conditions, Ahn et al. (Reference Ahn, Ryang, Chai and Sohn1989) and Lee et al. (Reference Lee, Chai, Hong and Sohn1990) reproduced the life cycle of a trematode, which they designated as E. cinetorchis. The starting point in their experiments was echinostomatid metacercariae from naturally infected molluscs. These metacercariae infected the final hosts, and adult worms were received. After that, the eggs obtained from the adult worms were used to infect different species of molluscs to determine the first intermediate hosts. However, by utilizing such a methodology in the experimental study of the life cycle of trematodes, there is a high probability of mixed invasion by metacercariae of different species in naturally infected second intermediate hosts, since, based on the morphology of metacercariae, it is almost impossible to determine the belonging of closely related species of trematodes. Members of Echinostoma are no exception. Several species with 37 spines on the collar are known of this genus, and they have a wide range of second intermediate hosts. The fact that Lee et al. (Reference Lee, Chai, Hong and Sohn1990) have obtained incorrect data as a result of methodological errors is testified by sizes of cercariae in their material, which are half the size of cercariae of E. cinetorchis according to Takahashi (Takahashi 1927 citation of Ito, Reference Ito1964) (Table 2). The above also applies to data obtained by Chung et al. (Reference Chung, Jung and Park2001a, Reference Chung, Jung, Park and Hwang2001b), who indicated that members of Lymnaeidae are the first intermediate hosts of E. cinetorchis in Korea. However, it is unlikely that worms of the same species would have used molluscs of different families as first intermediate hosts, Planorbidae (Takahashi 1927 citation of Ito, Reference Ito1964) and Lymnaeidae (Chung et al., Reference Chung, Jung and Park2001a, Reference Chung, Jung, Park and Hwang2001b), in connection with the specificity of trematodes to the first intermediate hosts. In addition, according to Chung et al. (Reference Chung, Jung and Park2001a, Reference Chung, Jung, Park and Hwang2001b), after the infection of molluscs with miracidia of echinostomes, the first cercariae began to emit from molluscs on day 21, which is also unlikely, because a longer period is required for the ripening of cercariae. Thus, besides data on the host composition and morphology of the developmental stages of the East Asian trematode E. cinetorchis obtained by Ando and Ozaki (Reference Ando and Ozaki1923) and Takahashi (Takahashi 1927 citation of Ito, Reference Ito1964), the remaining information on this species is insufficiently correct for species identification.
According to morphological characteristics, among echinostomes with 37 collar spines, the worms designated as E. cinetorchis in this study are most similar to the European E. paraulum at the adult stage. A defining characteristic for these species is the presence of such features as testes with a middle constriction and an elongate ovary (Dietz 1910 citation of Scrjabin 1947). In our material, all individuals of parasites had the same shape of testes and ovary. Cercariae of these species of trematodes have a similar morphometry; both of them had a swimming membrane on the tail. However, in contrast to E. paraulum, which has Lymnaeidae mollusc as the first intermediate host, E. cinetorchis uses molluscs from Planorbidae. This is a significant difference and indicates that these trematodes may belong to different species.
On the phylogenetic reconstruction performed based on 28S, E. cinetorchis from our study is clustered with other Echinostoma spp.: E. revolutum, E. miyagaway, E. paraulum, E. cinetorchis, E. trivolis, E. paraensei and E. novazealandense (Fig. 4). The first four species form a common branch with a low posterior probability, possibly reflecting the politomy of this group. E. cinetorchis (KX817344-KX817348) is closest to E. cinetorchis from this study based on the genetic distance between them (0.1%). The genetic distance between E. cinetorchis and E. paraulum was 0.9%, while the other species in the cluster mentioned above differed from trematode in this study by 0.16–0.20%.

Fig. 4. The phylogeny based on 28S sequences using the Bayesian algorithm. The outgroup species are listed in Table S1.
On the tree constructed using nad1, the trematode under study also formed a common Subcluster 14 with E. cinetorchis (KU519288-KU519292) and E. paraulum (KP065677-KP065679, KP065681), and sequences obtained in this study differ from E. cinetorchis from GenBank (KU519288-KU519292) and E. paraulum by 9.6 and 10.2%, respectively; in addition, there are seven non-synonymous substitutions in both cases (Figs. S5, 6). At the same time, the distance between sequences of E. cinetorchis and E. paraulum from GenBank reaches 11.0%, with 4 non-synonymous substitutions. These obtained values correspond with the level of interspecific difference. Unfortunately, the nucleotide sequences for E. cinetorchis in GenBank have no supporting publication with description of morphology, biology and phylogenetic relationships for this trematode. This situation creates difficulties in designating the species status of the worms as well as its phylogenetic relationship with members of the common cluster. Nevertheless, based on morphology, life cycles and molecular data, we assigned the trematode obtained in the Russian southern Far East to the species E. cinetorchis. In contrast, the nucleotide sequences designated as E. cinetorchis in GenBank may belong to another species close to E. cinetorchis and E. paraulum.
Echinostoma miyagawai Ishii, 1932
Definitive host: Anas platyrhynchos dom. (experimentally).
Site: lumen small intestine.
First intermediate host: Anisus centrifugops Prozorova et Starobogatov, 1997.
Second intermediate host: Rana dybowskii (Guenther, 1876), Lymnaea auriculaia (Linne, 1758), Anisus centrifugops (experimentally).
