Hemophilia A is an infrequent inherited disorder of blood coagulation, characterized by a permanent tendency to hemorrhage. The disease is more frequent in men, affecting one in every 5,000–10,000 male births. Classic hemophilia (also known as type A) results from a deficiency of clotting factor VIII (FVIII) as an X-linked inherited disorder (Reference Haemophilia1;Reference White, Rosendaal and Aledort2). Patients usually remain asymptomatic until the hemostatic system is stressed by surgery or trauma. However, severe hemophilic patients can also bleed spontaneously. Bleeding into the joints (such as knees, ankles, and elbows) leads to articular damage (hemophilic arthropathy) which is the most frequent long-term complication of the disease.
The mainstay of treatment is replacement therapy with exogenous clotting FVIII. Nevertheless, the chronic exposure to exogenous clotting factor can lead in some circumstances to the onset of antibodies (inhibitors) against the therapy, this is a serious and frequent complication of treatment. People with hemophilia A are categorized depending on their endogenous level of FVIII activity (LoA) as being either mild (LoA from 5 to 40 percent [0.05–0.4 IU/ml]), moderate (LoA from 1 to 5 percent [0.01–0.05 IU/ml]) or severe (LoA less than 1 percent [<0.01 IU/ml]) (Reference White, Rosendaal and Aledort2). For people with severe hemophilia A (SHA) bleeding (spontaneous or traumatic) can be life-threating.
In Colombia, hemophilia A care represents an important financial burden to the health system: In 2010, the costs associated with clotting factor replacement for this condition were 140,164,400,000 Colombian pesos (COP) (73.72 million USD). According to estimations from the Colombian League for Hemophilia (Reference Robledo3), there are between 2,000 and 2,600 hemophiliac patients in Colombia, of whom 800–1,040 are classified as severe cases. Healthcare coverage for this disease has attracted much media and political attention in recent times, however, not even the upcoming new health sector reform (HSR) has solved the issue of sustainable funding for orphan conditions such as SHA (Reference Castro, Briceño, Casas and Rueda4).
There are two main types of FVIII treatment for people with SHA. On-demand (OD), following a bleed, to stop it, or prophylaxis, when treatment is given before bleeding with the aim of preventing joint damage (Reference Haemophilia1;Reference Castro, Briceño, Casas and Rueda4). “Primary prophylaxis” (PP) represents treatment before the onset of repetitive bleeding. Although there is an approach so-called “Secondary prophylaxis” where treatment is initiated after the onset of joint damage, within this study only OD treatment after bleeding (without subsequent prophylaxis) was considered as an alternative.
Although the prophylactic replacement of clotting factor has been recommended as the gold standard of care by institutions such as the World Federation of Hemophilia (WFH) and the WHO (Reference Giangrande5), in Colombia, as in many other countries, PP is provided to less than 50 percent of all severe hemophilic patients (Reference Robledo3), largely because it is considered to be much more expensive than OD treatment. In Colombia, at the time of performing this study, there were no evidence-based clinical practice guidelines for SHA in place and hemophilic arthropathy with its subsequent negative impact on health-related quality of life (HR-QoL) was still a frequent finding (Reference Robledo3).
Knowledge about the clinical effectiveness of hemophilia A treatment has exponentially grown in recent decades (Reference Castro, Briceño, Casas and Rueda4). For example, several observational studies have shown that clinically observable signs of hemophilic arthropathy can be almost entirely prevented over prolonged periods (20 years) if exogenous replacement factor is provided early and frequently (Reference Nilsson, Berntorp, Löfqvist and Pettersson6–Reference Berntorp9). In contrast with this increasing stock of knowledge on the clinical effectiveness of prophylaxis, published economic evaluations of hemophilia care remain very limited and their results are inconclusive. A recent review of the literature by Miners (Reference Miners10) found that while several economic evaluations of prophylaxis with FVIII have been undertaken, their results vary enormously from it being “dominant” to costing over €1 million per quality-adjusted life-year (QALY) gained, reflecting the different perspectives, structural assumptions, time horizons, discount rates and sources of data used by previous studies. A salient finding from this review was that “all the reviewed studies contained methodological weaknesses, but some were considered weaker than others” and nearly all costs of treatment were attributable to clotting factor provision.
