INTRODUCTION
The distribution of a parasite population across a host population is characterized by its aggregation (Anderson and May, Reference Anderson and May1978; Shaw and Dobson, Reference Shaw and Dobson1995). Most parasite individuals occur on a few host individuals, while most host individuals have only a few or no parasites. In general, this suggests that some host individuals represent better habitat patches for parasites than other individuals. The aggregation of parasites among their hosts is commonly thought to arise due to heterogeneities in host populations and/or infection pressure (Shaw and Dobson, Reference Shaw and Dobson1995). For example, these heterogeneities may include among-host variation in such parameters as exposure risk and defensibility. Limited dispersal abilities of parasites can also result in aggregated distribution, although this may be true for some parasite taxa (e.g. lice) but not others (e.g. mosquitoes).
A concept of ideal free distribution (IFD; Fretwell and Lucas, Reference Fretwell and Lucas1970) has also been applied to the distribution of parasites across host individuals (Kelly and Thompson, Reference Kelly and Thompson2000). This concept predicts that animals that compete for resources distribute themselves among habitat patches in proportion to the amount of resources available to them, so that resource use per individual will be equal across all patches. In other words, animals are (a) ideal in assessing patch quality and (b) free to enter and use the resources on a regular basis. However, given the strict assumptions needed for IFD, it is doubtful that this concept may be applicable for endoparasites and permanent ectoparasites (which spend the entire life on the surface of a host, e.g. lice). However, the IFD approach can be useful in the examination of the distribution of temporary and periodic ectoparasites. The former are largely free living and visit the host for long enough to take a bloodmeal (e.g. mosquitoes, tabanids), whereas the latter spend a considerably longer time on the hosts than is required merely to obtain a bloodmeal, but nevertheless spend a significant amount of time off-host (e.g. most fleas, mesostigmatid mites) (Lehane, Reference Lehane2005).
Kelly and Thompson (Reference Kelly and Thompson2000) developed an IFD model of host choice by blood-sucking insects based on the premise that an individual haematophagous parasite evolved to choose the ‘best’ host to maximize feeding and, consequently, reproductive success. They suggested that an individual insect can improve its feeding success by choosing a host with a high intrinsic quality, a low defensiveness and a small number of competitors. An individual parasite's choice of which host to exploit is thus based on the ‘suitability’ of the host. In particular, suitability is assumed to be density dependent where higher densities of individuals will lower suitability within habitats. In other words, this model suggests intraspecific competition among blood-sucking arthropods and negative fitness-density relationships given that fitness of a haematophagous parasite is directly related to its feeding success.
Intraspecific competition among ectoparasites should thus result in a decrease in feeding success of an individual with an increase in the number of conspecifics. Intraspecific competition can be both exploitative and interfering. However, if ectoparasite insects are considered, intraspecific competition, at least between imagoes, appears to be interfering. It does not seem feasible that the blood in a host can be a limiting factor. The limiting factors can be those areas of a host body where blood is most readily available (e.g. thinnest skin or closest position of capillary to body surface). In addition, interference among parasite individuals can be mediated via the host. For example, if there is a threshold of host sensitivity to parasite attacks, then its defence systems (behavioural or immune) may be activated once exploiters attain certain abundance (Mooring, Reference Mooring1995; de Lope et al. Reference de Lope, Møller and de la Cruz1998). From this point on, the host defence may be the main force limiting parasite success.
The cost of mounting and maintaining anti-parasitic defences is high and there are numerous trade-offs between anti-parasitic defences and other concurrent needs of an organism (Mooring and Hart, Reference Mooring and Hart1995; Sheldon and Verhulst, Reference Sheldon and Verhulst1996; Schmid-Hempel and Ebert, Reference Schmid-Hempel and Ebert2003). Therefore, if the cost of suppressing the feeding of a great number of parasites is too high, then the strength of a host response can decrease with an increase in the number of attackers (e.g. Khokhlova et al. Reference Khokhlova, Spinu, Krasnov and Degen2004a). Furthermore, a great number of co-occurring attackers can suppress the defence system of a host by a cumulative effect of factors contained in their saliva (see reviews in Wikel, 1996 and Gillespie et al. Reference Gillespie, Mbow and Titus2000). As a result, co-occurrence of conspecific parasites can be facilitated via the host and the feeding success can be expected to increase with an increase in the number of co-feeding parasites.
