Families characterized by overt conflict, deficient nurturing, and cold and unsupportive relationships predict poor mental and physical health across the life span. The broad dimensions of conflict and warmth that characterize early rearing environments predict future health (Repetti, Taylor, & Seeman, Reference Repetti, Taylor and Seeman2002; Taylor, Way, & Seeman, Reference Taylor, Way and Seeman2011), as well as the health of adults currently living in those environments (Ailshire & Burgard, Reference Ailshire and Burgard2012; Fuller-Iglesias, Webster, & Antonucci, Reference Fuller-Iglesias, Webster and Antonucci2015; Yang, Schorpp, & Harris, Reference Yang, Schorpp and Harris2014). We and others have suggested a plausible biobehavioral mechanism: family environments characterized by higher conflict and less warmth may be related to an enhanced state of preparedness in innate immune cells (Repetti, Robles, & Reynolds, Reference Repetti, Robles and Reynolds2011) and reduced sensitivity to anti-inflammatory signals (i.e., resistance to the inhibitory effects of glucocorticoids; Miller, Chen, & Parker, Reference Miller, Chen and Parker2011), which may eventually result in hyperactive proinflammatory responses during infection. In this study, in both children and their parents, we examine whether conflict and lack of warmth within families are related to gene expression patterns in immune cells that are consistent with a proinflammatory phenotype. We also explore whether family conflict and lack of warmth are related to one possible clinical manifestation of a proinflammatory phenotype in children: severity of upper respiratory infection (URI) symptoms.
The family environment includes interactions between parents, between parents and children, and between children in the household. Numerous theoretical conceptualizations and measurement approaches have been applied to interparental/marital (Fincham & Linfield, Reference Fincham and Linfield1997; Mattson, Rogge, Johnson, Davidson, & Fincham, Reference Mattson, Rogge, Johnson, Davidson and Fincham2013), parent–child (Skinner, Johnson, & Snyder, Reference Skinner, Johnson and Snyder2005), and intersibling interactions (Brody, Reference Brody1998) in the family, and conflict and warmth appear as key dimensions across all those types of interactions (Repetti et al., Reference Repetti, Taylor and Seeman2002). Conflict is characterized by recurrent episodes of aversive behavior, including anger, hostility, and aggression. In contrast, warmth is characterized by interpersonal involvement, interest, and support. While conflict and warmth could be viewed as existing along a single dimension, most approaches to conceptualizing family and marital functioning consider them to be separate though correlated variables.
For both children and parents, several models suggest the family environment is a likely source of daily social adversity with implications for inflammation (Miller et al., Reference Miller, Chen and Parker2011; Repetti et al., Reference Repetti, Robles and Reynolds2011). Greater interpersonal, and particularly family-related conflict is related to a proinflammatory phenotype, including greater stimulated production of interleukin-6 (IL-6) in adults and adolescents (Davis et al., Reference Davis, Zautra, Younger, Motivala, Attrep and Irwin2008; Miller, Rohleder, & Cole, Reference Miller, Rohleder and Cole2009), and higher circulating C-reactive protein levels in adolescents (Fuligni et al., Reference Fuligni, Telzer, Bower, Cole, Kiang and Irwin2009). At the same time, low levels of warmth and support in the family are related to higher circulating inflammatory marker levels (Donoho, Crimmins, & Seeman, Reference Donoho, Crimmins and Seeman2013; Friedman et al., Reference Friedman, Hayney, Love, Urry, Rosenkranz, Davidson and Ryff2005; Whisman & Sbarra, Reference Whisman and Sbarra2012), as are high conflict and low warmth (Uchino et al., Reference Uchino, Bosch, Smith, Carlisle, Birmingham, Bowen and O'Hartaigh2013).
A key common pathway underlying these findings is expression of inflammation-related genes, which regulate production of inflammatory proteins such as IL-6 and C-reactive proteins. In adults, social adversity, including social isolation (Cole, Capitanio, et al., Reference Cole, Capitanio, Chun, Arevalo, Ma and Cacioppo2015; Cole et al., Reference Cole, Hawkley, Arevalo, Sung, Rose and Cacioppo2007; Cole, Levine, et al., Reference Cole, Levine, Arevalo, Ma, Weir and Crimmins2015), low socioeconomic status (SES) in childhood (Miller, Chen, et al., Reference Miller, Chen, Fok, Walker, Lim, Nicholls and Kobor2009), and familial caregiving for patients with brain cancer (Miller et al., Reference Miller, Chen, Sze, Marin, Arevalo, Doll and Cole2008) are related to upregulated expression of inflammation-related genes in leukocytes (immune cells). Similar findings are also observed in younger adults, with chronic interpersonal stressors predicting increased mRNA expression of the transcription factor nuclear factor kappa B (NF-κB; Miller, Rohleder, et al., Reference Miller, Rohleder and Cole2009), and in children with asthma, where low SES was related to overexpression of inflammation-related genes (Chen et al., Reference Chen, Miller, Walker, Arevalo, Sung and Cole2009). NF-κB plays a critical role within immune cells by regulating inflammatory responses to infection (Lawrence, Reference Lawrence2009); moreover, some data suggest that sympathetic nervous system stimulation of NF-κB may mediate inflammatory responses to psychological stress (Bierhaus et al., Reference Bierhaus, Wolf, Andrassy, Rohleder, Humpert, Petrov and Nawroth2003).
Social adversity in the studies described above was associated with increased expression of genes bearing NF-κB response elements (i.e., stereotyped DNA motifs that serve as genomic “targets” for transcription factor binding). Consistent with indications of glucocorticoid desensitization, these studies also found decreased expression of genes regulated by the glucocorticoid receptor (GR; i.e., bearing GR response elements). In this context, family environments may play a key role: among adults who grew up in low SES households, retrospective reports of more maternal warmth were associated with lower expression of genes bearing NF-κB response elements (Chen, Miller, Kobor, & Cole, Reference Chen, Miller, Kobor and Cole2011). Taken together, these data provide support for a transcriptional response to social adversity that involves increased expression of proinflammatory genes and decreased expression of anti-inflammatory, GR-responsive genes, with the function of promoting inflammation to protect individuals against short-term threats like microbial infections (Irwin & Cole, Reference Irwin and Cole2011). Moreover, the phenotype appears to be conserved throughout development, including childhood (Chen et al., Reference Chen, Miller, Walker, Arevalo, Sung and Cole2009), adolescence (Miller & Chen, Reference Miller and Chen2010), adulthood (Miller, Chen, et al., Reference Miller, Chen, Fok, Walker, Lim, Nicholls and Kobor2009; Miller et al., Reference Miller, Chen, Sze, Marin, Arevalo, Doll and Cole2008, Reference Miller, Murphy, Cashman, Ma, Ma, Arevalo and Cole2014), and old age (Cole, Capitanio, et al., Reference Cole, Capitanio, Chun, Arevalo, Ma and Cacioppo2015; Cole et al., Reference Cole, Hawkley, Arevalo, Sung, Rose and Cacioppo2007; Cole, Levine, et al., Reference Cole, Levine, Arevalo, Ma, Weir and Crimmins2015; O'Connor, Schultze-Florey, Irwin, Arevalo, & Cole, Reference O'Connor, Schultze-Florey, Irwin, Arevalo and Cole2014). However, most research at this point has examined gene expression in adults, with considerably less examination of whether family environments in childhood are concurrently related to gene expression in children and their parents.