Site: visceral muscle tissue.
Type-locality: the backwater in basin of the Bolshaya Ussurka River, Primorsky Region, the Russian southern Far East; 45°59′N, 134°07′E.
Adult worm (material examined: 8 specimens)
Fig. 1E, F; Table 1. Body elongate, with maximum width at level of ventral sucker, forebody short. Tegument armed with spines from anterior end to level of posterior testis distributed densely in forebody, but more sparsely in hindbody. Head-collar reniform, small. Collar spines 37. Five angle spines on each ventral lappet, lateral spines in single row, dorsal spines in double row. Oral sucker round, prepharynx short, pharynx elongate-oval, equal in size to oral sucker, oesophagus long. Intestinal bifurcation anterior to ventral sucker. Ventral sucker at beginning of second quarter of body. Testes elongated, in posterior third of body, tandem, separated. Surface of testes irregular with weakly expressed lobes. Cirrus-sac elongate-oval, between intestinal bifurcation and mid-level of ventral sucker. Internal seminal vesicle elongate-saccular. Genital pore median, at level of intestinal bifurcation or between intestinal bifurcation and ventral sucker. Ovary round, at short distance anterior to anterior testis. Uterus underdeveloped. Eggs are absent in uterus. Vitellarium from two lateral fields overlapping caeca and extending from short distance posterior to ventral sucker to the posterior body end. Excretory vesicle Y-shaped, pore terminal.
Redia (based on five specimens)
Body elongate, saccular, conspicuous collar present, two locomotory appendages at posterior third of body. Pharynx muscular, caeca long, extending to level of locomotory appendages. Birth pore just posterior to collar-region.
Cercaria (based on 10 specimens)
Fig. 2H, I; Table 2. Body elongate, covered with small spines. Head-collar well-expressed with spines. Oral sucker subterminal, round or elongate-oval, prepharynx short, pharynx elongate-oval, oesophagus long. Intestinal bifurcation just anterior to ventral sucker. Intestinal branches extending to level of anterior margin of excretory vesicle. Oesophagus and intestine do not have an internal lumen and consist of cells closely adjacent to each other. Ventral sucker round or transversally oval, larger than oral sucker. Genital primordia consists from two parts lying immediately anterior and posterior to ventral sucker. Cystogenic cells occupy entire space from posterior end of body to level of pharynx. Twelve gland cells located at oesophagus level and their ducts open at anterior end of body. Excretory vesicle saccular. Two collecting channels depart from excretory bladder, contain small granules. Caudal excretory canal opens two pores in anterior third of tail. Excretory formula [(3 + 3 + 3) + (3 × 3 + 3 + 3)] = 48. Tail longer than body, without fin-folds.
Metacercaria (based on 5 specimens)
Fig. 2J. Cyst 131–135 in diameter. Body covered with fine spines. Head-collar with spines. Oral sucker 30–38 × 38–42, pharynx oval, 22–25 × 140–17. Ventral sucker 39–42 × 42–54. Excretory bladder V-shaped. Excretory channels with granules.
Remark
In the morphology and structure of the life cycle, the worms obtained as a result of the experimental study are similar to E. miyagawai, which, as well as E. cinetorchis, were first discovered in Japan. For this trematode in the East Asian region, no data were obtained on the composition of the intermediate hosts and the morphology of the larval stages. Subsequently, worms of this species were found in birds in the territory of Europe (Bashkirova, Reference Bashkirova1941) and, in 1953, Nevostrueva (Reference Nevostrueva1953) obtained adult worms and designated them as E. miyagawai, when feeding chickens with Lymnaea molluscs naturally infected by echinostomatid metacercariae with 37 spines. In addition, Lymnaea-emitting cercariae of echinostomes were also found in the same pond from which molluscs infected with metacercariae were previously taken. On this basis, it was a priori suggested that the cercariae discovered belong to E. miyagawai. However, Nevostrueva (Reference Nevostrueva1953) neither provides a description nor drawings of the obtained metacercariae, cercariae and adult worms. In 1971, Kosupko (Reference Kosupko1971) conducted an experiment to confirm the validity of European E. miyagawai. The primary source of adult worms was metacercariae from naturally infected molluscs. Metacercariae were fed to various animals (birds and mammals). As a result, adult worms with 37 spines on the collar were obtained, some of which were identified as E. revolutum and others as E. miyagawai. Eggs were collected from these worms separately, and an experiment on the infection of different molluscs was carried out to detect the possible first intermediate hosts. In the first case, the molluscs Lymnaeidae were infected; in the second case, trematodes infected both Lymnaeidae and Planorbidae. The second intermediate hosts (molluscs and amphibians) were infected by cercariae from these molluscs, and the metacercariae from them were fed to chickens. Chickens were fed with 1-, 3- and 10-day-old metacercariae, as well as with larvae of an older age; in all cases, adult worms were obtained. Herewith, when determining trematodes as E. miyagawai, Kosupko (Reference Kosupko1969) refers to Nevostrueva (Reference Nevostrueva1953), where, as mentioned above, there is no description of cercariae, metacercariae or adult worms. Analysis of the research results of Nevostrueva and Kosupko showed that the methodology of the experiment was incorrect from the outset, as in the case indicated above in relation to E. cinetorchis, because the experiment was started by using metacercariae from naturally infected second intermediate hosts. The high probability of a mixed invasion by metacercariae of