Extended-spectrum β-lactamases (ESBLs) enable bacteria to hydrolyze penicillins, cephalosporins, and monobactams,Reference Paterson and Bonomo1, Reference Bradford2 thus conferring resistance to antimicrobials commonly used for empirical treatment. Because inadequate empirical antibiotic treatment increases the risk of mortality in patients with sepsis, the emergence of ESBL-producing Enterobacteriaceae (ESBL-PE) fosters incremental carbapenem consumption,Reference Paterson and Bonomo1 paving the way for carbapenem-resistant bacteria,Reference Candevir Ulu, Kurtaran and Inal3 for which hardly any treatment options remain.
The rapid emergence of ESBL-PE poses a particular challenge regarding the management of intensive care unit (ICU) patients, who are at high risk of developing infections requiring timely and adequate treatment.Reference Chelazzi, Pettini, Villa and De Gaudio4–Reference Richards, Edwards, Culver and Gaynes6 Unnecessary exposure to carbapenems in this population, however, may result in increased resistance rates on both individual and institutional levels and may negatively influence individual outcomes.Reference Johnson, Reichley, Hoppe-Bauer, Dunne, Micek and Kollef7–Reference Kritsotakis, Tsioutis, Roumbelaki, Christidou and Gikas9
Colonization with ESBL-PE has been identified as an important risk factor for invasive infections with ESBL-PE,Reference Biehl, Schmidt-Hieber, Liss, Cornely and Vehreschild10–Reference Vehreschild, Hamprecht and Peterson12 and different studies have shown that ICU patients have a particularly high risk of ESBL-PE colonization.Reference Ebrahimi, Mózes and Monostori13, Reference Bassetti, De Waele and Eggimann14
To inform recommendations on empirical use of carbapenems in the ICU setting, strategies for identifying patients at risk for ESBL-PE are needed. Thus, screening ICU Patients for ESBL-PE carriage on admission is a logical strategy. We sought to determine whether rectal colonization with ESBL-PE on ICU admission predicts the risk for ESBL-PE infection, impacts outcome, and/or has an effect on subsequent carbapenem prescription in an ICU cohort.
Methods
Setting and study design
This study was performed at the University Hospital Basel, a tertiary-care center in Switzerland with 855 beds and 35,000 admissions per year, including ∼5,300 admissions to the surgical and medical ICUs.
From November 2014 to August 2015, all patients admitted to the surgical and medical ICUs needing mechanical ventilation and expected to require intensive care for >48 hours were routinely screened on admission for ESBL-PE carriage by a single rectal swab. Routine screening of this patient population is outlined in our institutional guidelines and is conducted for surveillance purposes. Automated alerts were set in the computer-based monitoring and prescribing software (METAVISION by iMDSoft, Tel Aviv, Israel) and remained active until swabs were marked as performed by the ICU nurses. Screening results were available to the treating physicians through the laboratory information system.
This study was approved by the local ethics committee (Ethikkommission Nordwest- und Zentralschweiz, EKNZ BASEC 2016-00704), and informed consent was waived.