In children, one of the expected clinical consequences of a proinflammatory phenotype is greater inflammatory responses to challenge and more severe URI symptoms (Repetti et al., Reference Repetti, Robles and Reynolds2011). URIs are the most common acute illness in children, with an incidence rate of two to three illnesses per year after age 9 (Heikkinen & Jarvinen, Reference Heikkinen and Jarvinen2003), and they account for significant disruptions in normal functioning, including 22 million days of absence from school each year. Inflammation is the key mediator of all the major symptoms people experience during URI infection, including runny and stuffy nose and cough (Eccles, Reference Eccles2005). Accordingly, several studies suggest that stressful family environments are related to greater URI symptom severity in childhood. Greater parent-reported stressful life events over the past year were related to longer URI duration and severity in children between 1 and 11 years old (Boyce et al., Reference Boyce, Jensen, Cassel, Collier, Smith and Ramey1977). Children between 8 and 12 years experiencing recurrent URIs reported greater stressful life events compared to healthy children (Drummond & Hewson-Bower, Reference Drummond and Hewson-Bower1997). Overall, while links between early adversity and increased frequency and severity of URI symptoms have been observed in previous work (Caserta et al., Reference Caserta, O'Connor, Wyman, Wang, Moynihan, Cross and Jin2008; Wyman et al., Reference Wyman, Moynihan, Eberly, Cox, Cross, Jin and Caserta2007), biologically plausible underlying mechanisms related to URI have not been examined in children at the cellular and genomic level.
The primary aim of this study is to test whether conflict and warmth in the family environment is related to inflammatory gene expression in children and parents. The family social environment was assessed through different methods (interview, questionnaire, and diary-based methods) and multiple reporters (children and parents). In order to reduce Type I error in a small sample, composite conflict and warmth scores were based on multimethod ratings from independent reporters. Based on prior work, we hypothesized that high conflict and low warmth would be related to upregulated proinflammatory and downregulated anti-inflammatory gene expression, operationalized as greater expression of genes bearing NF-κB response elements and lower expression of genes bearing GR response elements, respectively. Our final aim was to explore whether conflict and lack of warmth in the family environment were related to greater URI symptom severity observed naturalistically over a 2-month period. We focused specifically on URI symptoms in children during clinical illness, which was more prevalent in children in our sample compared to parents. Based on prior work, we hypothesized that high conflict and low warmth would be related to more severe URI symptoms during clinical illness.
Method
Design
This correlational study tested whether exposure to conflict and warmth in the family social environment is associated with inflammation-related gene expression patterns and URI symptoms. Summary conflict and warmth scores combined standardized scores derived from different measurement methods; we then averaged individual reporter summary scores to create family-level composite scores.
Participants
Families with children 8–13 years old were recruited in the Los Angeles area during 2009–2012 through hardcopy and electronic flyers distributed at local elementary and middle schools, libraries and recreation centers, pediatrics and family medical clinics, newspaper advertisements, and 1,500 direct mailings using a marketing list of families within 5 miles of campus that were selected based on zip-code level income. At least one child and one parent in the target age range were required for the family to be included, although both parents were encouraged to participate. Participants were screened for medical conditions known to confound endocrine and immune measures, including chronic lung conditions (e.g., asthma), endocrine disorders (e.g., Cushing disease), metabolic disease, immunodeficiency, and heart disease or chronic heart conditions. Because the primary focus of the study was the frequency and severity of URIs in children, we applied the exclusion criteria in a more stringent manner for children compared to parents. As a result, several parents in the sample had chronic conditions that could impact the immune system, described in the Covariates section below. Caregivers from all families were screened over the phone; out of 207 initial contacts, 60 families were eligible to participate, and 47 families were enrolled in the study.
The overall sample included 47 mothers, 39 fathers, 47 target children (19 male, 28 female), and 12 siblings (5 male, 7 female). Target children participated in all study procedures, including interviews, questionnaires, diaries, and saliva sampling. The 12 siblings, who were included if they were in the same age range as the target child, but were positioned as younger than the target child in each family, also completed diaries, questionnaires, and saliva sampling. Because the study procedures constituted significant participant burden, the blood sample was not required for participation. Consequently, this paper focuses on participants who agreed to provide blood samples for RNA, which constituted 42 children (31 target, 11 siblings; demographics in Table 1) and 73 parents (demographics in Table 2) from 32 and 40 families, respectively. Children who provided blood samples were more ethnically diverse, χ2 (4) = 8.14, p = .04. The ethnicity of the children who provided blood samples was 16 mixed ethnicity (e.g., non-Hispanic White/Native American, Black/Puerto Rican, and non-Hispanic White/Filipino), 12 White non-Hispanic, 6 Black, 5 Latino, and 3 Asian. The ethnicity of the children who did not provide samples was 12 White non-Hispanic, 3 Black, 2 Latino, and 0 mixed ethnicity or Asian. Children who provided blood samples did not significantly differ in age, gender distribution, parental education, parent income, or body mass index (BMI) from children who did not provide samples. Parents who provided blood samples did not significantly differ in age, gender distribution, education, BMI, employment status (full-time, part-time, etc.), or income compared to parents who did not provide samples; those who provided samples were married for 5.02 fewer years, 95% confidence interval (CI) [1.35, 8.70]. Ethnicity in the subsample of parents was 31 non-Hispanic White, 17 Latino/Hispanic, 15 African American, 8 Asian, 1 Native American, and 1 self-identified mixed ethnicity. Parents’ median education was 12.4 years, and median personal income was within the $31,851–$64,250 tax bracket ($34,001–$82,400 in the third year of data collection).
Table 1. Descriptive statistics for children who provided RNA (N = 42)

Note: BMI, body mass index; URI, upper respiratory infection.
aN = 40.
bN = 29 target children only.
*p < .05.
Table 2. Descriptive statistics for parents who provided blood draws (N = 73)

a Income was assessed by income tax brackets. In the first 2 years of data collection the third income tax bracket was $31,851–$64,250 and in the third year of data collection $34,001–$82,400.
**p < .01.
Procedure
During an initial visit, typically at the family's home, researchers reviewed study procedures and obtained informed consent from parents and assent from children. Parents and children then completed interviews and questionnaires described below. A second visit involved training on daily diaries and saliva collection (full details can be found in Robles, Reynolds, Repetti, & Chung, Reference Robles, Reynolds, Repetti and Chung2013), after which families began completing the 8 weeks of daily diaries online (SurveyMonkey.com) each evening prior to bedtime. Paper diaries were available in case of technical difficulties. After the 8-week daily diary period, parents and children who elected to do so provided a blood sample at the University of California at Los Angeles (UCLA). One family provided samples 2 days before the end of the 8 weeks, the remaining samples were collected between 1 to 85 days after the end of the diary period (Mdn = 15 days). Each parent and child could earn up to $350 and $300, respectively, for completing all study procedures, including a $5 bonus gift card per week of 100% diary compliance (i.e., all diaries were completed on the evening due or before 9 a.m. the next morning). The 8-week diary period was chosen to balance maximizing URI detection with minimizing participant burden. Data collection took place during the 2009–2012 cold and flu seasons that span from October to May in Los Angeles County (http://publichealth.lacounty.gov/acd/FluSurveillance.htm). The protocol was approved by UCLA's Institutional Review Board.
Family conflict and warmth measures
Interview, questionnaire, and diary measures of family conflict and warmth administered to participants are described below. Descriptive statistics for all measures are shown in Tables 1 and 2 for children and parents, respectively.
Interviews
Target children (but not siblings) and parents were separately administered the 60- to 90-min semistructured UCLA Life Stress Interview (LSI; Hammen, Reference Hammen1991), used in 50 + studies of children (Rudolph & Hammen, Reference Rudolph and Hammen1999) and adults, which assesses chronic and episodic stressors in various domains over the past 6 months (e.g., family relationships, work, and school activity). Trained interviewers asked a series of open-ended questions for each domain, with additional probes as necessary to obtain sufficient information to score the domain from 1 (exceptionally good conditions) to 5 (extreme adversity). We adapted the interview to include questions that specifically ask about conflict and (lack of) warmth in parent–target child, target child–sibling, and intimate (marital or marital-like) relationships and used those data to create separate LSI family conflict and LSI family warmth variables based on child and parent reports.