closely related species makes the subsequent studies conducted by both Nevostrueva (Reference Nevostrueva1953) and Kosupko (Reference Kosupko1969) incorrect. As a result, the molluscs from different families are indicated as the first intermediate hosts for E. miyagawai, which is improbable. Moreover, obtaining adult worms from 1- to 3-day-old metacercariae of Echinostoma showed that, the naturally infected animals were apparently used in the experiment, because metacercariae of echinostomes at this age are not capable of infecting the final host. Kostadinova et al. (Reference Kostadinova, Gibson, Biserkov and Ivanova2000a, Reference Kostadinova, Gibson, Biserkov and Chipev2000b) also conducted an experimental study of the life cycle of European E. miyagawai. In this experiment, the authors initially designated echinostomes' cercariae from naturally infected molluscs Planorbis Müller, 1774 and Anisus as E. miyagawai. This was done based on the fact that the cercariae obtained have the tail membrane and were similar in the number of para-oesophageal gland cells to cercariae in Kosupko (Reference Kosupko1969), where data on the morphology of cercariae are limited to this information. In the experiment, Lymnaea molluscs were infected with cercariae emitted from Planorbis and Anisus, and metacercariae were obtained. According to Kostadinova et al. (Reference Kostadinova, Gibson, Biserkov and Ivanova2000a, Reference Kostadinova, Gibson, Biserkov and Chipev2000b), metacercariae of E. miyagawai became invasive on the 8th day. When chickens and pigeons were infected with them, as well as with older metacercariae, young and adult worms were found in pigeons. Worms reached sexual maturity on day 10 from the moment of pigeon infection.
Our experiments on the life cycle of an echinostome whose cercariae were produced by the Anisus centrifugops mollusc resulted in young 16-day-old worms, which are morphologically (number of spines on the collar, and structure and topology of organs) and metrically (Table 1) similar to the 8-day-old E. miyagawai presented in Kostadinova et al. (Reference Kostadinova, Gibson, Biserkov and Ivanova2000a, Reference Kostadinova, Gibson, Biserkov and Chipev2000b). The results of the conducted experiment showed that, in contrast to European E. miyagawai, which reaches maturity on day 8 after infection of the final host (Kostadinova et al., Reference Kostadinova, Gibson, Biserkov and Chipev2000b), East Asian worms remain undeveloped even on day 16. At the same time, cercariae of East Asian and European worms differ in the sizes of the body and organs, as well an absence and presence of swimming membrane on the tail, respectively (Table 2).
On the phylogenetic reconstructions performed based on both 28S and nad1, E. miyagawai is clustered with members of the same species obtained by other authors from European and Asian countries (Figs. 4, S5, 6). However, molecular data for a sample from the type locality (Japan) are not currently presented in GenBank. The 28S nucleotide sequences for E. miyagawai samples (KP065593, KT956916 and MH748722) found in the Czech Republic, Ukraine and China are identical to our data (Fig. 4). All listed individuals of E. miyagawai and E. miyagawai (KY436408) from New Zealand form a separate branch within this cluster with high support, but genetic differences between the samples have no statistical significance.
In accordance with to the conservative 28S gene data, no differences were found between E. miyagawai in our material and European E. miyagawai using the more variable mitochondrial marker nad1 (Subcluster 15) (Figs. S5, 6). However, it must be taken into account that the nucleotide sequences of this gene have different rates of mutation accumulation in closely related species of trematodes, as shown by the analysis of this mitochondrial marker for the family Opisthorchiidae Looss, 1899 (Sitko et al., Reference Sitko, Bizos, Sherrard-Smith, Stanton and Komorova2016; Besprozvannykh et al., Reference Besprozvannykh, Tatonova and Shumenko2019). Due to the non-uniform rate of nucleotide substitutions in the nad1 marker, there may be both a high value of genetic distances and the absence of any differences between some close species, or differences there are minimal. In contrast to nad1, cox1 has a more uniform evolutionary rate (Vilas et al., Reference Vilas, Criscione and Blouin2005), which leads us to suggest that this marker may identify differences between the populations of E. miyagawai from Europe and East Asia, which should have arisen as a result of their geographical isolation. On the reconstruction based on this marker (cox1), the samples obtained are clustered with E. miyagawai from China, with which they have no molecular differences (Fig. 3). Unfortunately, GenBank does not have data for cox1 of E. miyagawai samples from Europe, which does not allow us to confirm or refute our assumption about the molecular differences between the European and Asian populations using this marker.
Thus, although the 28S and nad1 markers do not separate the Asian and European populations of E. miyagawai, given the presence of morphological differences between them at the cercariae stage, the different times required for worms to reach maturity after infection of the final host and the geographical isolation of trematodes is associated with the migration routes of their final hosts (Winker et al., Reference Winker, McCracken, Gibson, Pruett, Meier, Huettmann, Wege, Kulikova, Zhuravlev, Perdue, Spackman, Suarez and Swayne2007). These factors provide strong grounds for considering East Asian and European worms to be of different species. Similar results were obtained for some representatives of Echinochasmidae Odhner 1910 (Tatonova et al., Reference Tatonova, Izrailskaia and Besprozvannykh2020).