Microbiological analyses
Rectal swabs were collected within the first 2 days of ICU admission. Standard culture methods using chromogenic screening agar plates (chromID ESBL, bioMérieux, Marcy-l`Etoile, France) were applied for detection of ESBL-PE. Species identification was conducted with matrix-assisted laser desorption/ionization time-of-flight mass spectroscopy (MALDI-TOF MS; Bruker Daltonics, Bremen, Germany). Susceptibility testing was performed using the Vitek 2 system (bioMérieux, Durham, NC). ESBL-PE was confirmed using Etest strips (bioMérieux, Marcy-l`Etoile, France) containing cefotaxime, ceftazidime, and cefepime, each tested with and without clavulanic acid. Unclear results were further evaluated using the Easyplex Superbug CPE panel including CTX-M1 and CTX-M9 genes. If these genes were absent, the result was considered negative, based on our local epidemiology.Reference Hinic, Ziegler, Straub, Goldenberger and Frei15
Clinical data
Pertinent data (baseline characteristics and clinical features) were collected retrospectively by reviewing the electronic patient records. Immunosuppression was defined as high-dose corticosteroids (prednisone equivalent of ≥1 mg/kg body weight in the previous 7 days) and all types of agents to prevent graft rejection in solid organ or human stem cell or bone marrow transplantation and to treat autoimmune diseases used in the last 3 months. Other data collected included (1) known colonization with multidrug-resistant bacteria (ie, methicillin-resistant Staphylococcus aureus [MRSA], ESBLs, vancomycin-resistant Enterococci, other multidrug-resistant [MDR] gram-negative bacteria as defined elsewhereReference Magiorakos, Srinivasan and Carey16), (2) antibiotic therapy since hospital admission prior to receipt of the screening result, (3) first course of empiric antibiotic therapy after receipt of the screening result, and (4) carbapenem exposure (days) during ICU stay. We also collected data regarding (5) causative pathogen and infection focus of the first empirically treated infection after receipt of the screening result, (6) presence of septic shock (defined as an increase in serum lactate level and new or increased need for vasopressors) at initiation of a first empiric treatment regimen after receipt of the screening result, and (7) death during hospital stay (ie, overall, attributable to any infection, or attributable to infection caused by ESBL-PE). Infections were defined according to the National Healthcare Safety Network Patient Safety Component Manual by the Centers for Disease Control and Prevention.17
Outcome measures
The primary outcomes were colonization and infection with ESBL-PE. Occurrence of any infection during ICU stay, exposure to carbapenems, length of ICU and hospital stay in survivors, as well as death (ie, overall or attributable to infection) were considered as secondary outcomes.
Statistics
Patients were categorized as colonized and not colonized with ESBL-PE. Comparisons were undertaken to identify risk factors for ESBL-PE colonization. Continuous variables were summarized as medians and interquartile ranges and were compared using the Mann-Whitney U test. Categorical variables were summarized as counts, and proportions and differences were assessed using χ2 tests or the Fisher exact test when appropriate. Hazard ratios (HRs) were calculated to assess the risk of any infection, infection with ESBL-PE, exposure to carbapenems, and death. Fine and Gray competing risk regression analyses were used to account for the competing risks of development of infection, as well as receipt of carbapenems and death during hospital stay, where appropriate.Reference Fine and Gray18
Time to development of ESBL-PE infection and to receipt of carbapenems in association with ESBL-PE colonization were described using a cumulative incidence function plot.Reference Coviello and Boggess19 Two-sided P values <.05 were considered significant. Analyses were performed using STATA version 14.0 statistical software (StataCorp, College Station, TX).
Results
Overall, 302 patients were screened for ESBL-PE carriage and were included in the study. Screening was performed within 2 days of ICU admission (median, 1 day; interquartile range [IQR], 1–2 days). Table 1 summarizes their baseline characteristics. Of the included 302 patients, 24 (8.0%) were colonized with ESBL-PE (22 with ESBL Escherichia coli, 1 with ESBL Klebsiella pneumoniae, and 1 with ESBL Providentia stuartii) on ICU admission. The only factor associated with ESBL colonization was known colonization with multidrug-resistant gram-negative pathogens (Table 2).
Table 1. Baseline Characteristics and Outcomes of 302 Patients Screened for ESBL-PE Carriage

Note. IQR, interquartile range; COPD, chronic obstructive pulmonary disease; SAPS II, simplified acute physiology score; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant Enterococci; ESBL, extended-spectrum β-lactamase; ESBL-PE, ESBL-producing Enterobacteriaceae.