Children
Example open-ended questions in each domain were as follows: parent–child conflict, “How often do you argue or fight with your mother/father?”; interparental relationship conflict, “How often do your parents fight?”; child–sibling conflict, “How often do you argue or fight with your siblings?”; parent–child warmth, “Do you talk to your mother and father about what is happening in your life?”; interparental relationship warmth, “Do your Mom and Dad spend time together?”; child–sibling warmth, “How do you get along with your siblings?” An LSI family conflict score was derived for children by averaging the interviewer conflict ratings from the parent–target child, interparental relationship, and child–sibling domains; and an LSI lack of family warmth score was computed by averaging interviewer warmth ratings from the parent–target child, intimate interparental relationship, and child–sibling domains. Higher scores indicated greater conflict and less warmth.
Parents
Relationships with the target child were assessed separately from relationships with other children in the family. Example open-ended questions in each domain were as follows: parent–child conflict, “How often do you argue or fight with your child?”; intimate relationship conflict, “Do you ever argue or fight with your spouse?”; parent–child warmth, “How do you feel about your time together with your child?”; intimate relationship warmth, “What is your relationship with your spouse like?” An LSI family conflict score was derived for parents by averaging the interviewer conflict scores from the parent–target child conflict, parent–other children conflict, and intimate relationship conflict domains; and an LSI family warmth score by averaging interviewer ratings from the parent–target child warmth, parent–other children warmth, and intimate relationship warmth domains. Higher scores indicated greater conflict and less warmth.
Questionnaires
Target children, siblings, and parents responded to a series of psychometrically sound measures of constructs related to family conflict and family warmth. Unless otherwise specified, scale scores were obtained by taking the mean of all items (after accounting for reverse-scoring when applicable). Descriptive statistics for all questionnaire scales for children and parents are shown in Tables 1 and 2, respectively.
Child report
Three measures assessed family conflict as reported by children, and higher scores on all scales indicated greater conflict. The 19-item Children Perception of Interparental Conflict Scale (α = 0.90; Grych, Seid, & Fincham, Reference Grych, Seid and Fincham1992) assessed perceived interparental conflict with items rated on a 1 (true) to 3 (false) scale. The 9-item Family Environment Scale (FES) conflict subscale (α = 0.70; Moos & Moos, Reference Moos and Moos1986) was adapted for the 8- to 13-year-old age range by using converting responses from the typical 1 (mostly true) to 4 (mostly false) scale to a format similar to the Harter Self-Perception Profile for Children (for more details, see Jaycox & Repetti, Reference Jaycox and Repetti1993). The 12-item Parental Environment Questionnaire (PEQ) conflict subscale (mother ratings, α = 0.91, father ratings, α = 0.81; Elkins, McGue, & Iacono, Reference Elkins, McGue and Iacono1997) was rated on a 1 (definitely false) to 4 (definitely true) scale.
Four measures assessed indicators of family warmth, and higher scores on all scales indicated greater warmth. The 7-item Child's View Questionnaire (Fauchier & Margolin, Reference Fauchier and Margolin2004) assessed the child's perception of interparental affection (α = 0.91) on a 1 (never) to 5 (always) scale. The 5-item Family APGAR Scale assessed satisfaction with family adaptability, partnership, growth, affection, and quality of time spent together on a 0 (never) to 4 (always) scale (α = 0.69; Austin & Huberty, Reference Austin and Huberty1989). The 23-item Parental Warmth Scale (mother ratings, α = 0.96, father ratings, α = 0.92; Repetti, Reference Repetti1996) assessed perceived parent involvement and interest on a 1 (disagree) to 3 (agree) scale. The 12-item PEQ involvement subscale (ratings of maternal involvement, α = 0.80; paternal involvement α = 0.78) was rated on a 1 (definitely false) to 4 (definitely true) scale.
Parent report
Three parent-reported measures assessed family conflict, with higher scores on all scales indicating greater conflict. The 9-item FES conflict subscale (α = 0.63) was rated on a 1 (mostly true) to 4 (mostly false) scale. The 10-item Parent Behavior Inventory (PBI) hostile/coercive subscale was rated on a 0 (not at all true) to 5 (very true) scale (α = 0.73; Lovejoy, Weis, O'Hare, & Rubin, Reference Lovejoy, Weis, O'Hare and Rubin1999). The 12-item PEQ conflict subscale was rated on a 1 (definitely false) to 4 (definitely true) scale (α = 0.93).
Four measures were used to assess indicators of family warmth, with higher scores indicating highly supportive, involved, and satisfying relationships unless otherwise specified. The 32-item Couples Satisfaction Inventory (CSI; Funk & Rogge, Reference Funk and Rogge2007) assessed marital satisfaction using items with multiple formats and the possible range of scores is 0–161 (α = 0.97). Parent–child involvement was assessed with the 10-item PBI supportive/engaged subscale (α = 0.89), and the 12-item PEQ involvement subscale (α = 0.90). The 7-item Parental Withdrawal Scale (PWS; Wood, Repetti, & Roesch, Reference Wood, Repetti and Roesch2004) assessed parent perceptions of disengagement from the target child, with 2 items rated based on frequency (1 = this never or almost never occurs, 4 = this almost always occurs) and 5 items rated on a 1 (definitely false) to 4 (definitely true) scale (α = 0.74). Items were averaged to yield the PWS score, and high scores indicate greater withdrawal. For the PBI, PEQ, and PWS, parents reported on interactions with the target child, and did not report on interactions with the siblings.
Daily diaries
The average child completed 51.8 of the 56 diaries with an average single diary completion time of 4.4 ± 3.1 min; the average number of diaries completed was 53.2 for mothers and 52.6 for fathers with an average completion time of 7.6 ± 4.2 min (see Reynolds, Robles, & Repetti, Reference Reynolds, Robles and Repetti2016, for more details regarding compliance). Reliability for diary measures was estimated using a generalizability theory framework (Shrout & Lane, Reference Shrout and Lane2012). The reliability of between-person differences (R KF), defined as the reliability of average scale ratings from all items and all days for a given scale, was .99 and above for all measures.
Child report
Items were taken from the Youth Everyday Social Interaction and Mood measure (Repetti, Reference Repetti1996). A total of 6 parent–child conflict items (“My [Mom/Dad] got mad at me today,” “I was angry at my [Mom/Dad] today,” and “My [Mom/Dad] punished me today”) and 6 parent–child warmth items, “I had fun with my [Mom/Dad] today,” “My [Mom/Dad] and I got along well today,” and “My [Mom/Dad] gave me love and attention today”) were rated on the following scale: 1 = not at all, 2 = some, 3 = a lot. Two interparental conflict items (“My mom and dad seemed angry with each other today” and “My mom and dad argued today”) and one interparental affection item (“My mom and dad kissed or hugged today”) were also rated on the same 3-point response scale. Within each domain, items were averaged within each day, yielding daily child-report measures of parent–child conflict, parent–child warmth, marital/interparental conflict, and marital/interparental affection scores.
Parent report
Items were primarily drawn from the Parent Home Data Questionnaire (Timmons & Margolin, Reference Timmons and Margolin2015), which assessed parents’ daily experiences and activities, including interactions with the target child and with spouses. Parent–child conflict (9 items; e.g., “Today I was angry with my child” and “Today how angry was your child at you”) and parent–child warmth (4 items; e.g., “Today I showed my child love and affection” and “Today I had fun with my child”) were rated on the following scale: 1 = not at all, 2 = some, 3 = a lot. Marital conflict (14 items; e.g., “I expressed anger or irritation at my partner” and “[My partner] ignored my wishes or needs”) and marital affection (1 item; “My partner and I kissed and hugged each other”) were rated on the same 3-point scale as the parent–child diary items. Within each domain, items were averaged within each day, yielding daily parent-report measures of parent–child conflict, parent–child warmth, marital conflict, and marital affection scores.