We studied the feeding success of 2 flea species, Xenopsylla conformis and Xenopsylla ramesis, when exploiting 2 species of gerbilline rodent hosts, Gerbillus dasyurus (average adult body mass 20 g) and Meriones crassus (average adult body mass 80 g). These flea and rodent species are common in the Negev desert. The 2 rodents co-exist in various non-sandy and non-rocky habitats. We tested 2 alternative hypotheses on the effect of flea density on blood consumption. In the first, there is intraspecific interference competition among fleas and in the second there is facilitation in blood consumption by fleas via suppression of the host defence system. We predicted that with an increase in the number of co-feeding fleas on a host, feeding success of fleas decreases in the first hypothesis but increases in the second hypothesis.
MATERIALS AND METHODS
Rodents and fleas
We used immune-naïve, adult male M. crassus and G. dasyurus from our laboratory colonies. Progenitors of the colony were captured at the Ramon erosion cirque, Negev Highlands, Israel (30°35′N, 34°45′E) in 1997. The rodents were maintained in plastic cages (60 by 50 by 40 cm) and offered millet seed and alfalfa (Medicago sp.) leaves ad libitum. No water was available as the alfalfa supplied enough for their needs. Each individual was used in an experiment only once.
Fleas were obtained from our laboratory colonies started in 1999 from field-collected specimens on M. crassus, using the rearing procedures described elsewhere (Krasnov et al. Reference Krasnov, Khokhlova, Fielden and Burdelova2001). In brief, a steel nest box with a wire mesh floor and a pan containing a mixture of sand and dried bovine blood (larvae nutrient medium) on the bottom was placed into a cage of an individual rodent host. The gravid females deposited eggs in the substrate and bedding material in the nest box. Every 2 weeks, we collected all substrate and bedding material from the nest box and transferred it to an incubator (FOC225E, Velp Scientifica srl, Milano, Italy), where fleas developed at 25°C air temperature and 75% relative humidity (RH). The newly emerged fleas were placed on clean animals. Colonies of rodents and fleas were maintained at 25°C and either 50% or 75% RH, respectively, with a photo-period of 12:12 h. Every year about 200 fleas captured in the field on both M. crassus and G. dasyurus were added to the colony to avoid local adaptation to the host species on which fleas were reared. Indeed, although both species were reared on 1 host species (M. crassus) for several years, no local adaptation seemed to rise and both fleas were able to reproduce on both host species (Krasnov et al. Reference Krasnov, Khokhlova, Burdelova, Mirzoyan and Degen2004).
Experimental design
We used fleas (24–48 h old) that did not feed from emergence until experimental treatments. After emergence and prior to experiments, the fleas were placed in an incubator and maintained at 25°C and 75% RH. Rodents were placed in wire mesh (5 by 5 mm) tubes (15 cm length and 5 cm diameter for M. crassus and 10 cm length and 3 cm diameter for G. dasyurus) that limited movement and did not allow self-grooming. Tubes with rodents were placed into individual white plastic baths. Then either 5, 10, 15, 25, 30, 35, 45 or 50 weighed fleas (X. conformis or X. ramesis) (±0·01 mg, 290 SCS Precisa Balance, Precisa Instruments AG, Switzerland) were placed on each rodent (maximal number of fleas recorded for M. crassus and G. dasyurus in the field was 35 and 25, respectively, see Krasnov et al. Reference Krasnov, Shenbrot, Medvedev, Vatschenok and Khokhlova1997). We collected the fleas after feeding on a host for either 30 or 60 min. Our previous study on Parapulex chephrenis (Krasnov et al. Reference Krasnov, Sarfati, Arakelyan, Khokhlova, Burdelova and Degen2003a) as well as preliminary observations on X. conformis and X. ramesis indicated that 60 min of staying on a host is enough for engorgement of the majority of fleas and that the proportion of engorged individuals does not change with an increase of this time. Consequently, we expected that the effect of density would be expressed more sharply, with a reduction of the time that fleas will stay on a host.
The rodent fur was brushed several times using a tooth-brush until all fleas were recovered. Fleas were then weighed again and the difference in mass was taken as blood consumption (see below). We assessed the level of flea midgut engorgement by examination of each flea under a light microscope (without dissection) as either low (less than 80% of midgut was filled with blood) or high (more than 80% of midgut was filled with blood). In addition, we counted fleas that did not feed.