It is still unclear if PP has reached the point of being considered “good value for money” or even if it is “worth-doing” at all (Reference Geraghty, Dunkley and Harrington11;Reference De Moerloose, Urbancik, Van Den Berg and Richards12). Therefore, the purpose of this article was to estimate the cost-effectiveness of PP compared with OD treatment for patients with severe hemophilia A from the Colombian health system perspective.
METHODS
A cost-utility analysis was performed comparing PP versus OD treatment for people with SHA, from the perspective of the Colombian health system (this means that only direct healthcare costs from the public payer's perspective and benefits perceived by patients were considered). This perspective was adopted because up to 95 percent of costs of long-term treatment for SHA may be associated with exogenous FVIII provision (Reference Daliri, Haghparast and Mamikhani13;Reference Miners14) and these costs are currently reimbursed by the Colombian health system (social health insurance).
To estimate the input parameters to populate the economic model (see Table 1), we performed a literature search to update effectiveness and cost-effectiveness information retrieved through a preliminary search conducted by Castro et al. in 2012 (Reference Castro, Briceño, Casas and Rueda4). In this search, we included publications considering the clinical effectiveness of different treatment options for hemophilia A. Search terms included hemophilia A, coagulation disorder, clotting factor disorder, and clotting factor deficiency. The following databases were searched: Medline, Embase, health economics and health technology assessment database, Ovid, ACP Journal Club, Cochrane Controlled Trials Register, The Cochrane Database of Systematic Reviews and Econlit (Reference Castro, Briceño, Casas and Rueda4).
Table 1. Input Parameters Used for the Economic Model

Alternatives Evaluated
The analysis compared the use of PP with OD treatment (both with plasma-derived FVIII) as current practice in Colombia. The cost of exogenous factor replacement using recombinant FVIII (rFVIII) was expected to be higher than for plasma-derived products, and because its use has steadily increased in recent years, it was considered as an alternative in a scenario analysis. For the purposes of this evaluation, PP is defined as treatment by intravenous injection of exogenous clotting factor to prevent bleeding in the first instance. It is assumed to begin in infancy and continue into adulthood. On the other hand, OD treatment is defined as FVIII administration whenever joint or extra-articular bleeding occurs.
Model Design
To estimate expected costs and outcomes of each strategy, a de novo Markov model was designed and populated using the best available evidence (Reference Lalezari, Coppola and Lin15–Reference Gringeri, Lundin and von Mackensen34). The model considered three health states: “Alive-no arthropathy” (which is the initial health state), “Alive-with arthropathy,” and “Dead.” The health state “Alive-no arthropathy” represents those patients with SHA that have not yet developed joint damage. “Alive-with arthropathy” represents those patients with SHA that have already developed joint damage.
Patients with SHA entered the model at 2 years of age, taking into consideration the international recommendation deemed as gold standard (Reference Nilsson, Berntorp, Löfqvist and Pettersson6;Reference Manco-Johnson, Abshire and Shapiro21;Reference Petrini22). Doses of FVIII per-year were adjusted to weight dependence as is normal when treating with exogenous clotting factor. The base case model ran for seventy yearly cycles accounting for the life expectancy of non–HIV-positive SHA patients, which is expected to be 15 years less than the general population (Reference Darby, Kan and Spooner20). Once treatment was allocated, patients fully complied with it and would not switch during their lifetime; clinical effectiveness and safety from treatment with either FVIII or rFVIII were expected to be similar (Reference Mingot, Heiniger, García and Fernández23;Reference Gouw, van der Bom and Ljung24).
Although the development of inhibitors is expected to be substantial and, according to some authors may reach up to 30 percent of cases (Reference Farrugia, Cassar and Kimber25), this complication was not considered in the model.