Summary and composite family conflict and warmth scores
All child interview, questionnaire, and diary scales were standardized relative to the entire sample of children (including siblings). Parent-reported interview, questionnaire, and diary scales were standardized relative to the entire sample of parents.
For each target child, we averaged standardized scores from the following six measures to create a child-reported family conflict summary score: LSI family conflict (based on the interview with the child), FES conflict, PEQ conflict, Children Perception of Interparental Conflict Scale, diary parent–child conflict, and diary marital/interparental conflict. For the target child-reported family warmth summary, we averaged standardized scores from seven measures: LSI family warmth score, Child's View Questionnaire, Family APGAR, Parental Warmth Scale, PEQ Involvement, diary parent–child warmth, and diary marital/interparental affection. Whenever child ratings of both mothers and fathers were available for the Parental Warmth Scale, PEQ, and diary measures, both sets of ratings were included in the summary scores. Siblings did not complete the LSI, so the composite for siblings did not include the interview-based measures. Descriptive statistics for child-reported summary scores are shown at the bottom of Table 1. Correlations among all child-reported measures are shown in online-only supplementary Table S.1.
For each parent, we averaged standardized scores from the following six measures to yield a parent-reported family conflict summary score: LSI family conflict score (based on the interview with the parent), FES conflict, PBI hostile/coercive, PEQ conflict, diary parent–child conflict, and diary marital conflict. For the parent-reported family warmth summary, we averaged scores from seven measures: LSI family warmth score, PBI supportive/engaged subscale, PEQ involvement subscale, CSI, PWS (reverse scored), diary parent–child warmth, and diary marital affection. Descriptive statistics for parent-reported summary scores are shown at the bottom of Table 2. Correlations among all parent-reported measures are shown in online-only supplementary Table S.2.
The child-reported and parent-reported conflict and warmth summary scores were used to create composite measures of family conflict and warmth that combined ratings from two family members. Even though between-reporter correlations in developmental research are typically in the small to moderate range, having reporters that provide unique (not correlated, perhaps even orthogonal) information is advantageous when combining across reporters (Kraemer et al., Reference Kraemer, Measelle, Ablow, Essex, Boyce and Kupfer2003). Rather than representing redundant information, data from multiple informants can help converge around a particular characteristic (De Los Reyes & Ohannessian, Reference De Los Reyes and Ohannessian2016). Correlations between child- and parent-reported measures are shown in online-only supplementary Tables S.3 and S.4. In this study, correlations between child-reported and parent-reported summary scores were generally small and not statistically significant, with the exception of child- and mother-reported warmth (target child/sibling and mother conflict r = .25, p = .12, warmth r = .53, p < .001; target child/sibling and father conflict r = .23, p = .17, warmth r = .20, p = .25). Composite measures of the family social environment were computed by incorporating perspectives from two reporters.Footnote 1 For the prediction of gene expression in target children, the child-reported and mother-reported summary scores were averaged and, for siblings, the sibling-reported and mother-reported summary scores were averaged.Footnote 2 For parents, we averaged the target child-reported and parent-reported summary scores together (e.g., for a given mother in the sample, composite family conflict was computed by averaging target child- and mother-reported summary scores; for a given father in the sample, composite family conflict was computed by averaging target child- and father-reported summary scores).
Composite conflict and warmth scores, as well as the interview, questionnaire, and daily diary scales making up those composites, did not differ between participants who provided blood draws and participants who did not provide blood draws (Tables 1 and 2). Figure 1 shows distributions and associations between conflict and warmth composites for children and parents. Conflict and warmth composites were strongly negatively correlated, with 56% and 43% shared variance for children and parents, respectively.

Figure 1. Associations between conflict and warmth composite z-scores for children (n = 42) and parents separately (n = 73). The broken x- and y-axis lines indicate the upper and lower tertiles for warmth and conflict, respectively. The solid black line indicates the best fit linear regression line, and the gray shaded band represents the 95% confidence interval. The gray line indicates the line of equality (perfect fit). Pearson correlation coefficients are reported, along with 95% confidence interval and p values.
URI measures
Each day children self-reported eight common URI signs and symptoms (“Jackson” symptoms; Cohen, Doyle, & Skoner, Reference Cohen, Doyle and Skoner1999; Turner Cobb & Steptoe, Reference Turner Cobb and Steptoe1996): congestion, runny nose, sneezing, cough, sore throat, malaise, headache, and chills (for children “malaise” was replaced with “felt sick” for age appropriateness) on a 0 = no or 1 = yes scale. Items were summed each day, yielding a daily child URI symptoms score ranging from 0 to 8. Parents self-reported the same signs and symptom for themselves, with items rated on a scale of 0 (none) to 4 (very severe). Items were summed each day, yielding a daily parent URI symptoms score ranging from 0 to 32.
Family members were instructed to contact the laboratory when a target child, sibling, or parent might be sick. In addition, URI symptoms from the online diaries were monitored by lab staff using a diagnostic algorithm based on prior empirical research. The algorithm assigned a URI diagnosis on each day when any two of runny nose, nasal congestion, or cough were present, or runny nose or nasal congestion was present given a prior-day algorithm-assigned URI diagnosis (Doyle & Alper, Reference Doyle and Alper2007). Runny nose, cough, and congestion were similarly identified as key diagnostic symptoms in prior studies of naturally occurring, biologically verified viral URIs (Pappas, Hendley, Hayden, & Winther, Reference Pappas, Hendley, Hayden and Winther2008; Taylor, Weber, Martin, McCarty, & Englund, Reference Taylor, Weber, Martin, McCarty and Englund2010).
When algorithm criteria were met or when a family member contacted the lab, two clinical researchers (typically nursing students trained by Dr. Chung, a pediatrician) visited the family's home no more than 48 hr after initial symptoms were reported to verify suspected URIs and collect a nasal wash sample. The physical examination included noting the child's temperature and respiratory rate; noting symptoms of cough, congestion, and sneezing; visual inspection of the eyes for redness and discharge, and the nose and throat for redness, edema, and secretions; palpitation of the neck to note size and tenderness of lymph nodes; visual inspection of the chest; and listening to participants’ lungs for signs of respiratory congestion. Each rater indicated his or her diagnosis; the clinical diagnoses and daily URI symptom data were reviewed by Dr. Chung, who resolved any discrepancies between raters. The primary diagnoses besides URI were allergic rhinitis and infections that were not upper respiratory in origin (e.g., lower respiratory tract infections).
Covariates
A number of factors could confound associations between family environments and gene expression. Demographic variables treated as covariates in the analyses presented below include parent and child ages and sex, and parent educational status. BMI was computed by dividing weight (kg; balance beam scale) by height (m2; stadiometer). For children, BMI z scores (standardized within age group; National Center for Chronic Disease Prevention and Health Promotion, 2016) were used as a covariate, which is appropriate for cross-sectional analyses (Must & Anderson, Reference Must and Anderson2006). The Pubertal Development Scale (PDS) assessed child physical changes associated with puberty (Petersen, Crockett, Richards, & Boxer, Reference Petersen, Crockett, Richards and Boxer1988). For parents, taking medication to treat chronic illness was also included as a covariate; conditions included hypertension (n = 5), inflammatory conditions (n = 5), hypothyroidism (n = 4), depression/anxiety (n = 3), and attention-deficit/hyperactivity disorder (n = 1). The average number of alcoholic drinks per day and smoking assessed over the 56 days were also included as parent covariates.
Gene expression measures
At the beginning of the final family visit (occurring between noon and 7 p.m.), blood was collected from participating children at the UCLA Clinical Laboratory through antecubital venipuncture in PAXgene Blood RNA tubes (two tubes, total 5 ml; Qiagen), which were chilled and transported to the UCLA Health Psychology Laboratory for storage at –80 °C. All PAXgene specimens were assayed in a single batch, with RNA extracted (Qiagen RNeasy), tested for suitable mass (Nanodrop ND1000) and integrity (Agilent Bioanalyzer), converted to fluorescence-tagged cRNA (Ambion TotalPrep), and hybridized to Illumina Human HT-12 v4 BeadArrays following the manufacturer's standard protocol in the UCLA Neuroscience Genomics Core Laboratory. All samples yielded valid results according to standard quality assurance methods (e.g., median probe fluorescence intensity > 100 units).