Treatments, therefore, differed in host species, flea species, flea density and time that fleas were allowed to stay on a host. Each treatment was replicated 5 times, totalling 2 host species×2 flea species×8 densities×2 time-periods×5 replicates=320 experiments. The order of treatments was selected randomly.
Data analysis
Feeding success was evaluated as (a) the mean amount of blood consumed by a flea and (b) the proportion of fleas with highly engorged midguts after a timed period of feeding. The mean amount of blood consumed by a flea (=mean bloodmeal size) per unit body mass was calculated as the difference between total mass of fleas after feeding and total mass of fleas prior to feeding, subtracting the number of fleas that did not feed×mean body mass of a starving flea; this value was divided by the number of fleas that took a bloodmeal×the mean body mass of a starving flea. The amount of blood consumed by a flea was log-transformed, whereas the proportion of fleas with low or high level of engorged midgut was arcsin-transformed prior to analysis. However, untransformed data are presented in the figures. The two parameters of feeding were not correlated (Spearman rank order correlation coefficients between the two parameters within a treatment r s=−0·47–0·41; all non-significant). This suggests that the mean bloodmeal size and the proportion of highly engorged fleas captured different facets of flea feeding.
Initially, we used 4-way ANOVA with mean bloodmeal size or the proportion of fleas with a highly engorged midgut as a dependent variable and flea and host species, time of feeding and number of fleas on a host as independent variables. Then, we carried out 1-way ANOVAs of one of the dependent variables in dependence of flea density (see below) within each flea and host species. To determine the border density at which feeding efficiency parameters were affected by flea density, we searched for the significant difference in dependent variables among different flea densities within each flea and host species using Tukey's HSD tests. Then we pooled data for treatments with different flea densities and feeding time but with similar mean bloodmeal size or proportion of fleas with highly engorged midguts and repeated the ANOVAs. We avoided an inflated Type I error by performing Bonferroni adjustments of alpha.
RESULTS
Size of a bloodmeal
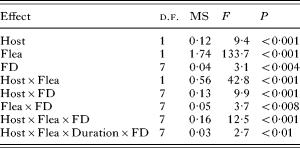
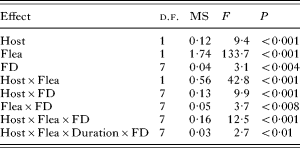
The amount of blood consumed by a flea differed significantly between flea species, and was dependent on host species and the number of fleas feeding on a host, but not on feeding duration (F 1,256=1·1, P>0·3) (Table 1). In addition, three 2-way interactions and two 3-way interactions were significant (Table 1). Four of these high-order interactions included flea species, 4 other interactions included host species and 1 included the feeding duration. This suggests that the pattern of the effect of flea density on feeding efficiency differed between flea and host species (Fig. 1). In the majority of treatments, this pattern was similar at 2 feeding durations. Pairwise comparisons demonstrated no significant differences in a bloodmeal size of the same flea feeding on the same host at the same density in dependence of feeding duration (Tukey HSD tests, non-significant) except for 10 X. ramesis on G. dasyurus and 30 X. ramesis on M. crassus (bloodmeal at 60 min of feeding larger or smaller than at 30 min of feeding, respectively; Tukey's HSD tests, P<0·02 for both).

Fig. 1. Mean (±s.e.) bloodmeal size per unit body mass of Xenopsylla conformis and X. ramesis when feeding on Gerbillus dasyurus and Meriones crassus at different densities.
Table 1. Summary of ANOVA of mean size of a bloodmeal (per unit body mass) consumed by an individual flea as affected by host species (Host – M. crassus or G. dasyurus), flea species (Flea – X. conformis or X. ramesis), duration of feeding (Duration – 30 or 60 min) and flea density (FD – 5, 10, 15, 25, 35, 45 or 50 fleas)
(Only significant (after adjustment) effects are shown.)