Table 2. Comparisons Between Baseline Characteristics, Exposures and Outcomes Between Patients Colonized and Not Colonized With ESBL-PE

Note. ESBL-PE, ESBL-producing Enterobacteriaceae; IQR, interquartile range; SAPS II, simplified acute physiology score; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant Enterococci; ESBL, extended-spectrum β-lactamase; NA, not applicable.
In total, 120 patients (39.4%) were diagnosed with an infection after the availability of the ESBL-PE screening; among them, 10 (3.3%) developed septic shock. In 66 of these 120 patients (55%), infection was related to a pulmonary source. Patients with ESBL-PE colonization were not at increased risk for developing any infection (HR, 1.21; 95% CI, 0.64–2.28; P = .567) or septic shock (0% vs 3.6%; P = 1.000). Infections with ESBL-PE occurred in 4 patients, of whom 3 (75%) had been identified as ESBL-PE-carriers on admission. ESBL-PE colonization on admission was associated with subsequent ESBL-PE infection (HR, 25.52; 95% CI, 2.40–271.41; P = .007) (Fig. 1).
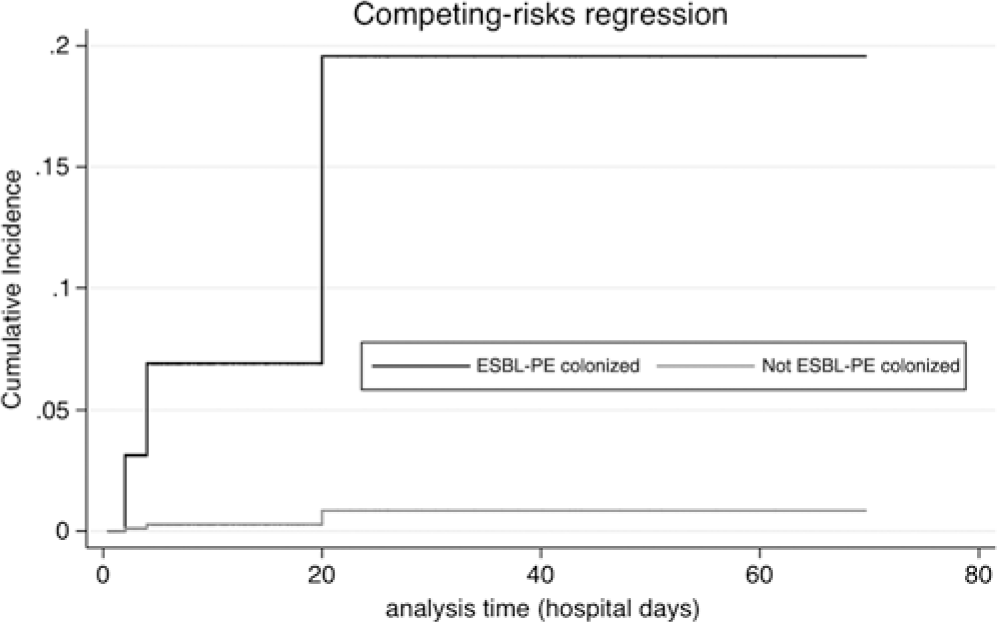
Fig. 1. Risk of infection with ESBL-producing Enterobacteriaceae (ESBL-PE) according to colonization with ESBL-PE on admission (hazard ratio, 25.52; 95% confidence interval, 2.40–271.41; P = .007).
In 120 patients (39.9%), empiric antibiotic treatment was started after the screening result was obtained. In 39 patients (32.5% of those receiving an empiric treatment) empiric therapy included a carbapenem. Colonization with ESBL-PE was associated with increased exposure to carbapenems (HR, 2.42; 95% CI, 1.01–5.79; P = .047) (Fig. 2), whereas the duration of carbapenem exposure (median 7, days [IQR, 3–8 days] vs median, 6 days [IQR, 3–9 days]; P = .983) did not differ between patients colonized and not colonized with ESBL-PE.