There is still controversy in the current published evidence about the differential rates of inhibitor development among those receiving replacement therapy by means of OD versus those with prophylactic schemes (Reference Lusher26–Reference Kurnik, Bidlingmaier and Engl28), in this case, it was assumed that inhibitors rates were roughly the same among regimens, thus not included.
Patients in the model could either experience active spontaneous or traumatic bleeding located within the joints (articular) or extra-articular. The primary assumption was that all patients bleed at some point in life, regardless of the treatment option allocated (Reference Olivieri, Kurnik, Pfluger and Bidlingmaier17). The difference between the two treatments is the annual rate at which bleeding occurs. In the model, once bleeding has occurred, patients could die, or recover (see Figure 1). A proportion of patients who have experienced articular bleeding and recover, develop arthrosis and enter the heath state “alive-with arthropathy” with the remaining proportion staying in the health state “alive-no arthropathy.”
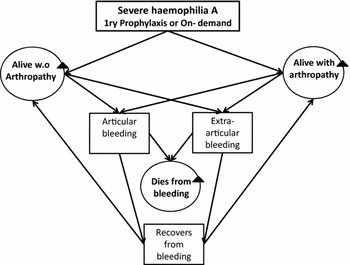
Figure 1. The Markov model for severe hemophilia type A.
Although dying from bleeding is expected to be similar for each treatment branch; chances of dying in the model were considered to be associated with the promptness of access to health care, the total number of bleeds per-cycle, and the severity of hemorrhage. In Colombia, the probability to die from bleeding is still expected to be significant and associated with logistic and cultural limitations that may prevent home-based provision of FVIII, these include, access to electricity, lack of cold-chain quality assurance, literacy, and a black market of costly medicines (Reference Robledo3;19). Therefore, this was incorporated in the base case model.
The annual probability of developing arthropathy was assumed to be constant and independent of the number of bleeds per cycle, as this relationship is complex and not well understood, also because we could not find enough evidence to estimate age or per-bleeding-dependant probabilities. In the base case model, it was further assumed that once people had entered the health state “alive-with arthropathy” they continued to experience bleedings, but that individual joints do not further detereiorate.
It was also assumed that once patients entered the health state “alive-with arthropathy” (irrespective of the treatment allocated), they remained in this same health state meaning that once arthropathy has occurred, it does not regress (Reference Hilberg and Czepa18). Eventually, throughout their life span, all patients without any previous joint damage, who present with additional articular bleed, would either develop arthropathy or die. The model assumes that fewer bleeding episodes leads to lower rates of joint-related complications, which in turn is assumed to lead to better HR-QoL (Reference Nilsson, Berntorp, Löfqvist and Pettersson6;Reference Lalezari, Coppola and Lin15;Reference Kempton, Valluri and Reding16).
Transition Probabilities
Transition probabilities were extracted from the literature search to update effectiveness and cost-effectiveness information described before. Input probabilities incorporated in the decision model included the annual probability of articular bleeding, the probability of extra-articular bleeding, the probability of dying from bleeding of any type, and the probability of developing arthropathy under each treatment branch. Whenever bleeding occurs, the probability of it being articular was extracted taking into consideration the data reported by Stachnik (Reference Haemophilia1), Wong and Recht (Reference Wong and Recht31), Vdovin et al. (Reference Vdovin, Andreeva and Chernova32), and Kempton et al. (Reference Kempton, Valluri and Reding16). These authors estimated the probability a bleeding to be located within the joints as around 80 percent of all bleeding episodes, this figure was derived and validated by a panel of clinical experts who considered it as plausible in this context, thus incorporated into the model. The probability of presenting with extra-articular bleeding was calculated as the complement of the probability of articular bleeding.