Isthmiophora hortensis (Asada, 1926)
Syn. Isthmiophora melis (Oshmarin, Reference Oshmarin1963; Besprozvannykh, Reference Besprozvannykh2001)
Definitive host: Rattus norvegicus Berkenhout, 1769 (experimentally).
Site: lumen small intestine.
First intermediate host: Lymnaea auricularia (Linnaeus, 1758).
Second intermediate host: fish Rhodeus sericeus (Pallas, 1776), Perccottus glenii Dybowski, 1877, tadpoles of Rana dybowskii (Guenther, 1876) (experimentally).
Site: head tissues of fish and tissues of body and organs of tadpoles.
Type-locality: the backwater in basin of the Karasik River, Primorsky Region, the Russian southern Far East; 42°35′N, 130°42′E.
Adult worm (material examined: eight specimens)
Fig. 1G, H; Table 1. Body elongate, with maximum width at level of ovary, forebody short. Tegument armed with spines from anterior end to level of posterior testis, distributed densely in forebody, but more sparsely in hindbody. Head-collar reniform, small. Collar spines 27 or 29. In first case, four angle spines on each ventral lappet, lateral spines in single row, dorsal spines in double row; in second case, between angle and rest spines, there is one underdeveloped spine on each side. Oral sucker round, prepharynx short, pharynx elongate-oval, equal in size to oral sucker, oesophagus long. Intestinal bifurcation anterior to ventral sucker. Ventral sucker in first quarter of body. Testes tandem, large, contiguous, irregular in outline with weakly expressed lobes. Anterior testis pre-equatorial, transversely elongated. Posterior testis equatorial, longitudinally elongated. Cirrus-sac elongate-oval, right to median line and partially covered by ventral sucker. Internal seminal vesicle elongate-saccular. Prostatic cells few. Genital pore median at level of intestinal bifurcation. Mehlis' gland sub-median, just anterior to anterior testis. Ovary transversely oval, anterior to testis. Uterine seminal receptacle present. Uterus between ovary and ventral sucker. Eggs unembryonated, operculated. Lateral fields of vitellarium between level of ovary and posterior extremity. Fields of vitellarium confluent in post-testicular region. Excretory vesicle Y-shaped, pore terminal.
Redia (based on 5 specimens)
Body elongate, saccular, conspicuous collar present, two locomotory appendages at posterior third of body. Pharynx muscular, caeca long extending to level of locomotory appendages. Birth pore just posterior to collar-region.
Cercaria (based on 10 specimens)
Fig. 2K, L; Table 2. Body oval or triangular, covered with small spines. Head-collar weakly expressed with 27 spines. Oral sucker subterminal, round or transversally oval, prepharynx short, pharynx oval, oesophagus long. Intestinal bifurcation at level of anterior half of ventral sucker. Intestinal branches extending to level of posterior margin of ventral sucker. Oesophagus and intestines do not have an internal lumen and consist of cells closely adjacent to each other. Ventral sucker transversally-oval, larger than oral sucker. Genital primordia lying dorsal to ventral sucker. Cystogenic cells occupy entire space from posterior end of body to level of pharynx. Twelve gland cells located at oesophagus level and their ducts open at anterior end of body. Excretory vesicle saccular, with thick walls. Two collecting channels depart from excretory bladder, contain large granules. Caudal excretory canal opens two pores in anterior third of tail. Excretory formula [(3 + 3 + 3) + (3 × 3 + 3 + 3)] = 48. Tail longer than body, without fin-folds.
Metacercaria (based on five specimens)
Fig. 2M. Cyst 290–320 in diameter. Сyst can be covered of thick fibrous shell. Body covered with fine spines. Head-collar with spines. Oral sucker 42–54 in diameter, pharynx oval 22–25 × 17–22. Ventral sucker 42–67 × 54–67. Excretory bladder V-shaped. Excretory channels with granules.
Remark
Worms in this study are identical to I. hortensis from Korea based on morphological and metric characters (Table 1). At the same time, according to the morphology and most of the metric indices of the adult individuals and cercariae, I. hortensis has no gap in the characters with I. melis (Tables 1, 2). There are insignificant differences between I. melis and I. hortensis at the adult stage. In I. hortensis, the collar spines and the eggs are slightly smaller than in I. melis. In addition, worms in our material may have one underdeveloped collar spine on each side between the angular and rest spines. Both species have identical life cycles, in which the molluscs Lymnaeidae play the role of the first intermediate host (Table 2); the second hosts are fish and amphibians, and the final hosts are mammals.
The East Asian region is considered to be the type locality for I. hortensis, while the type locality for I. melis is Europe. In addition to the first place of registration, I. melis was detected in North America and East Asia, including the Russian southern Far East (Beaver, Reference Beaver1941; Oshmarin, Reference Oshmarin1963; Besprozvannykh, Reference Besprozvannykh2001). Data on the life cycle and morphology of developmental stages were obtained for the worms in the Russian southern Far East, which were previously designated Euparyhium melis (Besprozvannykh, Reference Besprozvannykh2001). In our study, worms are identical to those presented in Besprozvannykh (Reference Besprozvannykh2001), both in terms of life cycle and morphology.
Molecular data on 28S, cox1 and nad1 markers also indicate that the obtained trematode belongs to I. hortensis (Figs 3–6, S5).