Fig. 2. Risk of carbapenem exposure according to colonization with ESBL-producing Enterobacteriaceae on admission (hazard ratio, 2.42; 95% confidence interval, 1.01–5.79; P = .047).
Duration of ICU and hospital stay in survivors did not differ between patients colonized with ESBL-PE (median, 8 days [IQR, 2–16] vs median, 6 days [IQR, 4–12]; P = .849) and not colonized with ESBL-PE (median, 20 days [IQR, 9–32] vs median, 20 days [IQR, 11–34]; P = .537). Patients colonized with ESBL-PE were not at increased risk for death by any cause (HR, 1.00; 95% CI, 0.44–2.30; P = .993) or death attributable to infection (HR, 1.20; 95% CI, 0.28–5.11; P = .808) (Table 2). Among the 4 patients with ESBL-PE infection, 2 patients died.
Discussion
Colonization with ESBL-PE was a reliable predictor for infection with ESBL-PE. Colonization with ESBL-PE was not associated with comorbid conditions, disease severity or prior exposure to antibiotics, but it was associated with an increased exposure to carbapenems after ICU admission. In contrast to prior studies, ESBL-PE colonization was not associated with increased length of ICU stay, length of hospital stay, or mortality.
Incidence of ESBL-PE colonization was 8% in our cohort. This finding is in line with the findings of a recent Swiss study reporting a prevalence of ESBL-PE colonization of 7.1% in HIV-positive outpatients and 10% in healthy individuals.Reference Pires, Kuenzli and Hauser20 Recent studies on colonization in ICU patients show rates varying between 2%Reference Harris, McGregor and Johnson21 and up to 56%,Reference Mulki, Ramamurthy and Bhat22 and this large range can be explained by different settings and countries, such as the United States and India, as well as different time frames (2001–2005 and 2016). In our cohort, as in most published studies on ESBL-PE colonization,Reference Mulki, Ramamurthy and Bhat22–Reference Repesse, Artiguenave and Paktoris-Papine24 22 of 24 ESBL-PE isolates were E. coli.
The only factor associated with ESBL-PE colonization in our study is known colonization with multidrug-resistant bacteria, including ESBL-PE. However, the most common previous colonizing multidrug-resistant bacteria were ESBL-PE. Previous colonization with multidrug-resistant bacteria might therefore not be an independent risk factor for a positive screening result but actually represents the same biologic event.
In our study, colonization with ESBL-PE was not a risk factor for development of any infection, septic shock, or death. In contrast to our findings, a number of studies show that infection and even colonization with ESBL-PE are associated with worse outcomes than infections with non–ESBL-PE.Reference Osthoff, McGuinness, Wagen and Eisen25–Reference Barbier, Pommier and Essaied28 A German study, however, did not find this association in ESBL-PE bloodstream infections.Reference Leistner, Bloch, Sakellariou, Gastmeier and Schwab29 A recent French study showed an increase in ICU length of stay in ESBL-PE–colonized patients but only a very small difference in crude mortality rates.Reference Barbier, Pommier and Essaied28 Although our study may be underpowered to show the latter, the former might be explained by the fact that we did not apply contact precautions in E. coli ESBL-PE–colonized patients. In the same study, the negative effect on outcomes of ESBL-PE infection was mostly associated with non–E. coli ESBL-PE. The small number of ESBL-PE other than E. coli in our cohort may therefore have lessened the effect. In addition, this French study analyzed from a period (1996–2013) when clinicians had less experience with ESBL-PE–colonized and –infected patients than they had during our study period (2014–2015). Most studies that found an association with negative outcomes also showed that the risk for receiving an ineffective empiric antibiotic treatment was higher for patients with ESBL-PE infections.Reference Tumbarello, Spanu and Di Bidino30 We speculate that the high percentage of patients empirically treated by carbapenems may have influenced the outcome.