Data analysis
Tests of family conflict and warmth as predictors of gene expression
Analysis of differential gene expression was conducted separately for children (siblings included) and parents. Raw gene expression data were quantile-normalized (Bolstad, Irizarry, Astrand, & Speed, Reference Bolstad, Irizarry, Astrand and Speed2003) and log2-transformed for standard linear model analyses quantifying the magnitude of differential gene expression as a function of predictors of interest while controlling for a priori specified covariates. The basic model for a specific gene was specified as follows:
where μ is the overall mean expression, i is the child, and ε i is the random error, normally distributed around 0. Here, the β1 family composite i represents expression of a given gene transcript as a function of the lowest to the highest tertiles on the family composite measure of interest (conflict or warmth composite; tertiles were coded as 0, 0.5, and 1.0 in linear models) after controlling for k covariates. Separate models were specified with the family conflict or family warmth composite as the primary independent variable, and then a third model was specified including both conflict and warmth composites as predictors in the same analyses. For child gene expression, a priori covariates included sex, age, child ethnicity, parent income, BMI percentile for age, and PDS score. Due to missing PDS data from 2 children, the total sample size for analysis was 40. For parent gene expression, a priori covariates included sex, age, income, BMI, taking medication to treat chronic illness, number of alcoholic drinks consumed per week, and parent smoking status. Due to missing BMI from 1 parent, the total sample size for analysis was 72.
Differentially expressed genes were identified as those showing a >1.2-fold difference in expression in samples from the lowest to the highest tertiles on each predictor of interest (conflict composite and warmth composite; tertiles were coded as 0, 0.5, and 1.0 in linear models). The 1.2-fold threshold is consistent with prior studies linking psychosocial factors to gene expression (e.g., Chen et al., Reference Chen, Miller, Kobor and Cole2011; Cole, Capitano, et al., Reference Cole, Levine, Arevalo, Ma, Weir and Crimmins2015). Differentially expressed genes were not interpreted or tested for statistically reliable association on an individual basis, but served only as inputs into higher order bioinformatics analyses (Transcription Element Listening System [TELiS]; http://www.telis.ucla.edu). Briefly, TELiS compares the promoter regions of differentially expressed genes to matrices of known transcription factor binding motifs (specific sequences that are known binding sites for transcription factors) in the promoter region of genes (Cole, Yan, Galic, Arevalo, & Zack, Reference Cole, Yan, Galic, Arevalo and Zack2005). Data that populates the matrices comes from the TRANSFAC v3.2 database (http://www.gene-regulation.com). Transcription factor binding motifs that are overrepresented in the promoter regions of differentially expressed genes are then used to infer whether a particular transcription factor is active. We tested a priori hypotheses regarding relative activity of proinflammatory NF-κB transcription factors (assessed by the prevalence of TRANSFAC V$NFKAPPAB_01 and V$NFKB_Q6 DNA motifs) and the anti-inflammatory GR (V$GR_Q6) that mediates hypothalamus–pituitary–adrenal (HPA) axis signaling (Cole et al., Reference Cole, Yan, Galic, Arevalo and Zack2005). Results were averaged over nine parametric variations of MatInspector scan stringency (0.80, 0.90, and 0.95) and promoter length (–300 nucleotides upstream of the gene's transcription start site, –600, and –1000 to +200; rationale described in Cole et al., Reference Cole, Yan, Galic, Arevalo and Zack2005). The pooled association estimate was tested for a statistically significant difference from 0 (null association) using a single-sample t test with bootstrap standard errors (200 cycles of resampled residual vectors, which controls for any potential correlation among residuals across genes; Efron & Tibshirani, Reference Efron and Tibshirani1993).
Transcript origin analysis was also applied to the differentially expressed gene sets to identify the specific peripheral blood mononuclear cell subtypes mediating the observed differences in gene expression, as previously described (Cole, Hawkley, Arevalo, & Cacioppo, Reference Cole, Hawkley, Arevalo and Cacioppo2011). Transcript origin analysis identifies the specific leukocyte subsets (e.g., cluster of differentiation 4 [CD4+] or CD8+ T cells, B cells, natural killer [NK] cells, monocytes, and plasmacytoid dendritic cells) that contribute to the observed transcriptome differences by quantifying the extent to which the genes with empirical differential expression in the present study of a heterogeneous leukocyte pool are expressed predominately by a single type of cell (based on a previously conducted reference study of physically isolated cell types; Cole et al., Reference Cole, Hawkley, Arevalo and Cacioppo2011).
Tests of family conflict and warmth as predictors of children's URI symptoms
Table 3 shows the frequencies of parents and children in the sample (the full sample and those with gene expression data) who had at least one algorithm-identified episode, more than one algorithm-identified episode, and clinically verified episodes. Episodes were defined as meeting algorithm criteria for at least 1 day. The beginning of the verified episode was the first day that the child met algorithm criteria; the end of the episode was the last day that the child met algorithm criteria and was followed by at least 2 days of not meeting algorithm criteria. The association between family conflict/warmth composites and children's URI symptom severity was computed for the 12 children with clinically verified URI episodes. Of the 15 children with at least one algorithm-identified episode, three had more than one episode (one with one additional episode and two with two additional episodes), and each of those children had only one verified episode. Thus, we focused on symptom severity during the verified episode. We computed a sum of the symptoms over the course of the entire verified episode, divided by episode duration in days, which yielded average URI symptoms per day during the verified episode for each child. URI episodes ranged in duration from 3 to 11 days, and the average episode was 6.5 days in length (SD = 3.12). Because the number of parents with verified URI episodes was extremely small (7 out of 87 parents [8%] compared with 12 out of 59 children [20%]), we did not conduct any further analyses on associations between family conflict/warmth and URI symptom severity in parents.
Table 3. Frequencies of URI episodes for parents and children

We tested whether there was a significant correlation between family conflict/warmth and average URI symptom severity on days that did not involve a clinically verified URI episode. Because we could include the entire sample in these analyses, we had greater power to test the alternative possibility that family conflict/warmth was related to greater symptom reporting in general, even when children where not verifiably ill with a URI. We then examined associations between family conflict/warmth and average URI symptom severity during verified episodes using Pearson and Spearman correlations. To test alternative explanations, particularly related to the role of negative affectivity, which in prior viral challenge work was related to greater symptom reports (Cohen et al., Reference Cohen, Gwaltney, Doyle, Skoner, Fireman and Newsom1995), we conducted partial correlations controlling for average negative affect over the 2-month diary.
For a more stringent test using all available daily diary data, we used a generalized mixed model (PROC GLIMMIX, SAS) based on a Poisson distribution described below, which is more appropriate for count data that is not normally distributed. The model was specified as follows:
 $$\eqalign{&\ln (\hbox {daily URI symptom count}_{ij}) = {\rm \beta }_0 + {\rm \beta}_{1} (\hbox{family composite}_i) \cr & \quad\quad\ \, + {\rm \beta }_2 ({\rm episode}_j) + {\rm \beta}_3 (\hbox {family composite}_i \times {\rm episode}_j).}$$
$$\eqalign{&\ln (\hbox {daily URI symptom count}_{ij}) = {\rm \beta }_0 + {\rm \beta}_{1} (\hbox{family composite}_i) \cr & \quad\quad\ \, + {\rm \beta }_2 ({\rm episode}_j) + {\rm \beta}_3 (\hbox {family composite}_i \times {\rm episode}_j).}$$
Family composite i refers to the family conflict or warmth composite; episode j = 0 during days that were not part of a verified URI episode; episode j = 1 during days that were part of a verified URI episode; and β3 represents the critical interaction term that tests whether the association between URI symptoms and greater family conflict (or lower warmth) is stronger during verified URI episodes. We also tested a model that included daily negative affect as a covariate.