On the phylogenetic tree using 28S (Fig. 4), worm obtained in this study forms a well-supported clade with I. hortensis (AB189982, Japan) and I. melis (KR092370, KT359583, AF151941, Lithuania, Poland and Ukraine, respectively). The genetic distances between these species from GenBank and the trematode obtained in this study are 0.1 and 0.2–0.3%, respectively. These results point towards the genetic closeness of the trematode from this work and I. hortensis from Asia. Additionally, this clade includes one of the Petasiger phalacrocoracis Yamaguti, Reference Yamaguti1939 samples (KY284000, Hungary). Other samples from the genus Petasiger Dietz, 1909 form the sister clade to Isthmiophora. The species identification of the worms designated as P. phalacrocoracis was carried out based on morphometry of the metacercaria. In this case, there is a high probability of erroneous determination of the species. Thus, based on 28S, the worm KY284000 should be assigned to the genus Isthmiophora.
In phylogenetic reconstructions based on both mitochondrial markers (cox1 and nad1), the trematodes obtained form a common clade with the I. hortensis from GenBank, which is separate from I. melis (Figs. 3, S5, 5). The genetic distance between I. melis and the trematode from this study is 48.6% when using nad1. For this marker, the distance is 1% between the worm obtained and I. hortensis from GenBank. Using cox1, the genetic distances between the obtained trematode and I. melis and I. hortensis from GenBank are 7.6% and 0.5–1%, respectively. These data confirm that the trematode in our material belongs to I. hortensis.
The molecular differences (particularly based on mitochondrial genes) between I. melis (Europe) and I. hortensis (Asia) most likely reflect the long geographical isolation of worms which may have previously belonged to the same species, as evidenced by their similarity at the morphological level. Our results indicate that I. melis is not a cosmopolitan species. This parasite was distributed in the European region, while I. hortensis inhabits East Asia.
Phylogenetic relationships in the Echinostomatidae
In 2016, Tkach et al. (Reference Tkach, Kudlai and Kostadinova2016) revised Echinostomatoidea Looss, 1899 using molecular data. Based on the 28S nucleotide sequences, these authors reconstructed the phylogenetic relationships dividing Echinostomatoidea into eight families. Based on the system of Tkach et al. (Reference Tkach, Kudlai and Kostadinova2016), all of the species studied in our work are Echinostomatidae. For the phylogenetic reconstruction using 28S, representatives from 16 genera of this family were used, which form two groups (groups 1 and 2) (Fig. 4). According to our data, the intergeneric distances for this marker are in the range from 0.9 to 8.7%.
Group 1 includes nine genera with high statistical support of each, which is consistent with the data of Tkach et al. (Reference Tkach, Kudlai and Kostadinova2016). On the tree of Tkach et al. (Reference Tkach, Kudlai and Kostadinova2016), Drepanocephalus auritus Kudlai et al., 2015 formed a well-supported clade with Euparyphium capitaneum Dietz, 1909. On the tree obtained in this study, including the new data for two species of Drepanocephalus Dietz, 1909, members of Drepanocephalus form a cluster with high statistical support, and E. capitaneum (KP009618-KP009620) occupies a basal position with respect to Drepanocephalus. This indicates the validity of the genus Drepanocephalus. At the same time, within the Drepanocephalus cluster, the genetic distances between the three American species are in the range from 0.1 to 0.2%. The presence of low distances for 28S does not clarify the situation with the taxonomic status of these worms. These distances may indicate that the worms either belong to a single species, such as the East Asian individuals of I. hortensis, or different species, for example the European and East Asian E. miyagawai (See remarks to Sections 3.3 and 3.4).
Group 2 combines members of seven genera, in which two subgroups are distinguished. Subgroup 1 has no clear division according taxonomy. This subgroup unites representatives of seven species of Echinoparyphium Dietz, 1909, as well as Euparyphium cf. murinum Tubangui, 1931 and three sequences of Hypoderaeum conoideum (Bloch, 1782). Five species of Echinoparyphium as well as Euparyphium cf. murinum Tubangui, 1931 and sequence KP065607 of Hypoderaeum conoideum are combined into a separate cluster with intrageneric distance varying in the range from 0.2 to 0.3%. The results of our studies are consistent with the conclusion of Tkach et al. (Reference Tkach, Kudlai and Kostadinova2016) that E. cf. murinum belongs to Echinoparyphium. The genetic distances between Echinoparyphium and H. conoideum under accession number KP065607 indicate that the latter is also representative of Echinoparyphium. This sample was mistakenly included in Hypoderaeum Dietz, 1909, which may be related to the identification of taxonomic status of the trematode based on the morphology of cercariae; in such a case, the probability of error is high due to the well-known morphological similarities between of cercariae of closely related genera. These data assume the genus Echinoparyphium unites E. ellisi (Johnston and Simpson, 1944) Johnston and Angel, 1949, E. recurvatum (Linstow, 1873) Dietz, 1909, E. cinctum Rudolphi, 1802, E. poulini Georgieva et al., 2017 and E. rubrum Cort, 1914, as well as Euparyphium cf. murinum and H. conoideum (KP065607).