Additionally, recent studies have indicated that β-lactam/β-lactamase inhibitor combination antibiotics might have a certain effectiveness in the treatment of ESBL infection.Reference Harris, Yin and Jureen31, Reference Ng, Khong and Harris32 As in our cohort, β-lactam/β-lactamase inhibitor therapy was the most frequent choice of empirical therapy, which may have further influenced the outcome.
In this study, of 4 patients that developed a documented ESBL-PE infection, 3 had a positive screening result, making ESBL-PE colonization the strongest risk factor for ESBL-PE infection. This finding is consistent with several studies that also identified ESBL-PE colonization as risk factor for ESPL-PE infection.Reference Harris, McGregor and Johnson21, Reference Barbier, Pommier and Essaied28, Reference Liss, Vehreschild and Cornely33–Reference Cornejo-Juarez, Suarez-Cuenca and Volkow-Fernandez35
Carbapenem exposure after availability of the screening result differed between patients with a positive screening result and patients with a negative screening result, which is supported by a recent French study that showed much greater carbapenem exposure for ESBL-PE–colonized patients.Reference Barbier, Pommier and Essaied28 This difference occurred despite screening not being accompanied by an antimicrobial stewardship intervention; however, the results were available to the treating physicians through the laboratory system and seem to have influenced their prescription strategies. Carbapenem exposure differed between patients colonized and not colonized with ESBL-PE, as determined by competing risk regression analyses but not by simple comparison of proportions. This finding points to the impacts of time to rather than simply presence or absence of carbapenem exposure, as well as death (representing a competing risk). The high number of patients in our study with exposure to in-hospital antibiotics prior to the ESBL screening made the choice of a carbapenem as an empiric therapy more likely as an escalation strategy in the case of suspected new-onset infection.
Our study has several limitations. The data were collected retrospectively and thus were subjected to documentation bias. The absolute number of patients in general, the number of patients with ESBL-PE colonization and even more ESBL-PE infection was low. This limited the study’s power for further multivariable analyses, but it reflects the situation in a low endemicity setting. This study was conducted at a single center; thus, our findings might not be generalizable to other settings and may have been influenced by the decisions of a few individual physicians. Screening was performed only once at ICU admission; thus, colonization by acquisition or selection of ESBL strains during an ICU stay may have been missed. Furthermore, we cannot rule out the possibility that initial colonization may have been missed because only 1 rectal swab was performed, an approach which has previously been shown to have a sensitivity ranging from 77% to 86%.Reference Grohs, Podglajen and Guerot36, Reference van Prehn, Kaiser, van der Werff, van Mansfeld and Vandenbroucke-Grauls37 Because only rectal carriage of ESBL-PE was screened for, colonization of other body sites might have been missed. Clinical staff performed the rectal swabs, and the manner in which the samples were collected may have differed, which might have influenced sample adequacy. In addition, a relevant number of patients were under an ESBL-PE active antibiotic therapy at the time of screening (ie, 20.9% of all patients received carbapenem treatment prior to ICU admission), which might have led to false-negative results. Our study design did not allow us to draw any conclusions regarding the number needed to screen: All patients were screened in our cohort, and carbapenem use after introduction of screening was not compared to the use prior to this intervention.
The strength of our study is the systematic inclusion of all consecutive patients in a relatively short time, which limited the effect of increasing ESBL-PE prevalence over time. In comparison to different studies, including the French study,Reference Barbier, Pommier and Essaied28 we distinguished between the antibiotic use before and after the availability of the screening result. Therefore, we were able to estimate the impact of the actual screening procedure.
Our study shows that screening for ESBL-PE colonization on ICU admission can help to identify patients at risk for ESBL-PE infection and may therefore facilitate the correct allocation of empiric carbapenem treatment.
Author ORCIDs
Aurélien Emmanuel Martinez, 0000-0001-9709-0644
Financial support
The study was conducted using internal funds of the University Hospital Basel.
Conflict of interest
All authors report no conflicts of interest relevant to this article.