Results
Child gene expression
Analyses relating children's gene expression to measures of family conflict identified 248 gene transcripts showing >1.2-fold difference in average expression between the highest and lowest tertiles after controlling for child age, sex, ethnicity, BMI, PDS score, and family income (157 upregulated and 91 downregulated, specific transcripts listed in online-only supplementary Table S.1a). TELiS promoter-based bioinformatics analyses linked these transcriptome differences to greater activity of proinflammatory transcription factor NF-κB (Figure 2a) but indicated no difference in GR activity. Transcript origin analyses identified monocytes and dendritic cells as the primary cellular source of differentially expressed genes (Figure 2b).

Figure 2. Among children in the sample, transcriptional activity of nuclear factor kappa B (NF-κB) and glucocorticoid receptor transcription factors as assessed by Transcription Element Listening System (a, c, e) bioinformatics analysis of transcription factor-binding motif (TFBM) prevalence in promoter DNA sequences and (b, d, f) transcript origin analyses identifying major leukocyte subset origins of genes showing ≥1.2-fold differential gene expression in high versus low tertiles of the following primary independent variable(s): (a, b) family conflict composite, (c, d) family warmth composite, and (e, f) family conflict and warmth when both composites are included in the same analyses. Numerical values along the x-axis (rotated) are p values.
Parallel analyses relating child gene expression to family warmth identified 617 gene transcripts showing >1.2-fold difference in average expression (278 upregulated, 339 downregulated, online-only supplementary Table S.1b). Bioinformatics analyses linked these transcriptome differences to lower NF-κB activity and no substantial difference in GR activity (Figure 2c). Transcript origin analyses identified monocytes, dendritic cells, and B-cells as primary cellular sources of differentially expressed genes (Figure 2d).
Simultaneous analyses of family conflict and warmth identified 422 gene transcripts showing >1.2-fold difference in average expression as a function of conflict (184 upregulated, 238 downregulated, online-only supplementary Table S.1c) and 1,224 transcripts showing >1.2-fold difference in average expression as a function of family warmth (640 upregulated, 584 downregulated, online-only supplementary Table S.1c). In contrast to the previous analyses with either conflict or warmth as predictors, bioinformatics analyses did not show associations between transcription factor activity (NF-κB or GR) and family conflict or warmth when both independent variables were included in the same analyses (Figure 2e). Transcript origin analyses identified monocytes, dendritic cell, NK cells, and B-cells as primary cellular origins of differentially expressed transcripts, with dendritic cells as sources of transcripts primarily linked to conflict and B cells as sources of transcripts linked to warmth (Figure 2f).
Child URI symptoms
The associations between family conflict/warmth and average URI symptoms per day for the entire sample, including children who did not provide a blood sample, are shown in Figure 3. The top half of Figure 3 shows that greater family conflict was related to greater average URI symptoms on days that did not meet algorithm criteria for a URI episode, r = .28, p = .03. The association was small in magnitude, and was no longer statistically significant after controlling for average negative mood over the 2-month diary period, partial r = .20, p = .13. The correlation between average negative mood and average URI symptoms on non-URI episode days was also small in magnitude, r = .24, p = .07. The family warmth composite was not related to URI symptoms on non-URI episode days, with or without controls for average negative mood.
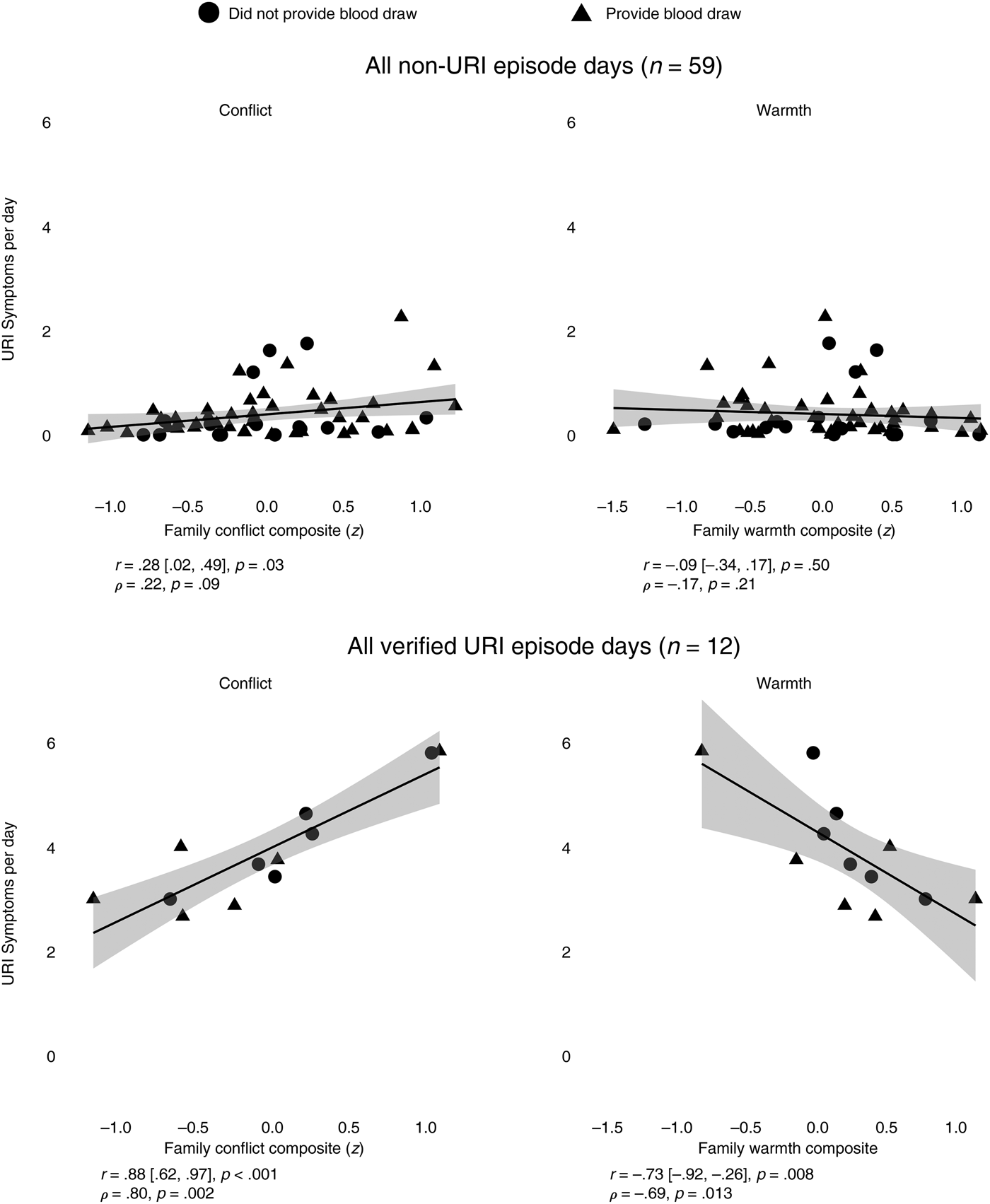
Figure 3. Associations between family conflict/warmth composites and children's upper respiratory infection (URI) symptoms per day, when averaged across all days without an algorithm-identified URI episode (top half), and when averaged across days with an algorithm- and clinically verified URI episode (bottom half). Only 12 children in the sample showed evidence of algorithm- and clinically verified URI episodes. The solid gray line indicates best fit linear regression line, and the gray shaded band represents 95% confidence interval. Pearson correlation coefficients are reported, along with 95% confidence interval and p values, followed by Spearman rank-order correlation coefficients and p values.