Two other samples of H. conoideum (KT956918, KT956919) form a sister clade with Echinoparyphium, consistent with data from Kostadinova et al. (Reference Kostadinova, Herniou, Barrett and Littlewood2003). Tkach et al. (Reference Tkach, Kudlai and Kostadinova2016) has suggested that the genera Echinoparyphium and Hypoderaeum are synonymous. However, the genetic distances between H. сonoideum (KT956918 KT956919) and five species of Echinoparyphium vary in the range from 0.8 to 1.1%. These values apparently correspond to intergeneric distances for the Echinostomatidae based on 28S and 2.6- to 5.5-times higher than the distances between species of genus Echinoparyphium. Thus, the currently available morphological and genetic data testify to the independence of the genus Hypoderaeum.
Two other separate branches in Subgroup 1, sister to Echinoparyphium, are formed by Echinoparyphium mordvilkowi Skrjabin, 1915 and Echinoparyphium aconiatum Dietz, 1909, the genetic distance between these branches is 1.7%. The genetic distance between E. aconiatum and E. mordvilkowi vs Hypoderaeum (KT956918, KT956919) are 1.3 and 1.7%, respectively. The distances between E. aconiatum, E. mordvilkowi and other Echinoparyphium are 1.6 and 1.9%, respectively. Thus, the samples designated as E. mordvilkowi and E. aconiatum should be assigned to two separate genera. However, it should be noted that species identification for E. mordvilkowi and E. aconiatum, as in the case of Hypoderaeum (KP065607), was based on the morphology of immature stages. In the present case, the final solution to the problems of taxonomy in the group of these worms will depend on new morphological and genetic data for developmental stages, which will be obtained in accordance with suitable methodology.
ITS2 (Fig. S4) was not used for analysis due to the low level of species separation in Echinostomatidae, which was also previously shown by Morgan and Blair (Reference Morgan and Blair1995), Nugaraite et al. (Reference Nugaraite, Mazeika and Paulauskas2017) and Mohanta et al. (Reference Mohanta, Watanabe, Anisuzzaman and Itagaki2019).
In contrast to the data based on 28S, phylogenetic analysis for the whole family Echinostomatidae was carried out for the first time using the mitochondrial marker nad1. Reconstruction based on this marker, as in the case with nuclear marker 28S, supports the subdivision of Echinostomatidae representatives into two groups (groups 1 and 2) (Figs 5, 6, S5). The distances between the analysed genera are in the range from 27.5 to 46%.

Fig. 5. Part of the phylogenetic reconstruction (Group 1) based on nad1 sequences using the Bayesian algorithm.

Fig. 6. Part of the phylogenetic reconstruction (Group 2) based on nad1 sequences using the Bayesian algorithm.
Group 1 includes the genera Isthmiophora, Drepanocephalus and Neopetasiger Bashkirova, 1941. The genus Neopetasiger [namely Neopetasiger islandicus (Kostadinova and Skirnisson, 2007)] joins samples JQ425587-JQ425590 and KT831342, forming Subcluster 1 with a high statistical support, as well as in the reconstruction based on 28S. Sample JQ425591, designated by Georgieva et al. (Reference Georgieva, Kostadinova and Skirnisson2012) as Neopetasiger neocomense Fuhrmann, 1927, is in a basal position, but this publication unfortunately does not contain data on the morphology and biology for this trematode, making it difficult to assess the species and generic affiliation for this worm. Nevertheless, the value of genetic distances between N. neocomense and other species from Group 1 is at the generic level (37.2–42.4%). To establish the taxonomic status of this trematode, a complex of data on morphological and genetic characterizations are necessary, including nad1 sequences for the genera Euparyphium, Petasiger, Ribeiroia Travassos, 1939, Cathaemasia Looss, 1899 and Rhopalias (Rudolphi, 1819), representatives of which are members of Group 1 in the tree based on 28S.
The genus Drepanocephalus on the tree is represented by three species, similar to the reconstruction based on 28S, and forms a separate cluster (Cluster 2) with four Subclusters, 2, 3, 4 and 5. Subcluster 2 combines only samples of Drepanocephalus mexicanus Lamothey Perez-Ponce de Leon, 1988, the validity of which is confirmed by distances of 16.7 to 17.8% between this species and members of Subclusters 3, 4 and 5. As for the remaining subclusters, the distances between them are at the interspecies level (2.4–4%). In so doing, Subcluster 5 included only Drepanocephalus spathans Dietz, 1909, while two others (3 and 4) combine the worms designated as D. spathans and D. auritus. There are no fixed nucleotide substitutions between any of these worms. There is a molecular basis to consider D. spathans and D. auritus as the same species according to the analysis of two markers (28S and nad1). These findings are supported by the study of Hernandez-Cruz et al. (Reference Hernandez-Cruz, Hernandez-Orts, Sereno-Uribe, Perez-Ponce de Leon and Garcia-Varela2017) who, based on the absence of morphological differences between D. spathans and D. auritus, as well as their molecular similarity based on nuclear and mitochondrial markers, proposed the consideration of D. auritus as a synonym of D. spathans. However, it is not excluded that the assumption about the validity of these species will be confirmed if new data are obtained, namely, the morphology of developmental stages, as well as the sequences of the cox1 gene.