In Colombia there is no available, updated, and reliable data about the mortality rates and causes of death among SHA patients. Hence, the probability of dying from bleeding of any type was extracted from Darby et al. (Reference Darby, Kan and Spooner20). Similarly, the overall probability of developing arthropathy was estimated and extrapolated from the published evidence. No publications have reported the specific risk of developing arthropathy with each new bleeding episode and only two recent randomized clinical trials reported the risk rates of developing joint damage with PP and OD after a period of observation (Reference Manco-Johnson, Abshire and Shapiro21;Reference Gringeri, Lundin and von Mackensen34) (see Table 1). The characteristics of the dosing scheme and profile of participants in the study by Gringeri et al. (Reference Gringeri, Lundin and von Mackensen34) limited extrapolation of results and left the study by Manco-Johnson et al. (Reference Manco-Johnson, Abshire and Shapiro21) as the most reliable source of evidence for the model.
Resource Use and Unit Costs
The dose of PP was based on the clinical trial of Manco-Johnson et al. (Reference Manco-Johnson, Abshire and Shapiro21): 25 IU/Kg three times per week for a minimum of 46 weeks per-year (3,450 IU/Kg year); this dosing schedule according to local experts is similar to the clinical approach of PP provision in Colombia. When bleeding occurred during prophylaxis patients received 40 IU/Kg of FVIII and the assigned prophylaxis scheme was resumed the next day, this approach was also incorporated in the model.
OD dosage in the base case departed from the enhanced episodic treatment presented by Manco-Johnson et al. (Reference Manco-Johnson, Abshire and Shapiro21). According to local experts, recovery after bleeding OD was assumed to happen in Colombia after 2 weeks of treatment instead of 4, thus this regimen was incorporated in the model to make it more realistic. This equated to a total of 200 IU/Kg of FVIII per articular bleeding episode OD (form onset to resolution). Any other kind of extra-articular bleeding OD dosage (soft tissue, intra-abdominal or intra-cranial or postsurgery [form onset to resolution]) was estimated in 100 IU/Kg of clotting factor per-episode (Reference Smith, Teutsch, Shaffer, Rolka and Evatt29–Reference Vdovin, Andreeva and Chernova32) and validated with the local experts who considered it as plausible.
The mean estimates of bleeding episodes of Manco-Johnson et al. (Reference Manco-Johnson, Abshire and Shapiro21) were also considered to calculate total costs of each alternative. This study reported results of treatment branches PP and OD, and median values of 1.15 and 17.13 (p < 0.001), respectively. Whereas with a mean value of 17.69 (confidence interval [CI] ± 9.25) for bleeding episodes OD was very close to the median, a higher mean value and wider interval was presented for prophylaxis 3.27 (CI ± 6.24). Therefore, after looking at other mean values reported by Fischer and Van Den Berg (Reference Fischer and Van Den Berg33), Coppola et al. (Reference Coppola, Di Capua and De Simone8) and Vdovin et al. (Reference Vdovin, Andreeva and Chernova32) in their observational studies that ranged between 2 and 5 episodes per-year, three bleeding episodes per-year with PP and 17 bleeding episodes per-year OD were selected as the input parameters for the base case model (closer to the mean estimates as reported by Manco-Johnson et al. in 2007). These seemed more realistic in the context of interest to clinical experts that participated in the study than more conservative estimates.
All relevant costs associated with replacement therapy with FVIII, follow-up, bleeding episodes, and treatment of complications from chronic bleeding or joint damage were gathered from local sources. Costing considered the quantities of resources used per-patient per-year, as well as the local unit cost of health services consumed from the health system´s perspective. See Table 2 for a detailed description of estimated costs and units used to populate the economic model.
Table 2. Resource Use and Unit Costs

The cost per IU of FVIII was estimated in 900 COP (0.47 USD. Currency exchange rate as per July 12th 2013. Source http://www.oanda.com/) and the cost per IU of rFVIII in 1,450 COP (0.76 USD). Prices in Colombia of FVIII and rFVIII had not been regulated or were publically available at the time of writing up; hence, a market reference of average costs per IU/Kg paid by healthcare insurers (EPS Spanish acronym) was used to populate the model. Because the difference in costs between FVIII and rFVIII were substantial, a variant of the base case model was run considering the costs of rFVIII solely. Exogenous replacement therapy represented the biggest burden of resources consumed.