In contrast, the bottom half of Figure 3 shows that greater family conflict and lower family warmth were related to greater URI symptoms on days that met algorithm criteria and were part of clinically verified episodes. The sample size was considerably smaller (n = 12) relative to the entire sample, and thus the large effect sizes for conflict and warmth (rs = .88 and –.73, respectively) should be interpreted with caution. Even after controlling for average negative mood over the 2-month diary period, both greater conflict (r = .88, p = .0003) and less warmth (r = –.69, p = .02) were related to more severe URI symptoms. The correlation between average negative mood and URI symptoms on verified URI episode days was not statistically significant (r = .36, p = .26).
Results from the generalized mixed model analyses of daily symptom data are shown in Table 4. In these more stringent tests, greater family conflict was related to greater URI symptoms during days when children did not have a URI episode in the entire sample and in the children that had verified URI episodes during the study; the effect held after accounting for daily negative affect. Greater family warmth was related to fewer URI symptoms during days when children did not have a URI episode, but only when the sample was restricted to children that had verified URI episodes during the study. The Family Composite × Episode interactions were in the expected direction, but were not statistically significant (ps = .12–.15). In sum, the results are partially consistent with the expectation that children in families with greater conflict and less warmth should show evidence of larger inflammatory responses to infectious threats. While greater conflict and less warmth were related to more severe URI symptoms during clinically verified URI episodes, the mixed models showed that greater conflict was related to more severe URI symptoms on days when children did not have a URI episode, and the Family Composite × Episode interactions were not statistically significant.
Table 4. Generalized mixed models predicting URI symptoms as function of URI episodes and family conflict/warmth composites

Note: All parameters are unstandardized ln(β) units from generalized mixed models based on a Poisson distribution. URI, upper respiratory infection.
Parent gene expression
Analyses relating parental gene expression to measures of family conflict identified 335 gene transcripts showing >1.2-fold difference in average expression between the highest and lowest tertiles after controlling for parental age, sex, income, BMI, presence of a chronic illness, alcohol consumption, and smoking (44 upregulated and 291 downregulated, online-only supplementary Table S.2a). TELiS promoter-based bioinformatics analyses linked these transcriptome differences to greater proinflammatory transcription factor NF-κB activity and lower GR activity (Figure 4a). Transcript origin analyses identified monocytes as the primary cellular source of differentially expressed genes (Figure 4b).

Figure 4. Among parents in the sample, transcriptional activity of nuclear factor kappa B (NF-κB) and glucocorticoid receptor transcription factors as assessed by Transcription Element Listening System (a, c, e) bioinformatics analysis of transcription factor-binding motif (TFBM) prevalence in promoter DNA sequences and (b, d, f) transcript origin analyses identifying major leukocyte subset origins of genes showing ≥1.2-fold differential gene expression in high versus low tertiles of the following primary independent variable(s): (a, b) family conflict composite, (c, d) family warmth composite, and (e, f) family conflict and warmth when both composites are included in the same analyses. Numerical values along the x-axis (rotated) are p values.
Parallel analyses relating parent gene expression to measures of family warmth identified 364 gene transcripts showing >1.2-fold difference in average expression (263 upregulated, 101 downregulated, online-only supplementary Table S.2b). TELiS promoter-based bioinformatics analyses found no significant link to differential activity of either NF-κB or GR (Figure 4c). Transcript origin analyses also failed to identify any particular leukocyte subset as a primary cellular source of differentially expressed genes except dendritic cells (Figure 4d).
Simultaneous analyses of family warmth and conflict identified 346 transcripts showing >1.2-fold difference in average expression as a function of conflict (97 upregulated, 249 downregulated, online-only supplementary Table S.2c) and 283 gene transcripts showing >1.2-fold difference in average expression as a function of warmth (152 upregulated, 131 downregulated, online-only supplementary Table S.2c). In promoter-based bioinformatics analyses, differential NF-κB activity was associated predominately with low family warmth (independent of conflict), whereas a trend-level difference in GR activity was associated predominately with family conflict (independent of warmth); both patterns were in the predicted directions (Figure 4e). Transcript origin analyses identified monocytes as primary cellular sources of transcripts associated with family conflict and dendritic cells as primary origin of transcripts associated with family warmth (Figure 4f).
Discussion
In a diverse sample of families, family conflict and warmth were related to differential expression of leukocyte genes bearing NF-κB response elements in children and parents. Greater conflict was related to upregulation, and greater warmth was related to downregulation of NF-κB responsive genes in immune cells. To our knowledge, this study is one of the first to demonstrate associations between concurrent family environment characteristics and patterns of leukocyte gene expression in children and parents, and provides additional support for conceptual frameworks that propose that adversity in the family environment is related to a proinflammatory phenotype (Miller et al., Reference Miller, Chen and Parker2011; Repetti et al., Reference Repetti, Robles and Reynolds2011). In children, the family environment, particularly family conflict, was related to greater upregulation of NF-κB responsive genes, with no statistically significant associations with GR responsive genes. For parents, the pattern of results for family conflict was similar to the upregulated NF-κB–responsive and downregulated GR-responsive gene expression pattern observed with other social adversities in adults, including chronic interpersonal stress in young adults (Miller, Rohleder, et al., Reference Miller, Rohleder and Cole2009), caregiving for a family member with brain cancer (Miller et al., Reference Miller, Chen, Sze, Marin, Arevalo, Doll and Cole2008), and the combination of low childhood SES and low maternal warmth in childhood (Chen et al., Reference Chen, Miller, Kobor and Cole2011). Extending the proinflammatory phenotype findings from the subset of children who provided RNA to plausible clinical manifestations, among children with a clinically verifiable URI, greater family conflict and lower family warmth were associated with more severe URI symptoms. Of note, greater family conflict was related to more severe URI symptoms, independent of negative affectivity, which often accounts for links between social factors and physical symptoms (Cohen et al., Reference Cohen, Gwaltney, Doyle, Skoner, Fireman and Newsom1995). However, the hypothesis that greater conflict and less warmth would have a stronger association with URI symptoms during URI episodes was not strongly supported.
This study and others implicating NF-κB as a key molecular mechanism suggest that pathways that (a) act on the NF-κB signaling pathway and (b) transduce signals about the social environment should be biologically plausible mechanisms linking family conflict and warmth to inflammation, as well as health more broadly. One candidate pathway is sympathetic nervous system (SNS) activation; norepinephrine is the major SNS neurotransmitter, and when norepinephrine acts on immune cells via binding to β-adrenergic receptors, this leads to increased transcription of proinflammatory genes (Cole et al., Reference Cole, Arevalo, Takahashi, Sloan, Lutgendorf, Sood and Seeman2010; Grebe et al., Reference Grebe, Takeda, Hickman, Bailey, Embryo, Bennink and Yewdell2010). Moreover, acute psychological stressors that increase SNS activation are associated with increased NF-κB activity in circulating leukocytes (Bierhaus et al., Reference Bierhaus, Wolf, Andrassy, Rohleder, Humpert, Petrov and Nawroth2003). Thus, variations in SNS activity as a function of the family environment, which have been observed in several large samples of children in early (Quas et al., Reference Quas, Yim, Oberlander, Nordstokke, Essex, Armstrong and Boyce2014) to middle childhood (Del Giudice, Hinnant, Ellis, & El-Sheikh, Reference Del Giudice, Hinnant, Ellis and El-Sheikh2012; El-Sheikh, Reference El-Sheikh2005) may explain how greater family conflict may be related to upregulated NF-κB responsive genes, and how greater family warmth may be related to downregulated NF-κB responsive genes. Alternatively, greater parasympathetic nervous system activation may play a mediating role linking family environments to gene expression. For example, in an adolescent sample increased high-frequency heart rate variability, an index of increased parasympathetic activation, in response to acute psychological stress was related to greater family warmth (Salomon, Matthews, & Allen, Reference Salomon, Matthews and Allen2000; cf. Del Giudice et al., Reference Del Giudice, Hinnant, Ellis and El-Sheikh2012), and acetylcholine (the major parasympathetic nervous system neurotransmitter) can downregulate NF-κB signaling during an inflammatory response to infectious stimuli (Czura & Tracey, Reference Czura and Tracey2005).