Group 2 includes the following genera: Artyfechinostomum (Lane, 1915), Euparyphium, Echinoparyphium, Hypoderaeum and Echinostoma, which are subdivided into three Clusters: 3, 4 and 5. The taxonomic status of Artyfechinostomum is clear. Members of the genus (JF412731-JF412732, JF412734) form a separate cluster on the tree (Cluster 3). Cluster 4 included echinostomatids, designated as Euparyphium, Echinoparyphium and Hypoderaeum. Euparyphium albuferensis Esteban et al., Reference Esteban, Toledo, Sanchez and Munoz-Antol1997 is part of a common branch with members of Echinoparyphium. The authors described this trematode (Esteban et al., Reference Esteban, Toledo, Sanchez and Munoz-Antol1997) by indicating the morphological similarity of the worm with Echinoparyphium and Euparyphium, but attributed it to the latter genus based on the morphological closeness with E. cf. murinum. Later, as in the present study, data were obtained which confirmed that E. cf. murinum is a member of Echinoparyphium as also shown by the Bayesian and ML 28S trees (Kostadinova et al., Reference Kostadinova, Herniou, Barrett and Littlewood2003; Tkach et al., Reference Tkach, Kudlai and Kostadinova2016). Taking this into account, as well as data on nad1 showing that the distances between the samples included in Echinoparyphium are the same as those between Echinoparyphium and Euparyphium albuferensis (4–5%), the latter should be included in the genus Echinoparyphium. Echinoparyphium aconiatum, as in the previous reconstruction based on 28S, forms a sister clade to other Echinoparyphium representatives, and the genetic distance between them is 27.9%. Hypoderaeum, also similar to data on 28S, occupies a basal position in relation to Echinoparyphium spp. and E. aconiatum, with distances in the range from 29.8 to 27.5%. On the one hand, these data confirm the validity of Hypoderaeum, while, on the other hand, they point to an erroneous assignment of samples designated as E. aconiatum to Echinoparyphium.
Cluster 5 united members of the genus Echinostoma, represented by 12 species in our current work. The type species of this genus is E. revolutum (Fröhlich, 1802) Rudolphi, 1809, for which a large amount of molecular data has been accumulated. On phylogenetic reconstruction, this species is divided into two subclusters, 11 and 12. These subclusters combine Eurasian and North American representatives, respectively, which is consistent with the data of Georgieva et al. (Reference Georgieva, Faltynkova, Brown, Blasco-Costa, Soldanova, Sitko, Scholz and Kostadinova2014). The distance between these subclusters (5.5%) is comparable to the distances between the samples of D. spathans and D. auritus (2.4–4%), which, in our opinion, belong to the same species. At the same time, the samples of E. revolutum from North America and Eurasia have five fixed nucleotide substitutions between each other, which may indicate differentiation between the populations from geographically isolated regions.
On the phylogenetic reconstruction, Echinostoma cf. friedi Toledo et al., 2000 (AY168937) occupies a basal position relative to other Echinostoma. Unfortunately, molecular data for this sample were obtained at the cercariae stage (Kostadinova et al., Reference Kostadinova, Herniou, Barrett and Littlewood2003), which does not make it possible to objectively determine not only the species status, but also the genus. The position of AY168937 regarding Echinostoma points to the need for assigning this sample to a separate genus.
Echinostoma bolschewense and E. chankensis nom. nov., as shown in Section 3.1, have low genetic distances between themselves, according to the nuclear marker (28S), but according to the mitochondrial marker (nad1), the distances between them are at the interspecies level (13.3%). With regard to Echinostoma caproni Richard, 1964, almost all samples designated as this species form a common Subcluster 10, with a genetic distance within it of 1.4%. At the same time, the sample of E. caproni AF025836 occupies a basal position to this subcluster, with a distance of 24.3% between AF025836 and other samples of E. caproni. Molecular data for E. caproni under accession numbers AF025836 and AF025837 were obtained by Morgan and Blair (Reference Morgan and Blair1998a) for adult individuals with no morphology description; the latter, in contrast to the former, clusters with other E. caproni samples. The species determination of sample AF025836 as E. caproni is probably erroneous.
Samples of Echinostoma trivolvis Cort, 1914 are divided into two subclusters, 7 and 8, with high statistical support. The genetic distance between these subclusters (9.9%) is higher than the distances between populations of E. revolutum from different continents (5.5%), and in the range of distances between different species of Echinostoma. The number of fixed nucleotide substitutions between sequences in both subclusters is also high (n = 16), and two of them lead to amino acid substitutions. Molecular data for E. trivolvis were obtained for worms collected from naturally infected animals in North America, which means either existence of two E. trivolvis more or less isolated populations or the presence of two morphologically similar species in this region. The probability of the existence of several morphologically similar species, designated as E. trivolvis, was also indicated by Detwiler et al. (Reference Detwiler, Bos and Minchella2010) and Detwiler et al. (Reference Detwiler, Zajac, Minchella and Belden2012). To solve this problem, further molecular and morphological studies of worms united under the name E. trivolvis are necessary.
Subcluster 9 included Echinostoma paraensi Lie et Basch, 1967, which occupies a basal position in relation to E. trivolvis. This subcluster has high statistical support; the genetic distance between E. paraensi and E. trivolvis is 17.1%. Thus, the systematic position of E. paraensi is clear, as well as the systematic position of Echinostoma novaezealandense Georgieva et al., 2017, which forms a separate well-supported Subcluster 13 on reconstruction. Genetic distances between E. novaezealandense and species from neighbouring subclusters with E. revolutum and E. miyagawai are in the range from 10 to 12.2%, which confirms the validity of E. novaezealandense.