The mean size of a bloodmeal of X. conformis feeding on G. dasyurus was affected by flea density (F 7,64=26·4, P<0·0001). Fleas consumed significantly less blood at low (5–15 fleas per host) than at high (25–50 fleas per host) densities (0·20±0·01 versus 0·46±0·01 mg of blood per mg of flea body mass, respectively; F 1,78=173·6, P<0·0001). The effect of density was also significant for X. conformis feeding on M. crassus (F 7,64=10·7, P<0·0001). However, the pattern of blood consumption was opposite to that observed when fleas fed on G. dasyurus. On M. crassus, fleas consumed significantly more blood at low than at high densities (0·63±0·05 versus 0·37±0·01 mg of blood per mg of flea body mass, respectively, F 1,78=22·3, P<0·0001).
In contrast, mean bloodmeal size of X. ramesis feeding on either G. dasyurus or M. crassus was not affected either by flea density (F 7,64=1·1 and F 7,64=0·2, respectively; P>0·3 for both) or by host species (F 1,158=0·2, P>0·67). In general, X. ramesis consumed the same amount of blood from both rodents (0·39±0·07 mg of blood per mg of flea body mass when feeding on G. dasyurus and 0·41±0·01 mg of blood per mg of flea body mass when feeding on M. crassus).
Level of engorgement
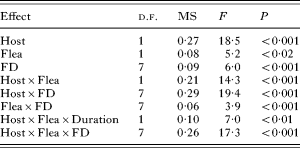
The proportion of fleas with highly engorged midguts was affected by flea and host species and flea density. Three 2-way interactions, one 3-way interaction and a 4-way interaction were also significant (Table 2). This, again, suggests that the effect of flea density on feeding success was manifested differently in different treatments (Fig. 2). The only significant difference on the proportion of highly engorged fleas as affected by feeding duration ceteris paribus was found in a treatment with 25 X. conformis on G. dasyurus (greater at 60 min of feeding; Tukey's HSD test, P<0·02).

Fig. 2. Proportion (±s.e.) of Xenopsylla conformis and X. ramesis with highly engorged midguts when feeding on Gerbillus dasyurus and Meriones crassus at different densities.
Table 2. Summary of ANOVA of the proportion of fleas with highly engorged midgut as affected by host species (Host – M. crassus or G. dasyurus), flea species (Flea – X. conformis or X. ramesis), duration of feeding (Duration – 30 or 60 min) and flea density (FD – 5, 10, 15, 25, 35, 45 or 50 fleas)
(Only significant (after adjustment) effects are shown.)
X. conformis feeding on both hosts demonstrated a strong density effect on the proportion of highly engorged individuals (F 7,72=15·1 for G. dasyurus and F 7,72=8·5 for M. crassus; P<0·001 for both). When feeding on G. dasyurus, a lower proportion of highly engorged fleas was observed at lower (5–15 fleas) than at higher (25–50 fleas) densities (0·39±0·02 versus 0·60±0·02, respectively; F 1,78=47·1, P<0·001). The opposite was true for X. conformis feeding on M. crassus, where a greater proportion of fleas attained a high degree of engorgement at lower than at higher densities (0·73±0·03 versus 0·59±0·02, respectively; F 1,78=16·6, P<0·001). In general, a larger proportion of X. conformis filled their midgut when feeding on G. dasyurus than on M. crassus at low densities (F 1,58=58·7, P<0·001), whereas at high densities no between-host difference was found (F 1,98=0·1, P=0·75).
Proportions of highly engorged X. ramesis were similar at all flea densities when feeding on a particular host (F 7,72=2·2 for G. dasyurus and F 7,72=1·3 for M. crassus; P>0·05 for both), but differed between host species (F 1,144=7·2, P<0·01). In general, slightly, albeit significantly higher proportions of X. ramesis filled their midgut when feeding on G. dasyurus than on M. crassus (0·75±0·01 versus 0·71±0·01, respectively; F 1,158=5·2, P<0·02).
DISCUSSION
Predictions of each of the alternative hypotheses were true for a particular flea-host association. There was an effect of density on feeding success in X. conformis but not in X. ramesis. Furthermore, when X. conformis parasitized M. crassus, the effect of density on feeding suggested intraspecific competition, but on G. dasyurus this effect suggested apparent facilitation. In addition, both measures of feeding success demonstrated similar patterns, although they reflected different aspects of flea feeding. Bloodmeal size reflected mainly the blood loss of a host, whereas the proportion of highly engorged fleas reflected mainly their individual feeding efficiency.