Another mechanism beyond direct autonomic input to circulating immune cells that may operate in both children and adults involves increased production and/or activation of monocytes and dendritic cells: myeloid lineage immune cells that serve as primary mediators of proinflammatory gene expression. Previous experimental studies have linked chronic social threat to increased production of these myeloid lineage cells via increased SNS activity (Cole, Capitanio, et al., Reference Cole, Capitanio, Chun, Arevalo, Ma and Cacioppo2015; Powell et al., Reference Powell, Sloan, Bailey, Arevalo, Miller, Chen and Cole2013), and bioinformatics analyses in the present sample implicated monocytes and dendritic cells as origins of the differential gene expression profiles observed here. Finally, while adipose tissue is a major source of inflammatory mediators, the statistical analyses controlled for BMI (O'Connor et al., Reference O'Connor, Bower, Cho, Creswell, Dimitrov, Hamby and Irwin2009), which suggests other pathways linking family environments to proinflammatory gene expression beyond adiposity itself.
Family conflict and warmth were conceptualized as separate but correlated dimensions, consistent with prior theory and research (Brody, Reference Brody1998; Fincham & Linfield, Reference Fincham and Linfield1997; Mattson et al., Reference Mattson, Rogge, Johnson, Davidson and Fincham2013; Repetti et al., Reference Repetti, Taylor and Seeman2002; Skinner et al., Reference Skinner, Johnson and Snyder2005). That said, the warmth and conflict summary (within reporter) and composite scores (between family) had high levels of shared variance, particularly among the children in our sample. That source of multicollinearity, combined with a small sample size, likely contributed to the loss of the statistically significant association between downregulated NF-κB expression and family warmth after controlling for conflict. At the same time, the direction of associations was similar to the direction found for parents, who showed stronger and statistically significant associations, perhaps due in part to a larger sample size. For parents, family warmth and family conflict had somewhat distinct biological correlates. Proinflammatory NF-κB–related gene expression was most strongly related to low family warmth (i.e., independent of high levels of family conflict) in both children and parents. In contrast, GR-related gene expression was more strongly related to family conflict (independent of warmth) among parents. The basis for such fine-grained biological differentiation remains to be explored in future research, but may potentially involve a differential impact of conflict and warmth on different neuroendocrine signaling pathways to the immune system. However, the differential associations between conflict and warmth were not tested for statistical significance in this study (due to limited statistical power) so the observed “dissociations” may also reflect sampling variability or differences in statistical power (e.g., related to potential differences in the reliability of the measures of conflict vs. warmth). As such, the notion of distinct biological correlates of different dimensions of the family social environment should be treated as preliminary and subject to future confirmatory studies.
Both children and parents showed associations between family characteristics and NF-κB related gene expression, but only adults showed downregulation of GR related to family functioning (primarily greater family conflict). Proinflammatory NF-κB–related gene expression was related to risky family conditions (conflict or low warmth) for both children and adults. In adults, these effects are paralleled to some extent by reduced GR activity, suggesting a potential role for long-term failure of the HPA axis to inhibit proinflammatory signaling dynamics. However, this study found no evidence of lower GR activity in children exposed to risky family characteristics, suggesting that other pathways may contribute to proinflammatory transcriptome skewing early in life (e.g., increased production and/or activation of myeloid lineage immune cells). With that in mind, it is conceivable that the decreased GR activity observed in adults is a late-maturing feature of the same cellular dynamic that plays out over decades. The HPA axis undergoes changes during puberty and adolescence (Gunnar & Quevedo, Reference Gunnar and Quevedo2007; Gunnar, Frenn, Wewerka, & Van Ryzin, Reference Gunnar, Frenn, Wewerka and Van Ryzin2009), as do the neural regions that regulate HPA axis output, such as the amygdala and hippocampus (Goddings et al., Reference Goddings, Mills, Clasen, Giedd, Viner and Blakemore2014).
Our results come from a cross-sectional, modestly sized sample (with a smaller sample of children), and need to be replicated in future studies. With multiple methods of measurement (interview, multiple questionnaires, and diary scales), a structural equation modeling approach creating latent conflict and warmth variables would be ideal; however, our sample size precluded such an approach. In addition, the sample of children included 10 pairs of siblings from the same family (out of 32 families), and 33 spousal pairs from the same family (out of 40 families), which could potentially result in correlation among observed gene expression profiles. Preliminary analyses indicated no detectable empirical correlation in gene expression among siblings or couples, and given the limited sample size available and the sporadic nature of these relationships (i.e., most sampled participants did not have another family member present in the data set), it was not feasible to control for such effects in the data analyses. Nonetheless, potential intrafamilial correlations in gene expression should be assessed in future studies using more structured sampling strategies and larger sample sizes. Despite having a 2-month period, only one-fifth of the sample developed clinically verifiable URI episodes, suggesting the need for longer observation periods in naturalistic URI studies. Not requiring the blood sample as a requirement for participation may have introduced selection bias, although we found no problematic differences between participants who did and did not provide blood samples in demographic or family environment measures. While our sample drew from a wide range of socioeconomic strata and race/ethnic groups, children and parents in our sample were generally healthy and were on favorable ends of both conflict and warmth dimensions, which combined with generating sample-specific summary and composite scores limits the immediate generalizability of our findings (Evans, Li, & Whipple, Reference Evans, Li and Whipple2013). In addition, range restriction likely biased our estimates of the association between family functioning and gene expression in a downward direction. Thus, our findings may actually underestimate differences in inflammatory processes and clinical URI outcomes between high conflict/low warmth and low conflict/high warmth families, suggesting the need for including more “risky” families in future work.
Our findings provide preliminary evidence at the molecular level that family environments characterized by higher conflict and less warmth are related to an enhanced state of preparedness in innate immune cells (Miller et al., Reference Miller, Chen and Parker2011; Repetti et al., Reference Repetti, Robles and Reynolds2011). Moreover, for children, stressful family environments may manifest at a clinical level as well, as indicated by more severe URI symptoms. Similar to other research on social adversity and inflammation, NF-κB regulation of inflammation appears to be a key common pathway for children and adults, as well as GR regulation in adults. The preliminary URI symptom findings tentatively suggest that children with greater family conflict (and presumably a proinflammatory phenotype), when challenged with infectious threats, may respond with heightened inflammation, which underlies all URI signs and symptoms (Eccles, Reference Eccles2005). However, more definitive conclusions will require more adequately powered samples with a larger number of children with verified URI episodes. Ultimately, experimentally manipulating family conditions through family-based interventions will be critical for defining the causal relationships involved in the associations observed here. For example, inflammatory responses might alter affective or social processes via inflammatory regulation of central nervous system function (Dantzer, O'Connor, Freund, Johnson, & Kelley, Reference Dantzer, O'Connor, Freund, Johnson and Kelley2008), and thereby affect the family environment rather than vice versa. Finally, a key direction for future work will be documenting whether similar principles apply for other acute and chronic illnesses that involve stimulated innate immune cells (e.g., monocytes and dendritic cells) as a key player in their pathophysiology. Innate immune cells respond to other types of stimulation besides viruses and bacteria, such as airborne environmental pollutants (Wu et al., Reference Wu, Muller, Berhane, Fruin, Liu, Jaspers and McConnell2014) and lipids in high-fat meals (Herieka & Erridge, Reference Herieka and Erridge2014). Accordingly, the link between family environments and proinflammatory signaling may have wide-ranging implications for health across the life span.
Supplementary Material
To view the supplementary material for this article, please visit https://doi.org/10.1017/S0954579417000591.