With regard to E. cinetorchis and E. paraulum (Subcluster 14), as shown above (Section 3.2), despite low distances between them on the basis of 28S, there is an interspecies level of difference based on the mitochondrial marker (nad1).
Among the members of Echinostomatidae, one of the most complex subclusters regarding taxonomy and systematics is Subcluster 15, uniting samples of E. miyagawai-likely. This group includes worms found in East Asian, South Asian, European and Australian regions and was originally set out as E. miyagawai, E. revolutum, Echinostoma robustum Yamaguti, 1935 and Echinostoma friedi Toledo et al., 2000. Subsequently, the worms of E. revolutum (AF025832, AF026286-AF026288) and E. friedi (AJ564379) were recognized as synonyms of E. miyagawai (Faltynkova et al., Reference Faltynkova, Georgieva, Soldanova and Kostadinova2015) based on the morphology of adult individuals. With regard to E. robustum from Bangladesh (LC224086-LC224096), E. revolutum from Germany (AF025832, AF026286-AF026288) and E. friedi from Spain (AJ564379), these species clustered with samples assigned as E. miyagawai (Subcluster 15) on the phylogenetic reconstruction. The genetic distances within this group of worms are at the intraspecific level (0.3–1.7%). When considering this group of worms based only on the results of genetic studies, including data of nuclear (28S) and mitochondrial (nad1) markers, all individuals represented in the tree should be classified as one species, E. miyagawai. However, our data for East Asian E. miyagawai point to a number of significant differences in morphometry of cercariae and the timing of reaching maturity in comparison with European worms designated as E. miyagawai. Based on this, it is possible that the worms included in E. miyagawai-likely in Subcluster 15 may belong to different species, despite having no differences in the considered markers. To resolve the issue, comprehensive research is needed, including data on the morphology of developmental stages, the composition of the hosts and molecular markers for worms in all regions where they were found.
Subclusters 16 and 17 include representatives of E. robustum (GQ463053-GQ463055) with obtained genetic data was found in North America. The type locality E. robustum is Japan, but, unfortunately, molecular data are not available for samples from this region. The distances between E. miyagawai-likely and E. robustum GQ463053 are in the range from 4.9 to 6.7%, while the distances between E. miyagawai-likely and E. robustum GQ463054-GQ463055 range from 8.4 to 11.1%. Whether these worms (GQ463053-GQ463055) belong specifically to E. robustum or to some another species can be defined based on additional data for individuals of this species from the type locality.
It is also important to note that the genetic distances between Groups 1 and 2, based on nad1 and 28S, are 63.4% and 6%, respectively. For the nad1, this distance is similar to values for distances between Groups 1 and 2 and species of outgroup (Fasciola hepatica), which are equal to 50 and 70%, respectively. Based on 28S, genetic distances between Groups 1 and 2 are 2–3 times higher than the distances within Groups and comparable to the distances between them and Fasciola hepatica (6.8 and 7.9%, respectively). Thus, we assume that the above may indicate the family level of differences between representatives of Groups 1 and 2, which are currently combined in Echinostomatidae.
Conclusion
The family Echinostomatidae comprises a large number of genera. In this family, different species from the same genus or classified as different genera are often morphologically similar, and their precise species identification is impossible without a comprehensive analysis, including data on the morphology of different developmental stages and life cycles as well as molecular data. This has also been shown by analysing the systematic position of the detected trematodes, the species identification of which would be difficult or erroneous using only morphological or genetic data. Our studies, as well as previously published results (Morgan and Blair, Reference Morgan and Blair1998b; Georgieva et al., Reference Georgieva, Faltynkova, Brown, Blasco-Costa, Soldanova, Sitko, Scholz and Kostadinova2014), indicate that for members of Echinostomatidae, nuclear markers 28S and ITS2 separately from mitochondrial markers are ineffective both at the level of interspecific and intergeneric differentiations. At the same time, based on the results of the current study, both 28S and ITS2 are acceptable markers at a higher systematic level for this group of trematodes. It is necessary to use the more informative nad1 gene in the separation of related species of echinostomatids. However, even this marker may be ineffective in resolving the species affiliation in some cases. The nucleotide sequences of this gene accumulate mutations at a non-uniform rate, which, in our opinion, may cause the inability to resolve contentious issues at the species level in some cases. As for cox1 mtDNA, its role in the taxonomy and systematics of Echinostomatidae is currently difficult to estimate due to the limited number of cox1 sequences in GenBank. At the same time, the data already available for Echinostoma confirm a more uniform accumulation of mutations for this marker in comparison with nad1. Genetic distances between species of the genus Echinostoma for nad1 vary in a wide range from 4.9 to 40.3%, while the distances between species of Echinostoma for cox1 range from 7.3 to 16.8%. The latter also separated two species of Isthmiophora. We therefore conclude that this marker is more promising for solving taxonomic problems in the structure of Echinostomatidae.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182021000950.
Data
DNA sequences: GenBank accessions MT577825-MT577836, MT577585-MT577590, MT592852-MT592857.
Financial support
This research was funded by the Government basic research program (project no. 0267-2019-0018).
Conflicts of interest
The authors declare no conflict of interest.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals including birds and mammals.