Unit costs of central venous implantation, MRI (Magnetic Resonance Imaging), radiology, ultrasonography, physiotherapy sessions, arthrocentesis, sinovectomy, and arthroplasty per joint were estimated using the reference tariff manual ISS 2001 (35), which is a widely used reference manual by insurers and healthcare providers to purchase healthcare services in Colombia. The global tariff was adjusted by informal consensus with local experts (purchasers) adding a 30 percent factor to control for inflation and regional variability of prices. Other costs were obtained from local market reference prices and SISMED 2013 (the official reference website for prices of medicines in Colombia). Costs are presented in 2013 COP and corrected for inflation if not gathered for the year 2013.
Estimation of HR-QoL
Collecting and gathering utilities for the health states incorporated into the model (see Table 1) represented a major challenge because no general instruments to assess preferences for health states had been formally applied in Colombia (either to patients or general population). Under these circumstances, the second best option was to use primary or secondary sources of local data of hemophiliac patients to populate the model. Therefore, to estimate health utilities, a cohort of forty-eight Colombian patients that had been followed up since 2009 was considered (a total of thirty-one severe hemophilic A patients were included, eighteen treated OD and thirteen with PP) (Coomeva EPS- MEDEX cohort 2012 unpublished).
Patients answered the Hemophilia Joint Health Score (HJHS) questionnaire and a Spanish version of the EQ-5D to elicit their preferences about health states associated with hemophilia and joint damage. It should be noted that in Colombia the EQ-5D 3 level questionnaire had not been formally applied to a random sample of population; hence, utility values for each of the four possible health states of interest were estimated using the UK tariffs (Reference Dolan, Gudex, Kind and Williams36). Because joint damage is expected to have a further negative impact on the overall quality of life of those who develop it, a lower utility value was considered for those patients with a score of 2 or more in the mobility dimension of this generic questionnaire. This assumed that the main differences in quality of life among sub-groups were associated with joint damage and not derived from the type of treatment received or any other variable or comorbidity.
The HJHS (Feldman et al. (Reference Feldman, Funk and Bergstrom37) is a multidimensional clinic-metric tool that is reported to be more sensitive than the WFH questionnaire to diagnose early joint damage among the haemophiliac population. HJHS evaluates six “index joints” (elbows, knees, and ankles) using a quantitative scale from 0 to 124 points to capture joint damage progression by a trained physiotherapist. The scale evaluates swelling, duration of swelling, muscle atrophy, axial alignment, and crepitus of motion, flexion loss, extension loss, instability, joint pain, strength and gait. HJHS also relies on a physician's global score of joint health consisting of a series of six 10-cm visual analogue scales (VAS), one for each index joint, whereby 1 equals “no complaints, no findings” and 0 equals “continuous pain, severe limitations, worst damage”, and one VAS for the overall assessment of arthropathy on HR-QoL whereby 1 equals “no impact on life” and 0 equals “most severe impact on life”. Each yields an individual score and the average of the 6 joints yields the total physician's score
Previously published utility values for hemophilia A (Reference Miners14;Reference Farrugia, Cassar and Kimber25;Reference Risebrough, Oh and Blanchette38) and the VAS scores from physicians and patients reported through the HJHS were set as parameters to be used in the sensitivity analyses. The disutility values related with bleeding were not included in the model because of the lack of evidence.
Time Horizon and Discount Rate
A lifetime horizon was considered as clinically relevant because SHA is a lifelong disease, and the long-term benefits, costs and complications of treatment are expected to occur throughout the life span of patients. We used a 1.5 percent discount rate for health effects and 3.5 percent for costs, with a range of 0–5 percent for health effects and costs in the sensitivity analyses (these are commonly used figures in economic evaluations and health care, and also were in line with ranges for discount rates suggested by the Colombian health technology assessment agency, IETS). This approach is plausible because when health effects are valued in monetary terms, as is the case of cost-benefit analysis, these should be discounted at the same rate as the costs (Reference Gravelle and Smith39). However, if health effects are measured in quantities (e.g., QALYs), as in this case, discounting benefits at a lower rate than costs is a valid approach for taking into consideration the future value of health effects (as it would be the case of quasi-normal life spans with preserved joint mobility).