The difference between fleas in their response to density may be associated with their different strategies of host selection. Earlier studies indicated that X. conformis behaved as a density-dependent host selector (Krasnov et al. Reference Krasnov, Khokhlova and Shenbrot2003b). It showed sharp selectivity at low densities and parasitized M. crassus only, whereas with an increase in flea population size, the less preferable host, G. dasyurus, was also parasitized. Furthermore, X. conformis produced more eggs per female when parasitizing M. crassus than G. dasyurus (Krasnov et al. Reference Krasnov, Khokhlova, Burdelova, Mirzoyan and Degen2004). In addition, food requirements necessary for successful egg development were lower and egg survival was higher when a flea exploited M. crassus rather than G. dasyurus (Krasnov et al. Reference Krasnov, Khokhlova, Burdelova, Mirzoyan and Degen2004). However, development time of X. conformis larvae did not depend on host species.
In contrast, X. ramesis at low density chose a host species randomly (Krasnov et al. Reference Krasnov, Khokhlova and Shenbrot2003b). However, with an increase in flea population, their pressure on M. crassus increased at a faster rate than that on G. dasyurus and, thus, a preference of M. crassus over G. dasyurus occurs (Krasnov et al. Reference Krasnov, Khokhlova and Shenbrot2003b). According to the definition of Morris (Reference Morris1988), X. ramesis was a density-independent host selector with a direct correspondence of density with host quality (Krasnov et al. Reference Krasnov, Khokhlova and Shenbrot2003b). Indeed, X. ramesis did not have any direct reproductive advantage when feeding on either M. crassus or G. dasyurus, although less bloodmeals were necessary for oviposition and development rate of pre-imago was faster by fleas exploiting M. crassus than G. dasyurus, thus providing indirect reproductive benefits (Krasnov et al. Reference Krasnov, Khokhlova, Burdelova, Mirzoyan and Degen2004).
Consequently, our previous studies suggested that the difference between the two flea species in their strategy of host selection was associated with the difference in the relative delay of reproductive benefit received when exploiting the ‘higher-quality’ host (M. crassus) than the ‘lower-quality’ host (G. dasyurus) (see discussion on relative quality of M. crassus versus G. dasyurus for X. conformis and X. ramesis in Krasnov et al. Reference Krasnov, Khokhlova, Burdelova, Mirzoyan and Degen2004). When fleas fed on the ‘higher-quality’ host, reproductive advantage for X. conformis was almost immediate (egg production), whereas that for X. ramesis was delayed (shorter development time and, thus, benefit in competitive ability of larvae, see Krasnov et al. Reference Krasnov, Khokhlova, Burdelova, Mirzoyan and Degen2004 for details). The between-flea difference in the impact of density on feeding success found in the present study may be one of the mechanisms behind the between-flea difference in the effect of density on host selection.
Between-flea differences in reproduction-related traits when feeding on the 2 hosts explained differences in patterns of host selection that, in turn, indicated that fleas behaved in an IDF-like manner (see Krasnov et al. Reference Krasnov, Khokhlova and Shenbrot2003b, Reference Krasnov, Khokhlova, Burdelova, Mirzoyan and Degen2004). However, the necessary assumption of an IDF is a negative fitness-density relationship. Although fitness parameters were not measured in this study, we assume that the pattern of feeding-density relationship might be indicative of the pattern of fitness-density relationship. Our present study demonstrated that this might be the case for X. conformis but not for X. ramesis. However, the above-mentioned delayed fitness response to the host species in X. ramesis suggested that the response to density may also be delayed and manifested in the number and survival ability of offspring rather than in bloodmeal size.
The most surprising result of this study was the difference in the response to density in X. conformis when feeding on different hosts. Both the bloodmeal size and proportion of highly engorged fleas increased with density when fleas were on G. dasyurus, but decreased with density when on M. crassus. This between-host difference may be due to the differential effect of fleas on the host energy balance and difference in the pattern of mounting an immune response against fleas.