Model and Input Validation
Three physicians (two hematologists and a public health specialist) familiar with SHA participated as local clinical experts to validate the model structure and refine or estimate some of the input parameters. Three third-party payer senior managers of health insurers (EPS) also participated as experts on local costs and tariffs. This panel of experts was virtually allocated and requested to consent in at least two rounds of enquiry about unit consumption and costs whenever needed, participants were anonymous to each other.
Decision Rule
A theoretical cost-effectiveness threshold of one up to three times the current Colombian gross domestic product (GDP) per-capita was set as decision rule. Although WHO recommendations used this threshold as willingness to pay, WTP for disability-adjusted life-years averted, it could serve as a point of reference to inform decision makers in Colombia where no cost-effectiveness threshold was in operation by time of writing up. This figure for 2013 was equivalent to 48,458,861 COP (25,335 USD).
Sensitivity Analysis
We performed deterministic and probabilistic sensitivity analysis (PSA) to estimate the impact of uncertainty of parameters and structural assumptions of the model in the cost-effectiveness results (see Supplementary Table 1 for distributions used for the sensitivity analyses). We present results of PSA as cost-effectiveness acceptability curves. All analyses were performed using TreeAge Pro 2013 software.
RESULTS
In the base case, the incremental cost-effectiveness ratio (ICER) calculated for PP versus OD treatment throughout life using FVIII was 105,081,022 COP (55,204 USD) per QALY gained, and thus not considered cost-effective. When PP was provided using rFVIII, the ICER reached 174,159,553 COP (91,494 USD) per QALY gained (see Table 3).
Table 3. Results of the Cost Utility Analysis over a lifetime horizon.

Modifying the probability of developing arthropathy with both treatment approaches did not show a significant impact on the final ICER, neither did changing discount rates for QALYs and costs from 0 to 5 percent. Only with a discounting rate of the effects very close to 0, PP was considered to be cost-effective. The ICER results are sensitive to cost of FVIII: a reduction of 50 percent or more on the cost of FVIII would make PP cost-effective in Colombia. In the case of rFVIII, only a price discount of 75 percent or more would make PP cost-effective in this context.
Varying the utility range of being alive without arthropathy OD from 0.5 to 0.942 does not reduce the ICER of PP up to a point of being considered cost-effective; this only happens with a utility as low as 0.2 or less in this health state. The opposite occurs with the utility of being alive with arthropathy PP: the higher the value of this health state the lower the ICER of PP, although not even when the utility value of this health state is closer to 1 PP becomes cost-effective. The model results are sensitive to the location of bleeding episodes. Modifying the probability of having an articular bleed from 0.5 up to 1.0 shows that the higher the chance of bleeding within the joints, the lower the ICER of PP, making it cost-effective above 0.95.
The tornado diagram (Supplementary Figure 1) shows that the discount rates for costs, cost of FVIII, number of bleeding episodes OD, probability of presenting with an articular bleeding, dosage/Kg per joint bleeding OD, probability to die from bleeding and dosage/Kg per any other bleeding OD had the biggest impact on the final reported ICER.
The PSA shows PP is more effective, but also costlier than OD in almost all iterations, irrespective of the WTP (Supplementary Figure 2): At a WTP of 48,458,861 COP (25,335 USD) the incremental cost-effectiveness scatterplot shows PP is not considered cost-effective in any iteration when compared with OD. The cost-effectiveness acceptability curve (Supplementary Figure 3) shows that PP would only be considered as cost-effective if the Colombian threshold were higher than 95,000,000 COP (49,908 USD). A formal willingness to pay for an additional QALY threshold does not currently exist in Colombia, but using WHO recommendations in all model iterations, PP was found not to be cost-effective at such a WTP.