Khokhlova et al. (Reference Khokhlova, Krasnov, Kam, Burdelova and Degen2002) demonstrated that G. dasyurus parasitized by fleas had higher energy requirements than non-parasitized conspecifics by 16%. Flea-infested G. dasyurus also lost more body mass (Khokhlova et al. Reference Khokhlova, Krasnov, Kam, Burdelova and Degen2002) and had a greater amount of white blood cells than parasite-free animals (Khokhlova et al. Reference Khokhlova, Spinu, Krasnov and Degen2004b). However, the pressure of flea parasitism on G. dasyurus in terms of blood consumed by the fleas was low (Khokhlova et al. Reference Khokhlova, Krasnov, Kam, Burdelova and Degen2002). Thus, the major effect of fleas on the energy expenditure of the host was a result of causes other than blood deficiency, such as the stimulation of an immune response to derived molecules from salivary glands of the fleas (Jones, Reference Jones and Wikel1996). Given the high energetic cost of immune responses in general (Oppliger et al. Reference Oppliger, Christe and Richner1996; Lochmiller and Deerenberg, Reference Lochmiller and Deerenberg2000; Moret and Schmid-Hempel, Reference Moret and Schmid-Hempel2000) and in G. dasyurus in particular (Khokhlova et al. Reference Khokhlova, Krasnov, Kam, Burdelova and Degen2002), a highly parasitized individual may give up immune responses. This is analogous to the decrease of the effectiveness of energy allocation to immune defence with an increase of the diversity of attack types when the optimal strategy of a host may be merely to tolerate damage (Jokela et al. Reference Jokela, Schmid-Hempel and Rigby2000). In other words, if the rate of attacks is too high, a host would not have enough energy resources to invest in immune responses. No empirical data supporting this mechanism in any flea-mammal association are available. However, G. dasyurus has been shown to demonstrate ‘post-invasive’ immunity against fleas and to mount immune responses immediately after flea attacks (Khokhlova et al. Reference Khokhlova, Spinu, Krasnov and Degen2004b). Thus, we envisioned the following scenario to have occurred. When a G. dasyurus was attacked by a relatively low number of fleas, it mounted an immune response that suppressed the feeding success of fleas in terms of bloodmeal size (e.g. Rechav et al. Reference Rechav, Heller-Haupt and Varma1989). When, however, the number of fleas was high, the energy available to the host was insufficient to mount an effective immune response, so the feeding success of the fleas increased with density. This pattern of flea co-feeding represents apparent intraspecific facilitation mediated via the host.
In contrast to G. dasyurus, energy requirements of M. crassus were not affected by flea parasitism (I.S. Khokhlova, unpublished data), although it was characterized by ‘pre-invasive’ immunity and maintained a certain level of immune ‘readiness’ against fleas even when not being attacked (Khokhlova et al. Reference Khokhlova, Spinu, Krasnov and Degen2004a). Furthermore, most immunological parameters of this species (except for phagocytic activity of leukocytes) were not affected by flea burden (Khokhlova et al. Reference Khokhlova, Spinu, Krasnov and Degen2004a). Consequently, the scenario envisioned for M. crassus was as follows. The magnitude of the immune response in this species did not change with the number of haematophagous attackers, but they competed with each other, for example, for areas of host body where the blood was more readily and/or easily available. As a result, feeding success per flea decreased with an increase in density. Another scenario was related to body size differences between hosts: M. crassus being 5 times larger than G. dasyurus and usually much more heavily infested by fleas in the field (Krasnov et al. Reference Krasnov, Shenbrot, Medvedev, Vatschenok and Khokhlova1997). Consequently, the number of fleas used in our experiments perhaps was not high enough to cause the termination of the immune response by M. crassus as was the case for G. dasyurus. These two scenarios are not mutually exclusive. It should be noted that a paradoxical situation when fleas compete with each other on larger rather on smaller host suggests greater feasibility of the scenarios that link feeding-density pattern in X. conformis and (a) relationship between flea density and immune response in G. dasyurus and (b) lack of this relationship in M. crassus. Physiological and immunological mechanisms behind the phenomena reported in this paper remain to be supported by experimental studies. In particular, it is unknown whether the period of 30 or 60 min of flea feeding is sufficient to produce the response of a host. Nevertheless, the suppression of the defence system of a host by a cumulative effect of factors contained in the saliva of ectoparasites can be rather rapid (Wikel, Reference Wikel1996).
In conclusion, this study showed that density dependence of feeding success of a flea (a) varied both between fleas and within-fleas between hosts, and (b) indicated intraspecific competition in some cases, but facilitation via host in other cases.
This study was supported by the Israel Science Foundation (Grant no. 249/04 to B. R. K., I. S. K. and A. A. D.). This is publication no. 563 of the Mitrani Department of Desert Ecology and no. 228 of the Ramon Science Center.