DISCUSSION
Methodological recommendations from available guidelines in Colombia at the moment of developing this study were followed. In the base case, our analysis suggests that PP using FVIII generates an ICER of 105,081,022 COP (55,204 USD) per QALY. When PP was provided using rFVIII, the ICER reached 174,159,553 COP (91,494 USD) per QALY gained. PP would only be considered as potentially cost-effective if the Colombian threshold were higher than 95,000,000 COP (49,908 USD). A formal threshold does not currently exist in in this context, but using WHO recommendations of a threshold of up to three times the current Colombian GDP per-capita, PP was found not to be cost-effective at such a WTP.
For more than three decades the early initiation of PP in children has proven to be beneficial in preventing hemophilic arthropathy. However, in Colombia PP is still not usual practice and no previous health technology assessment report has been produced for this health condition in this context, the results from this cost-utility analysis (CUA) could be used to assist decision making in this country.
Model results were within the wide range of published studies that have estimated incremental costs per QALYs gained. For instance, Miners in 2002 estimated an ICER of £46,500 (Reference Miners14), while in 2009 Risebrough et al. (Reference Risebrough, Oh and Blanchette38) estimated an ICER of 542,938 Canadian Dollars for an escalating dose regime versus OD. In 2009, Miners updated his previous publication and re-calculated an ICER near £10,000 lower than his previous estimates due to the use of different structural assumptions and methods to fit the various model parameters (Reference Miners14).
As in other publications, the results of the base case model were sensitive to input parameters. Initially, the model attempted to estimate differential utility values for all health states of interest but after further consideration about the limited size of the sample and evidence of heterogeneity among sub-groups, an average utility estimate was used for those without joint damage and for those with it irrespective of treatment allocated. This latter assumption is more conservative; however, it may have led to different results from the primary intention of analysis. The estimation of utilities probably represented the major limitation of this study because the health states preferences to estimate QALYS were gathered from a local cohort of only 31 patients, nonetheless this was considered as the best available evidence in the context of interest.
Only one economic evaluation had been previously published for low and middle income countries (Reference Daliri, Haghparast and Mamikhani13) and no CUA had been published in these context. According to Miners (Reference Miners10), studies that might be of use for this condition should collect utility data derived from discrete patient cohorts, link intermediate outcomes such as bleeds with longer-term likelihood of developing arthropathy and, if necessary, gather expert opinion using formal elicitation methods to fill data gaps for insertion into appropriately designed decision models. Some of these methodological recommendations were considered in the model.
The main limitations of this CUA arose from the structural assumptions (i.e., the model assumed a constant annual probability of developing arthropathy), methods (i.e., we could not find enough evidence to estimate properly age or per-bleedingdependant probabilities) and parameters used to populate the model (i.e., we used utility values from a local but small sample of Colombian patients). Blood-borne infections, were expected to be nonexistent if proper viral attenuation methods were used or if recombinant exogenous factor was supplied. Hence, they were not considered albeit proper viral attenuation or recombinant FVIII supply, may not be available in all regions in a country like Colombia, additionally uncertainty still remains about prions or yet-to-be discovered pathogens (Reference Van der Poel, Reesink and Mauser-Bunschoten40;Reference Mannucci and Tuddenham41); hence, this could also be considered as another limitation.
CONCLUSION
In conclusion, using a decision rule of up to three times the Colombian GDP per capita, PP (with either FVIII or rFVIII) would not be considered as cost-effective in this country. The estimation of a combined index of health benefits and costs, as an ICER, should be considered as just one input parameter in to decision-making process in Colombia, a final decision on providing or preventing patients from PP as a gold standard of care for SHA should also consider broader criteria than the ICER results itself. Only a price reduction of exogenous FVIII of 50 percent or more would make PP cost-effective in this context.
SUPPLEMENTARY MATERIAL
Supplementary Table 1: https://doi.org/10.1017/S0266462316000544
Supplementary Figure 1: https://doi.org/10.1017/S0266462316000544
Supplementary Figure 2: https://doi.org/10.1017/S0266462316000544
Supplementary Figure 3: https://doi.org/10.1017/S0266462316000544
CONFLICTS OF INTEREST
No conflicts of interest declared.






